Abstract
The aim of the study was to examine genetic, pharmacokinetic and demographic factors that influence sensitivity to nicotine in never smokers. Sixty never smokers, balanced for gender and race (Caucasian, Blacks and Asian), wore 7 mg nicotine skin patches for up to 8 hours. Serial plasma nicotine concentrations and subjective and cardiovascular effects were measured, and genetic variation in the CYP2A6 gene, the primary enzyme responsible for nicotine metabolism, was assessed. Nicotine toxicity requiring patch removal developed in 9 subjects and was strongly associated with rate of rise and peak concentrations of plasma nicotine. Toxicity, subjective and cardiovascular effects of nicotine were associated with the presence of reduced function CYP2A6 alleles, presumably reflecting slow nicotine metabolic inactivation. This study has implications for understanding individual differences in responses to nicotine medications, particularly when the latter are used for treating medical conditions in non-smokers, and possibly in vulnerability to developing nicotine dependence.
INTRODUCTION
Individuals differ in their susceptibility to the acquisition of tobacco addiction. Twin studies indicate that 50% or more of the variance in likelihood of developing tobacco dependence is genetic, and that genetic factors influence not only who becomes a smoker, but how much they smoke and how hard it is for them to quit.1–3 The genetic determinants of individual differences in vulnerability to nicotine addiction are incompletely understood. One possibility includes individual differences in pharmacologic responses to initial exposure to nicotine.
Smoking prevalence, cigarette consumption among smokers and the risk of developing smoking-related disease differ among racial groups. Relevant to the present study, Asian-Americans are less likely to become smokers, smoke fewer cigarettes per day and are less likely to develop lung cancer compared to Caucasians.4,5 Black Americans have a similar prevalence of smoking, smoke fewer cigarettes per day but are more likely to develop lung cancer compared to Caucasians 5,6. One factor influencing individual differences in smoking behavior may be the rate of metabolism of nicotine. Nicotine is metabolically inactivated to its major proximate metabolite, cotinine (COT), primarily by the liver enzyme CYP2A6.7 Cotinine is metabolized to 3-hydroxycotinine (3HC) by the same enzyme. Compared to Caucasians, Asians and Black Americans have a higher prevalence of CYP2A6 gene alleles that are associated with slower metabolism of nicotine.8 Slower metabolism of nicotine is expected to result in the need to smoke fewer cigarettes per day to achieve a desired level of nicotine in the body, and has been associated with greater rates of quitting compared to faster metabolizers.9
Differences in pharmacologic response to early nicotine exposure may also be important determinants of differential vulnerability to addiction. Several studies have reported that an initial pleasant nicotine response (for example, feeling high, a pleasurable buzz or rush or feeling dizzy) among novice smokers is associated with continued smoking and the development of nicotine dependence 10–15. DiFranza et al also reported that aversive responses to smoking the first cigarette, such as nausea and dizziness, were predictors of greater symptoms of nicotine dependence, consistent with the idea that a general sensitivity to nicotine predicts a higher likelihood of development of dependence11.
Differences in response to nicotine are difficult to study in people who are already using tobacco because they have developed considerable tolerance to many of the effects of nicotine 16. To explore intrinsic individual differences in kinetics and response to nicotine, we conducted a study of transdermal nicotine in never-smokers. We also examined variation in CYP2A6 genotype which cause differences in the rate of nicotine metabolism and which has been reported to influence the likelihood of developing nicotine dependence 17–19.
RESULTS
Demography
Sixty participants (29 females and 31 males) were enrolled in the study. Participants were Asian (n = 20), Black (n = 20), and White (n = 20). The average age of the participants was 26.5 years (range, 19–39) and did not differ by race (p = 0.78). The average BMI was 24.2 kg/m2 (range, 17.3–30.7) and was significantly higher among Blacks (mean, 25.9) compared to Asians (23.8) and Whites (22.7) (p = 0.003). Four participants did not have data for the entire 8 hour study period: three participants who had their patch removed left before 480 minutes and the respective times of last sample collection are 180 minutes, 240 minutes, and one 420 minutes. One other subject with patch still in place chose to terminate the study after their 420 minute blood sample.
CYP2A6 genotype
CYP2A6 genotype was determined in 58 participants; in two subjects genotyping was not conclusive (both Blacks). Thirty-five participants, (60%), had the wild-type genotype (*1/*1) and 23 participants (40%), had one or two variant CYP2A6 alleles. The allele frequencies and their distribution by race and gender are presented in Table 1. The proportion of *1/*1 vs. variant alleles was significantly different by race (p = 0.004); 70% of Asian participants had a variant allele compared to 28% of Blacks and 20% of Whites.
