Abstract
In previous calculations of how the O2 transport system limits V̇O2max, it was reasonably assumed that mitochondrial PO2 (PmO2) could be neglected (set to zero). However, in reality, PmO2 must exceed zero and the red cell to mitochondrion diffusion gradient may therefore be reduced, impairing diffusive transport of O2 and V̇O2max. Accordingly, we investigated the influence of PmO2 on these calculations by coupling previously used equations for O2 transport to one for mitochondrial respiration relating mitochondrial V̇O2 to PO2. This hyperbolic function, characterized by its P50 and V̇MAX, allowed PmO2 to become a model output (rather than set to zero as previously). Simulations using data from exercising normal subjects showed that at V̇O2max, PmO2was usually < 1 mm Hg, and that the effects on V̇O2max were minimal. However, when O2 transport capacity exceeded mitochondrial V̇MAX, or if P50 were elevated, PmO2 often reached double digit values, thereby reducing the diffusion gradient and significantly decreasing V̇O2max.
Keywords: bioenergetics, mitochondrial respiration, mitochondrial PO2, oxygen transport, V̇O2max
1. Introduction
At rest or during exercise, production of ATP requires both physical O2 transport from the environment to the mitochondria and subsequent chemical utilization of O2 by oxidative phosphorylation. Oxygen transport has been well described (Dejours, 1966; Gnaiger et al., 1998; Weibel et al., 1981) based on the O2 transport pathway, consisting of the lungs/chest wall, the heart, vascular tree and blood, and the tissues. These structures conduct O2 as an in-series system in which the main sequential transport steps are ventilation, alveolar-capillary diffusion, circulatory transport, and tissue capillary to mitochondrial diffusion. At each step, the mass of O2 must be conserved, and this allows a set of simple equations to be defined (Wagner, 1993, 1996b) that quantifies how the transport process at each step integrates with those of the other steps to determine how much O2 is delivered to the mitochondria per minute (Wagner, 1996a). In this construct, it is shown that each of the four steps contributes to limitation to V̇O2max and that the quantitative effects of changes at each step are similar.
Systems physiological investigations (Wagner, 1993, 1996b) targeting the understanding of the limits to maximal V̇O2, have previously been performed on the basis of an important simplifying approximation. This has been that the downstream mitochondrial PO2 (PmO2) is so small in comparison to tissue capillary PO2 that it can be ignored and therefore set to zero, thus making the analyses of O2 transport much more tractable. However, because O2 is one of the molecules that drive oxidative phosphorylation according to the law of mass action, this approximation cannot be physiologically correct, or otherwise V̇O2 would itself be zero.
Given that PmO2 must exceed zero, the PO2 difference between red cells and mitochondria must be less than when PmO2 is assumed to be zero, and thus the diffusive movement of O2 between them must also be reduced. Therefore, if PmO2 is now considered as greater than zero, there is an additional resistance, from the process of mitochondrial respiration, to O2 movement through the entire pathway of O2 transport and utilization. We therefore hypothesize that this additional resistance must reduce maximal V̇O2 below that which would be expected if this resistance were ignored. Clearly, the degree to which V̇O2max would be reduced will depend on how the high mitochondrial PO2 rises above zero. This in turn will depend broadly on the capacity for O2 transport (how many O2 molecules can be delivered to the mitochondria per minute) compared to the capacity for metabolism (how many O2 molecules can be consumed by the mitochondria per minute).
The importance of including consideration of oxidative phosphorylation goes beyond asking how much does mitochondrial respiration contribute to the overall impedance to V̇O2. Because the value of PmO2 is dependent on the mitochondrial respiration curve/O2 transport interaction, hypoxia-induced biological changes may be affected by this interaction. Thus, the significance of the present study is in the degree to which V̇O2max is reduced by the resistance imparted by oxidative phosphorylation and the consequent effect on mitochondrial PO2, which in turn may affect processes such as generation of reactive oxygen species and hypoxia-induced gene expression.
The purpose of the present paper is therefore to expand the prior theoretical analysis of the integrated O2 transport pathway (Wagner, 1993, 1996a) by analyzing the consequences for O2 transport of allowing mitochondrial PO2 to be greater than zero. This requires integration of the previously described O2 transport equations with an equation for mitochondrial respiration, followed by the application of mass conservation principles to solve this new equation system. The same data that were used in (Wagner, 1993, 1996a) are used here.
2. Material and methods
2.1. Principles
Oxidative phosphorylation ensues via the following equation 1 that embodies the law of mass action:
| eq. 1 |
In this equation, PmO2 corresponds to O2. Clearly, this mass action equation can only move from left to right and produce ATP if PmO2 is greater than zero.