TABLE 1.
CYP2A6 genotype, nicotine-induced toxicity [#], plasma nicotine AUC0→90 and AUC0→360, Cmax, and Tmax by sex and race
| Variable | All subjects | Asian | Black | Caucasian | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| F | M | Total | F | M | total | F | M | total | F | M | total | |
| CYP2A6 genotype | ||||||||||||
| *1/*1 | 18 [1] | 17 [2] | 35 [3] | 2 | 4 | 6 | 8 [1] | 5 | 13 [1] | 8 | 8 [2] | 16 [2] |
| All variants | 10 [3] | 13 [3] | 23 [6] | 8 [3] | 6 [1] | 14 [4] | 1 [0] | 4 [0] | 5 [0] | 1 [0] | 3 [2] | 4 [2] |
| Variant alleles | ||||||||||||
| *1/*4 | 1 | - | 1 | 1 | - | 1 | - | - | - | - | - | - |
| *1/*7 | 1 | - | 1 | 1 | - | 1 | - | - | - | - | - | - |
| *1/*9 | 3 [1] | 6 [2] | 9 [3] | 2 [1] | 3 | 5 [1] | - | - | - | 1 | 3 [2] | 4 [2] |
| *1/*10 | 1 | 1 | 2 | 1 | 1 | 2 | - | - | - | - | - | - |
| *1/*17 | - | 2 | 2 | - | - | - | - | 2 | 2 | - | - | - |
| *1/*25 | 1 | - | 1 | - | - | - | 1 | - | 1 | - | - | |
| *4/*9 | 1 [1] | 1 | 2 [1] | 1 [1] | 1 | 2 [1] | - | - | - | - | - | - |
| *4/*10 | 1 [1] | - | 1 [1] | 1 [1] | - | 1 [1] | - | - | - | - | - | |
| *4/*12 | - | 1 | 1 | - | - | - | - | 1 | 1 | - | - | |
| *4/*17 | - | 1 | 1 | - | - | - | - | 1 | 1 | - | - | |
| *9/*9 | 1 | - | 1 | 1 | - | 1 | - | - | - | - | - | |
| *10/*10 | - | 1 [1] | 1 [1] | - | 1 [1] | 1 [1] | - | - | - | - | - | - |
|
| ||||||||||||
| AUC0→90 (min•ng/mL) | ||||||||||||
| mean | 275 | 285 | 280 | 302 | 291 | 297 | 273 | 262 | 267 | 248 | 300 | 276 |
| SD | 103 | 183 | 149 | 125 | 179 | 151 | 87 | 192 | 145 | 97 | 194 | 156 |
| AUC0→360 (min•ng/mL) | ||||||||||||
| mean | 2171 | 1791 | 1975 | 2222 | 2028 | 2124 | 2513 | 1715 | 2113 | 1734 | 1645 | 1686 |
| SD | 1148 | 620 | 926 | 633 | 733 | 674 | 1744 | 700 | 1356 | 615 | 381 | 487 |
| Cmax (ng/mL) | ||||||||||||
| mean | 7.71 | 7.02 | 7.35 | 8.93 | 7.56 | 8.24 | 7.52 | 6.92 | 7.21 | 6.55 | 6.62 | 6.59 |
| SD | 2.41 | 2.34 | 2.38 | 2.71 | 2.70 | 2.73 | 1.56 | 2.34 | 1.98 | 2.35 | 2.10 | 2.15 |
| Tmax (min) | ||||||||||||
| mean | 236 | 276 | 257 | 265 | 303 | 283 | 192 | 282 | 237 | 253 | 245 | 249 |
| SD | 139 | 156 | 148 | 150 | 158 | 151 | 125 | 155 | 145 | 143 | 164 | 151 |
NOTES:
number of participants who removed nicotine patches due to toxic responses
Nicotine Toxicity
Acute nicotine toxicity was evidenced by nausea and/or vomiting, necessitating patch removal in nine subjects. The number of participants who removed their patch by CYP2A6 genotype is presented in Table 1. The times of patch removal by genotype and race were 44 min (*10/*10, Asian), 60 min (*1/*9, White), 89 min (*1/*1, White), 91 min (*1/*1, White), 93 (*1/*9, Asian), 100 (*4/*10, Asian), 131 min (*1/*1, Black), 372 min (*4/*9, Asian) and 384 (*1/*9, White). The median time to patch removal was 93 minutes and the average time to patch removal was 152 (SD 131) minutes.
Nicotine levels over time
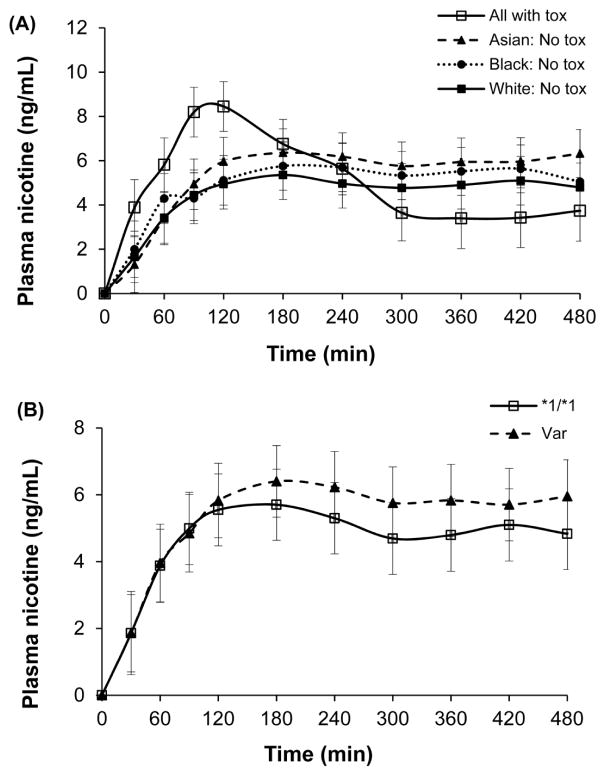
Figure 1A displays the average plasma nicotine concentration over time for all participants who became ill and for all those who did not, by race. Average peak plasma nicotine concentration was higher (p = 0.006) and average time to peak nicotine concentration was shorter (p = 0.018) in participants who developed nicotine-induced toxicities compared to those who did not. Average plasma nicotine concentration over time by CYP2A6 genotypes (*1/*1 vs. variants) is displayed in Figure 1B. Average peak plasma nicotine concentration was not significantly different with CYP2A6 genotype. Table 1 displays nicotine pharmacokinetic data (AUC, Cmax, Tmax) for all subjects and by race.
FIGURE 1.
A. Plasma nicotine concentration over 8 h of all participants with toxicity and participants without toxicity by race. Values are geometric means and standard errors.
B. Time course of plasma nicotine concentration by CYP2A6 genotype group (wildtype, *1/*1, versus those with at least one variant allele, Var)
Subjective (VANES) and cardiovascular responses
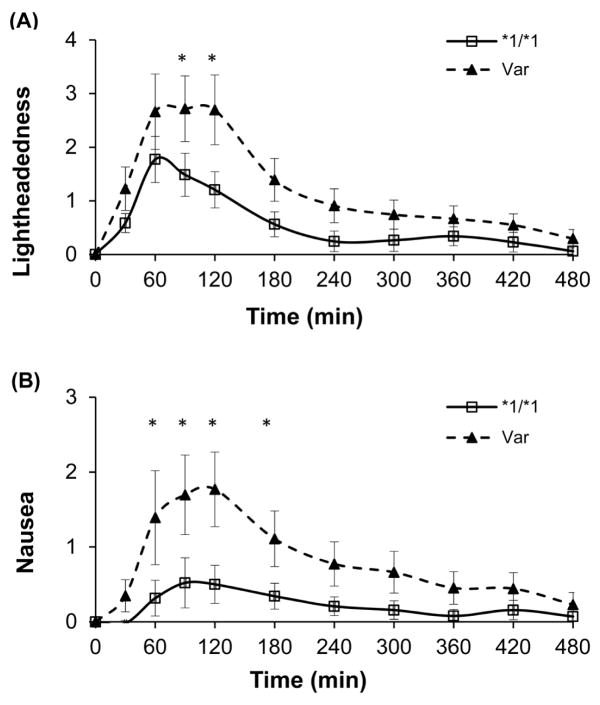
At baseline there was no significant difference in the eleven individual VANES parameters between those with variant alleles and those with the *1/*1 genotype (wild type). At 30 minutes, two VANES parameters (calmness and dose perception) were significantly different; at 60 minutes, five parameters (anxious, concentrate, nausea, stimulated, and palpitations) were significantly different; and at 90 minutes, seven parameters were significantly different in subjects with variant compared to *1/*1 genotypes. The group differences were greatest at 120 minutes, when eight of 11 VANES parameters were significantly different. The differences in changes in subjective responses to nicotine from baseline to 90 and 120 minutes comparing subjects with wildtype vs variant CYP2A6 alleles are presented in Table 2. Those in the variant group had significantly more unpleasant subjective responses when compared to those with *1/*1 genotype. Specifically, those in the variant group reported being more anxious, less calm, less able to concentrate, more light headed, experienced more nausea, and reported more palpitations. By 180 minutes, these differences were no longer significant. The time-course of perceived lightheadedness and nausea by genotype group are presented in Figure 2.