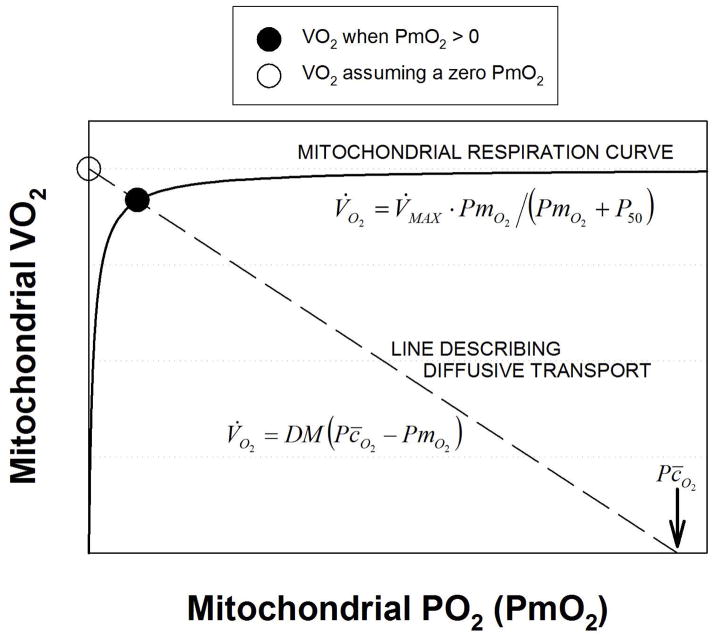
To illustrate this effect, a graphical depiction of mitochondrial respiration is presented in Figure 1. Here, the solid line is the relationship between velocity of the reaction (i.e., mitochondrial V̇O2), and PmO2, similar to what has been found experimentally (Gnaiger et al., 1998; Scandurra and Gnaiger, 2010; Wilson et al., 1977). It shows how V̇O2 is a positive but non-linear function of mitochondrial PO2, and indicates that at low PmO2, V̇O2 is very sensitive to (and thus limited by) PO2, while at higher PmO2, V̇O2 becomes independent of PO2, and is limited by factors other than O2.
Figure 1.
Graphical analysis of diffusive transport of O2 from muscle capillary to the mitochondria (dashed line) and subsequent utilization of O2 through oxidative phosphorylation (solid line). See text for details.
The hyperbolic curve through the origin displayed in Figure 1 represents mitochondrial respiration. It is of note that despite mitochondrial respiration kinetics is not really a Michaelis-Menten type (Johnson and Goody, 2011; Michaelis and Menten, 1913), experimental data (Gnaiger et al., 1998; Scandurra and Gnaiger, 2010) are well fitted by such a curve. As a hyperbola, it can be represented by equation 2:
| eq. 2 |
Where V̇O2 is mitochondrial V̇O2 (the ordinate in Figure 1); V̇MAX is the asymptote of the curve, and represents the maximal rate of use of O2 when O2 is in excess; PmO2 is mitochondrial PO2 (the abscissa in Figure 1) and P50 is the PO2 at 50% of V̇MAX. Thus, the mitochondrial respiration curve is defined by two parameters: V̇MAX and P50.
Also shown in Figure 1 is a straight (dashed) line of negative slope. It represents the Fick law of diffusion and depicts diffusive O2 transport between the tissue capillary and the mitochondria as a function of mitochondrial PO2 for a given tissue O2 diffusional conductance (DM) and a given tissue mean capillary PO2 (Pc̄O2), both at maximal exercise. We previously utilized this representation as a tool for interpreting intracellular oxygenation data obtained using magnetic resonance spectroscopy (Richardson et al., 1999). The equation is as follows:
| eq. 3 |
As the figure indicates, as PmO2 is increased, V̇O2 in eq (3) must fall because the PO2 difference between mean capillary and mitochondrial PO2 is reduced. Thus, Figure 1 shows how V̇O2 increases with mitochondrial PO2 according to oxidative phosphorylation, but decreases with mitochondrial PO2 according to the laws of diffusion.
The key concept in Figure 1 is that in a steady state of O2 consumption, V̇O2 given by both equations 2 and 3 must be the same at the same mitochondrial PO2 (i.e., the law of mass conservation applies). This can occur only at the single point of intersection between the two relationships, as indicated by the solid circle placed there. If, as previously approximated (Wagner, 1996b), mitochondrial PO2 were truly zero, V̇O2 would be higher, as indicated by the open circle at the left end of the dashed straight line in Figure 1. For a given O2 transport system defined by the conductances for O2 allowed by ventilation, alveolar-capillary diffusion, circulation, and capillary to mitochondrial diffusion, the values of mitochondrial V̇MAX and P50 (equation 2) will thereby influence maximal rate of O2 utilization, V̇O2max. In the remainder of this paper, it will be important to distinguish between V̇MAX (the asymptote to the mitochondrial respiration curve) and V̇O2max (actual maximal rate of O2 utilization, solid circle in Figure 1) to avoid confusion. In general, V̇MAX can exceed V̇O2max, but V̇O2max cannot exceed V̇MAX.
2.2. Modeling the O2 transport/utilization system
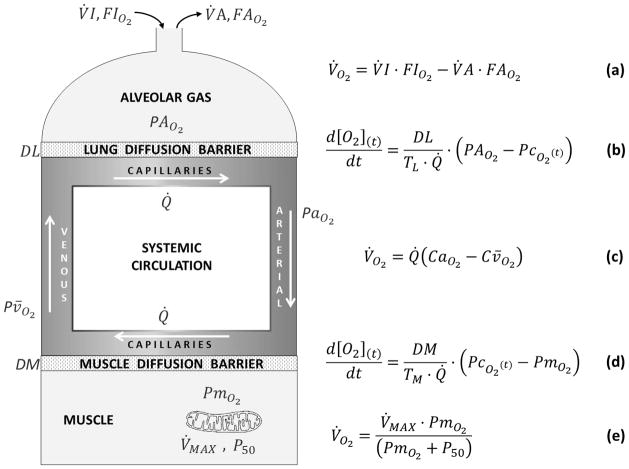
The present study augments our prior approach (Wagner, 1993, 1996b) by adding equation (2) to the equation system used previously. Figure 2 recapitulates the O2 transport pathway, and the associated four mass conservation equations governing O2 transport at each step. It adds Equation (2), describing O2 utilization as a function of PmO2. The important point is that in this way, the system has expanded from four equations with four unknowns into a system of five equations and five unknowns.
Figure 2.
Schematic representation of the oxygen transport and utilization system considered in this study and the five associated mass conservation equations governing O2 transport (equations a–d) and utilization (equation e).
Briefly, using specified input values for O2 transport step parameters (i.e., values of inspired O2 fraction (FIO2), ventilation (V̇I, inspired; V̇A, expired), lung diffusing capacity (DL), cardiac output (Q̇), [Hb], acid base status, tissue (muscle) diffusing capacity (DM), and mitochondrial respiration curve parameters (V̇MAX and P50)), five mass conservation equations are written for O2 (see Figure 2). They describe (a) ventilatory transport; (b) alveolar-capillary diffusion; (c) circulatory transport; (d) muscle capillary-mitochondrial diffusion; and (e) mitochondrial respiration. There are five unknowns in these equations: Alveolar PO2 (PAO2), arterial PO2 (PaO2), venous PO2 (Pv̄O2), mitochondrial PO2 (PmO2) and V̇O2 itself. In Figure 2, equations (b) and (d) are differential equations describing the process of diffusion across the lung blood: gas barrier and across the tissue capillary wall respectively. They specifically describe the time rate of change of O2 concentration, [O2], along the respective capillary as a function of the diffusing capacity, blood flow, red cell capillary transit time (TL (lungs); TM (tissues)) and the instantaneous difference between upstream and downstream PO2 values (alveolar and pulmonary capillary in (b); capillary and mitochondrial in (d)). The two equations are each expressions of the Fick law of diffusion.
The additional inputs of mitochondrial V̇MAX and P50, and the additional coding for the fifth equation were added to the prior model, and the same (numerical) method of solution employed before (Wagner, 1996b) was used to find the solutions for any set of input variables, defined as the unique values of the five unknowns listed above that simultaneously satisfy all five equations for the given input data defining O2 transport and utilization.
2.3. Input data for simulations
The input data defining the O2 pathway parameters used in this analysis were essentially identical to those used previously (Wagner, 1996b), and come from Operation Everest II (Sutton et al., 1988). They reflect maximal exercise by normal subjects at sea level, at a chamber “altitude” of 4,573 m(approximately 15,000 ft.) and at the chamber altitude of the Everest summit, 8,848 m (approximately 29,000 ft.). They are reproduced in Table 1. It is clear that data do not exist for the two new key variables: mitochondrial V̇MAX and P50. Therefore, for each of the three data sets we computed solutions to the equation system over a systematic range of five mitochondrial V̇MAX (1000, 2000, 3000, 4000, and 5000 ml/min) and four mitochondrial P50 values (0.1, 0.3, 0.5 and 1.0 mm Hg), resulting in 20 combinations of the two, and thus 20 mitochondrial respiration curves. The values of V̇MAX were chosen to encompass the range of V̇O2max from the very sedentary to the elite athlete. Values of P50 on the other hand were based on physiological studies inscribing the mitochondrial respiration curve from samples of normal muscle (Gnaiger et al., 1998; Scandurra and Gnaiger, 2010).
TABLE 1.
| Normal subjects at… | |||
|---|---|---|---|
|
| |||
| Parameter | Sea Level | 15,000 ft. | Everest summit |
| Barometric pressure (PB), Torr | 760 | 464 | 253 |
| Fractional Inspired Oxygen (FIO2) | 0.2093 | 0.2093 | 0.2093 |
| Alveolar ventilation (V̇), BTPS, L·min−1 | 112 | 125 | 165 |
| Blood flow (Q̇), L·min−1 | 23 | 21 | 16 |
| Hemoglobin concentration ([Hb]), g·dl−1 | 14.5 | 15.5 | 18.0 |
| Body temperature (T), °C | 38 | 38 | 37 |
| O2 dissociation curve P50, Torr | 26.8 | 26.8 | 26.8 |
| Total Lung O2 diffusing capacity (DL), ml·min−1·Torr−1 | 51 | 80 | 100 |
| Total muscle O2 diffusing capacity (DM), ml·min−1·Torr−1 | 102 | 88 | 62 |
| Maximum O2 uptake (V̇O2 max), L·min−1 | 3.82 | 2.81 | 1.46 |
A typical example from one of these papers is reproduced with permission in Figure 3, where the hyperbolic character of the curve and its P50 can both be seen by the fitted curve. In this and other similar published cases (Gnaiger et al., 1998; Scandurra and Gnaiger, 2010), P50 is close to 0.3 mm Hg. This accounts for our choice of P50 values - from a third of this typical value to about threefold greater. However it should be stressed that the modeling can be based on any combination of P50 and V̇MAX, and need not be limited to the choice of specific parameters appearing here.
Figure 3.
Graphical depiction of the hyperbolic equation for oxidative phosphorylation fitted to the data of Scandurra & Gnaiger (Scandurra and Gnaiger, 2010). p16. fig 3B).
2.4. Analysis
Across the matrix of V̇MAX and P50 values, we posed two questions: First we asked how much would PmO2 have to rise above zero to satisfy mass conservation and drive mitochondrial respiration for the given set of physiological O2 transport variables, V̇MAX and P50- and as a result, how much would that cause V̇O2 to be reduced (compared to assuming PmO2 = 0) as per Figure 1 (comparing the open and closed circles). This question allows a quantitative description of the theoretical consequences for V̇O2max of any combination of mitochondrial V̇MAX and P50. While this is a very useful question to answer, in reality V̇O2max is a directly measured variable. Therefore, asking how much would it be reduced by any pair of V̇MAX and P50 values is hypothetical. On the other hand, muscle diffusing capacity, DM, is a variable calculated on the assumption that mitochondrial PO2 can be neglected and set to zero – the very approximation that the present study is addressing.
Thus, another way to interrogate the model system can be proposed, leading to a second question: It recognizes that the muscle O2 diffusion step was previously modeled, and muscle diffusing capacity estimated, on the basis of PmO2 = 0. However, if PmO2 is greater than zero, the capillary to mitochondrial O2 diffusion gradient would be reduced, and this would necessitate, by the Fick law of diffusion, a higher value of DM to accomplish a given, measured V̇O2max (compared to the value calculated assuming PmO2 = 0).
Therefore, for each of the combinations of V̇MAX and P50, we asked how much would muscle diffusing capacity have to increase to maintain V̇O2 constant at the measured value as a result of PmO2 being greater than zero.
3. Results
3.1. Effects of mitochondrial respiration on PmO2 and maximal V̇O2
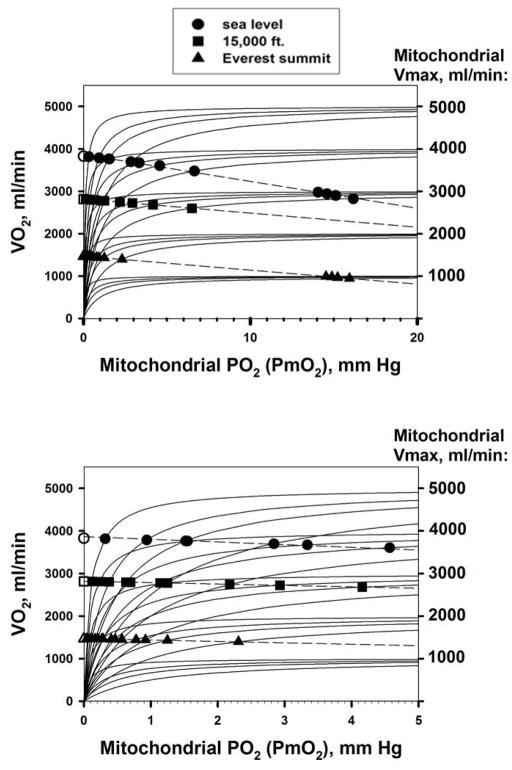
Figure 4 shows how the different combinations of mitochondrial V̇MAX and P50 affect V̇O2 max. The upper panel covers the mitochondrial PO2 (PmO2) range from zero to 20 mm Hg; the lower panel shows the same data, but expands the abscissa to better reflect the lower PmO2 range between zero and 5 mm Hg. In both panels, each solid curved line emanating from the origin represents one of the twenty mitochondrial respiration curves (as in Figure 3) for a particular V̇MAX and P50 combination. Solid circles reflect sea level conditions; solid squares represent moderate altitude and solid triangles are for the equivalent of the Everest summit. It turns out that at each altitude, an approximately straight line can be drawn through the resulting V̇O2max/PmO2 solution points for each mitochondrial respiration curve. These are the dashed lines in the figure.
Figure 4.
Effects of considering mitochondrial respiration on maximal V̇O2 and mitochondrial PO2. For each V̇MAX value, the four hyperbolic curves represent P50 values of 0.1, 0.3, 0.5 and 1.0 mm Hg, left to right. See text for details.
The values of V̇O2max at each altitude at the point where PmO2 equals zero (open symbols at zero PmO2) are the same as those described in (Wagner, 1996b) where PmO2 was taken to be zero. The figure shows how relaxing that approximation affects V̇O2max for each combination of mitochondrial V̇MAX and P50.
At sea level (solid circles), results show that allowing for a non-zero PmO2 has a small but significant impact on V̇O2max. For example, V̇O2max at PmO2 = 0 mm Hg (open circle) would be 3,827 ml/min, but if V̇MAX were 4,000 ml/min and P50 1.0 mm Hg, V̇O2max would be significantly less, by 9%, and would be 3,477 ml/min. Moreover, this would require a mitochondrial PO2 of 6.7 mm Hg to drive oxidative phosphorylation, as the figure shows. In general, for the fixed set of O2 transport parameters used (see Table 1), the lower the V̇MAX and the higher the P50, the greater is the reduction in V̇O2, and the higher is the PmO2 required to drive ATP generation. The range of possible values of mitochondrial PO2 is considerable, from a fraction of a mm Hg to more than 10 mm Hg, depending on V̇MAX and P50.
The same outcome is seen at each altitude, but with V̇O2 lower at any PmO2 as PIO2is reduced. The reduction in V̇O2 per unit change in PmO2 is somewhat less at altitude than at sea level, but if examined as a percent of V̇O2 at PmO2 = 0 at each altitude, the effects of allowing for mitochondrial respiration on maximal V̇O2 are relatively similar across altitudes.
In summary, the higher the mitochondrial V̇MAX and the lower the P50, the more O2 can be metabolized for a given upstream (heart, lungs, blood, muscle) transport system. Mitochondrial PO2 at V̇O2 can be neglected when considering O2 transport only when mitochondrial P50 is low and mitochondrial V̇MAX is high. When V̇MAX is low and/or P50 is high, the mitochondrial PO2 required to drive oxidative phosphorylation may reach double digit values, and the impact on V̇O2max can be considerable.
3.2. Maintenance of maximal V̇O2 in the face of non-zero PmO2
The preceding subsection showed how V̇O2max would have to decrease as a function of mitochondrial V̇MAX and P50 with constant values for all O2 transport conductances. In this subsection we investigate how much higher the muscle O2 diffusing capacity would have to be to maintain V̇O2max constant over the same range of V̇MAX and P50 values as PmO2 increases above zero.
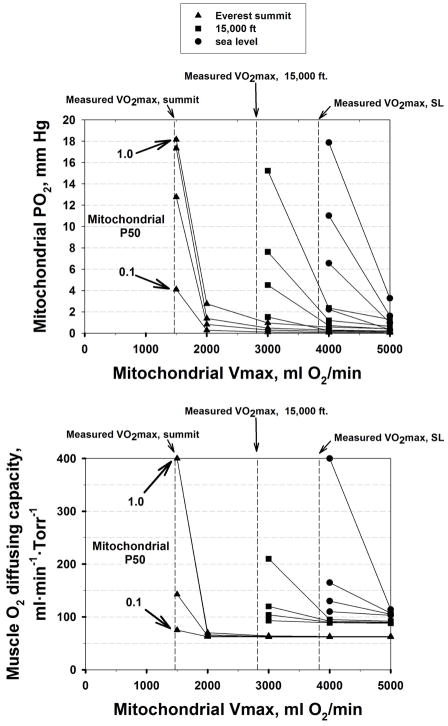
The results are shown in Figure 5, which displays the simulation outcomes across the entire matrix of V̇MAX and P50 values, using V̇MAX on the abscissa and isopleths for each P50. Results are shown for each altitude as indicated by the different symbols. The top panel shows mitochondrial PO2 for every combination of V̇MAX and P50 examined, and the bottom panel the corresponding values of muscle diffusing capacity (DM), that would have to exist to maintain V̇O2max at measured levels (indicated at each altitude by the vertical dashed lines). Comparing panels shows that when PmO2 is high (thus reducing the PO2 gradient between capillaries and mitochondria), DM must also be high to maintain diffusive O2 transport.
Figure 5.
Mitochondrial PO2 (upper panel) and muscle O2 diffusing capacity (lower panel) required to maintain V̇O2 constant at the measured value across the domain of V̇MAX and P50 values at each altitude studied (see text for details).
Also, when mitochondrial V̇MAX substantially exceeds measured V̇O2max (at each altitude), PmO2 remains low, and therefore DM does not need to be substantially increased to maintain O2 flux. However, the closer mitochondrial V̇MAX is to measured V̇O2max, the higher PmO2 must be (see Figure 4), and therefore, DM is also required to be elevated to maintain O2 transport. When V̇MAX and actual V̇O2max are very close, the required DM may be as much as four times the value needed when PmO2 is (close to) zero, and the associated PmO2 would reach double digit values.
4. Discussion
4.1. Summary of major findings
This study shows that including mitochondrial respiration in analyzing O2 transport and utilization generally poses a very small additional resistance to the system (over that of the transport pathway alone), only slightly reducing V̇O2max below that computed ignoring this contribution (Figure 4). The associated mitochondrial PO2 is also usually low (< 1 mm Hg). If however mitochondrial V̇MAX is low in relation to O2 transport capacity, or if mitochondrial P50 is high, V̇O2max may be considerably reduced. Mitochondrial PO2 would then increase more, and may reach double digit values.
In order to maintain V̇O2 max when mitochondrial PO2 is high, muscle diffusing capacity (DM) would need to be higher than when PmO2is assumed to be zero. Under most conditions, the necessary increase in DM would be minimal, being significant only when PmO2 is considerably elevated (Figure 5).
4.2. Unifying principles
The main principle demonstrated in the present study is that the final step in the O2 pathway – mitochondrial respiration – may contribute a non-negligible resistance to O2 movement through the system from the air to its conversion to CO2, resulting in a lower V̇O2max compared to a system where metabolism imposed no resistance to overall O2 flow. The higher the mitochondrial PO2 required to drive oxidative phosphorylation, the greater would be the relative resistance and thus the more effect there will be on reduction in V̇O2max. When mitochondrial P50 is about 0.30 mm Hg as reported by Gnaiger (Gnaiger et al., 1998; Scandurra and Gnaiger, 2010) the effects are generally minor.
The simulations at the three inspired PO2 values shown here demonstrate that it is the relative capacities (rather than individual absolute values) of the physiological transport system and the mitochondrial respiratory chain that effectively determine both the mitochondrial PO2 and the associated effect on V̇O2max, and that both variables, but especially mitochondrial PO2, may vary over a wide range depending on mitochondrial respiratory function.
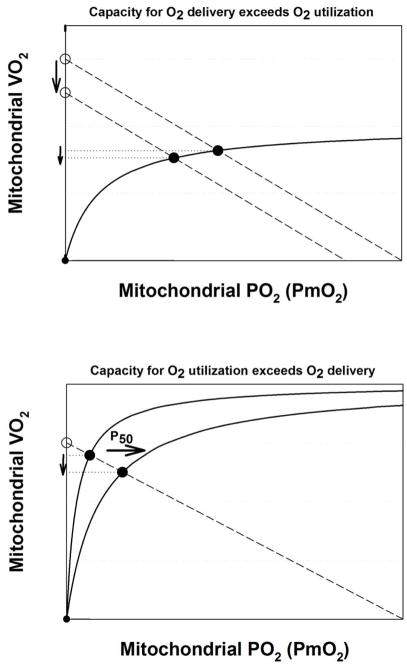
An additional important principle is shown in Figure 6: Even when O2 transport capacity (i.e., potential for O2 delivery) is considerably greater than mitochondrial respiratory capacity (i.e., potential for O2 utilization), as illustrated in concept in the top panel, a change in the former will change overall V̇O2. The converse is also true – that when mitochondrial respiratory capacity exceeds O2 transport capacity, (lower panel), a change in the former will have an effect on V̇O2. It is thus not correct to think that when one component is greater than the other, only the lesser of the two determines overall V̇O2max. This conclusion is much the same as described for individual components of the physiological transport pathway of the lungs and chest wall, the heart, blood and circulation, and the muscles, where we previously showed (Wagner, 1996a, b) that all components affect V̇O2max, not just the step with the least transport capacity.
Figure 6.
Graphical depiction of the concept that even when the capacity for O2 delivery exceeds O2 utilization (upper panel) a change in O2 delivery will change actual V̇O2. Conversely, when the capacity for O2 utilization exceeds O2 delivery (lower panel) a change in O2 utilization (increase in P50 in this example) will change actual V̇O2. Open circles: maximal O2 delivery to mitochondria if PmO2 was zero. Closed circles: actual V̇O2. Solid and dashed lines: as in Figure 1.
4.3. Effects of mitochondrial respiration kinetics on both V̇O2max and PmO2 may be small or large
For the examples shown – fit normal subjects – the effects of considering mitochondrial respiration are generally less on V̇O2 than on the associated PmO2 (Figure 4). Examining the sea level results for the example of V̇MAX = 4,000 ml/min and P50 increasing from 0.1 to 1.0 mm Hg, V̇O2max would fall by 9% while PmO2 would increase by an order of magnitude, from less than 1 mm Hg to more than 6 mm Hg. Just how much variation there is in mitochondrial P50 in the normal population is unknown, let alone whether this may change systematically with training, or in chronic diseases such as chronic obstructive pulmonary disease (COPD) or chronic heart failure. The calculations presented herein however point out that the quantitative nature of the mitochondrial respiration curve may be a critical determinant of the values of mitochondrial PO2 and V̇O2max, over and above any influence of upstream O2 transport.
Even if the effects on V̇O2max are numerically small, they would likely be important in the competitive endurance athlete where very small differences may separate success from failure. But possibly even more significant might be the potentially large variation in mitochondrial PO2 depending on P50 and V̇MAX due to known hypoxia-induced biological effects (Semenza, 2011). Thus, hypoxia-induced gene expression or reactive O2 species generation may vary according to mitochondrial PO2.
4.4. Potential for estimating mitochondrial P50 based on the current modeling approach
The analysis presented here suggests a possible method for estimating the characteristics of the mitochondrial respiration curve in vivo. Currently, mitochondrial V̇MAX and P50 are measured in vitro in respirometers where mitochondria are exposed to different levels of O2 and V̇O2 measured (as in Figure 3), (Gnaiger et al., 1998; Scandurra and Gnaiger, 2010; Wilson et al., 1977). To obtain this information in humans would therefore necessitate a muscle biopsy, and even if that were done, the result would be subject to the usual sampling constraints as for any other measure of muscle structure or function determined from a single biopsy.
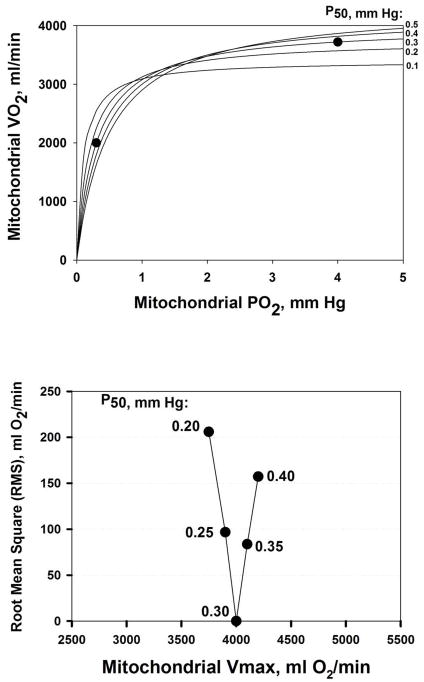
The fitting of a hyperbolic function to paired measured values of V̇O2max and mitochondrial PO2 has the potential for estimating P50 and V̇MAX in vivo, and this is illustrated in Figure 7. The intervention to garner several points on the curve would come from acutely varying FIO2 and measuring V̇O2 and mitochondrial PO2 during maximal exercise at each FIO2, as indicated by the theoretical example of the two solid circles in the upper panel of Figure 7. These two points reflect a mitochondrial respiration curve with P50 of 0.30 mm Hg and V̇MAX of 4,000 ml/min. If such data were to span both the steep and flat parts of the respiration curve, as shown in the figure, identifying the V̇MAX and P50 of a hyperbola that resulted in a least squares best fit to the data points would be possible, as shown in the lower panel of Figure 7. Here, over a range of trial values of both V̇MAX and P50, the root mean square (RMS) residual V̇O2 between the data and the hyperbola corresponding to each trial combination of P50 (and the V̇MAX providing the lowest RMS for that P50) is shown. In this error-free theoretical case, one could quite accurately estimate V̇MAX (4,000 ml/min) and P50 (0.3 mm Hg) from the values at the nadir of the relationship in the figure. However, if measured data happened to lie on only the flat or only on the steep parts of the curve, ability to estimate V̇MAX and/or P50 would be considerably reduced.
Figure 7.
Estimation of mitochondrial P50. Upper panel: least squares best fit (solid lines) to data (solid circles) for five trial P50 values. Lower panel: closeness of fit to data reflected by the Root Mean Square error, showing that a P50 of 0.30 mm Hg provides the best estimate.
While whole body or large muscle mass V̇O2 can be measured relatively easily, the experimental challenge would be to measure mitochondrial PO2 (during exercise) (Mik, 2013). The closest approach to date in intact subjects has used MRS-based determination of myoglobin O2 saturation (Jue et al., 1994; Richardson et al., 1995), where the signal comes from a relatively large muscle region. This approach gives intracellular PO2 estimates of 3–4 mm Hg during exercise (Richardson et al., 1995), but this is the PO2 associated with myoglobin, inferred from the finding of about 50% myoglobin saturation during peak exercise combined with accepted values of myoglobin P50 of about 3 mm Hg (Rossi-Fanelli and Antonini, 1958). This PO2 is an order of magnitude greater than that projected at the mitochondria based on the preceding discussion. In the end, a method would have to be developed for direct measurement of mitochondrial PO2. Whether a candidate signaling atom or molecule can be found for an MRS-based approach is currently unknown.
4.5. Limitations of the analysis
As in previous work (Wagner, 1993, 1996b), the entire analysis is applicable only to steady state conditions (meaning, that O2 partial pressures are constant in time as is V̇O2 itself). Therefore, the analysis cannot be used to study transient changes in metabolic rate. Another limitation is not taking into account ventilation-perfusion mismatch in the lung and/or metabolism-perfusion mismatch in the muscle as contributors to impaired oxygen transport. However, using methods to quantify both of these phenomena, this limitation could be removed. A final limitation is that non-muscle blood flow during maximum exercise is neglected.
5. Conclusions
Considering the hindrance to overall O2 flux caused by mitochondrial respiration using an established model of O2 transport to the mitochondria revealed that in normal subjects exercising maximally, the step of oxidative phosphorylation, with its requirement for a mitochondrial PO2 > 0, likely plays only a small role in total O2 flux resistance. However, we identified conditions in which mitochondrial PO2 can rise to double digit values. This occurs particularly when the mitochondrial respiration curve has either a low V̇MAX (relative to O2 transport), or a high P50, and under such conditions, mitochondrial function may significantly impair O2 flux and cell function may be affected, for example, in reactive oxygen species generation and/or oxygen-sensitive gene expression.
HIGHLIGHTS.
We developed an integrative model of O2 transport and utilization.
We simulated healthy fit subjects exercising at sea level and altitude.
Mitochondrial PO2 likely plays only a small role in total O2 flux resistance.
If O2 transport capacity exceeds V̇MAX, PmO2 may reach double digit values.
The approach offers the potential for estimating mitochondrial P50 and V̇MAX.
Acknowledgments
We would like to acknowledge the contributions made by the Synergy-COPD consortium and NIH P01 HL091830.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Dejours P, Kayser C. Respiration. Oxford University Press; 1966. [Google Scholar]
- Gnaiger E, Lassnig B, Kuznetsov A, Rieger G, Margreiter R. Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J Exp Biol. 1998;201:1129–1139. doi: 10.1242/jeb.201.8.1129. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Goody RS. The Original Michaelis Constant: Translation of the 1913 Michaelis–Menten Paper. Biochemistry. 2011;50:8264–8269. doi: 10.1021/bi201284u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jue T, Kreutzer U, Chung Y. 1H NMR approach to observe tissue oxygenation with the signals of myoglobin. Adv Exp Med Biol. 1994;361:111–118. doi: 10.1007/978-1-4615-1875-4_15. [DOI] [PubMed] [Google Scholar]
- Michaelis L, Menten ML. Die kinetik der invertinwirkung. Biochem Z. 1913;49:352. [Google Scholar]
- Mik EG. Measuring Mitochondrial Oxygen Tension: From Basic Principles to Application in Humans. Anesthesia and analgesia. 2013 doi: 10.1213/ANE.0b013e31828f29da. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Leigh JS, Wagner PD, Noyszewski EA. Cellular PO2 as a determinant of maximal mitochondrial O2 consumption in trained human skeletal muscle. J Appl Physiol. 1999;87:325–331. doi: 10.1152/jappl.1999.87.1.325. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J Clin Invest. 1995;96:1916–1926. doi: 10.1172/JCI118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-Fanelli A, Antonini E. Studies on the oxygen and carbon monoxide equilibria of human myoglobin. Arch Biochem Biophys. 1958;77:478–492. doi: 10.1016/0003-9861(58)90094-8. [DOI] [PubMed] [Google Scholar]
- Scandurra FM, Gnaiger E. Cell respiration under hypoxia: facts and artefacts in mitochondrial oxygen kinetics. Adv Exp Med Biol. 2010;662:7–25. doi: 10.1007/978-1-4419-1241-1_2. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Oxygen Sensing, Homeostasis, and Disease. New England Journal of Medicine. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- Sutton JR, Reeves JT, Wagner PD, Groves BM, Cymerman A, Malconian MK, Rock PB, Young PM, Walter SD, Houston CS. Operation Everest II: oxygen transport during exercise at extreme simulated altitude. J Appl Physiol. 1988;64:1309–1321. doi: 10.1152/jappl.1988.64.4.1309. [DOI] [PubMed] [Google Scholar]
- Wagner PD. Algebraic analysis of the determinants of VO2max. Respiration physiology. 1993;93:221–237. doi: 10.1016/0034-5687(93)90007-w. [DOI] [PubMed] [Google Scholar]
- Wagner PD. Determinants of maximal oxygen transport and utilization. Annu Rev Physiol. 1996a;58:21–50. doi: 10.1146/annurev.ph.58.030196.000321. [DOI] [PubMed] [Google Scholar]
- Wagner PD. A theoretical analysis of factors determining VO2 MAX at sea level and altitude. Respiration physiology. 1996b;106:329–343. doi: 10.1016/s0034-5687(96)00086-2. [DOI] [PubMed] [Google Scholar]
- Weibel ER, Taylor CR, Gehr P, Hoppeler H, Mathieu O, Maloiy GM. Design of the mammalian respiratory system. IX. Functional and structural limits for oxygen flow. Respiration physiology. 1981;44:151–164. doi: 10.1016/0034-5687(81)90081-5. [DOI] [PubMed] [Google Scholar]
- Wilson DF, Erecinska M, Drown C, Silver IA. Effect of oxygen tension on cellular energetics. American Journal of Physiology - Cell Physiology. 1977;233:C135–C140. doi: 10.1152/ajpcell.1977.233.5.C135. [DOI] [PubMed] [Google Scholar]