TABLE 2.
Differences in changes in subjective responses (VANES) and heart rate from baseline between participants with CYP2A6 wildtype alleles and those with variant alleles
| Differences in VANES responses between *1/*1 and variants
|
||||
|---|---|---|---|---|
| at 90 minutes | p-value | at 120 minutes | p-value | |
| VANES, mean (95% CI) | ||||
| Alert | 1.0 (−0.3, 2.2) | 0.13 | 1.7 (0.4, 2.9) | 0.01 |
| Anxious | −0.5 (−1.1, −0.02) | 0.04 | −0.5 (−1.1, −0.03) | 0.04 |
| Calm | 1.5 (0.2, 2.8) | 0.02 | 1.9 (0.6, 3.1) | 0.004 |
| Concentrate | 1.9 (0.6, 3.2) | 0.004 | 1.4 (0.1, 2.7) | 0.035 |
| Contented | 1.5 (0.1, 2.8) | 0.03 | 1.2 (−0.2, 2.5) | 0.09 |
| Dose perception | −0.6 (−1.6, 0.4) | 0.26 | −1.4 (−2.5, −0.4) | 0.006 |
| High | −0.4 (−1.0, 0.2) | 0.22 | −0.2 (−0.8, 0.5) | 0.68 |
| Lightheaded | −1.1 (−2.0, −0.2) | 0.02 | −1.3 (−2.3, −0.4) | 0.005 |
| Nausea | −1.0 (−1.7, −0.3) | 0.005 | −1.1 (−1.8, −0.4) | 0.002 |
| Stimulated | −0.5 (−1.2, 0.3) | 0.23 | −0.4 (−1.1, 0.4) | 0.36 |
| Palpitation | −0.5 (−0.9, −0.01) | 0.046 | −0.6 (−1.1, −0.2) | 0.01 |
| Cardiovascular parameters, mean (95% CI) | ||||
| Heart rate | −4.0 (−8.0, −0.1) | 0.046 | −4.2 (−8.1, −0.3) | 0.035 |
NOTES: VANES = Visual Analog Nicotine Effect Scale; values presented are the differences in changes in VANES between *1/*1 and variants (*1/*1 minus variants); changes in VANES for subjects were computed from baseline to each time-point.
FIGURE 2.
Time course of perceived lightheadedness and nausea by CYP2A6 genotype group (wildtype, *1/*1, versus those with at least one variant allele, Var).
For the cardiovascular parameters, at baseline there was no difference between the two genotype groups (p values range: 0.88 to 0.98). Thereafter, heart rate acceleration was significantly greater (change from baseline) from 30 minutes through 180 minutes post patch placement for the variant group compared to the wild type group (Table 2). Blood pressure did not show consistent differences between the two genotype groups.
Predictors of nicotine toxicity
Univariate analyses to investigate the association between demographic data and CYP2A6 genotype and nicotine-induced toxicity (patch removal) found no significant effect for age (p = 0.6), gender (p = 1.0), BMI (p = 0.5), or race (p = 0.4). Although 6 of 9 participants who had their patch removed had at least one variant allele, this difference in toxicity was not statistically significant across genotype groups. Table 3 presents further univariate analyses for the association between nicotine toxicity and possible predictors (race, genotype, and pharmacokinetic parameters). AUC and Cmax were significantly higher and Tmax shorter in participants with toxicities compared to those without.
TABLE 3.
Comparison of demographic and pharmacokinetic variables between participants with nicotine toxicity (removed patch) and those without.
| Variable | No toxicity (n = 51) | Toxicity (n = 9) | p-valuea |
|---|---|---|---|
| Age | |||
| mean (range) | 26.5 (19–39) | 26.6 (19–31) | 0.617 |
| Sex | |||
| female (n, %) | 25 (41.7%) | 4 (6.7%) | 1.000 |
| male (n, %) | 26 (43.3%) | 5 (8.3%) | |
| Race | |||
| Asian (n, %) | 16 (26.7%) | 4 (6.7%) | 0.360 |
| Black (n, %) | 19 (31.7%) | 1 (1.7%) | |
| White (n, %) | 16 (26.7%) | 4 (6.7%) | |
| BMI | |||
| mean (range) | 24.0 (17.3–30.5) | 24.8 (21.0–30.7) | 0.49 |
| Genotypeb | |||
| *1/*1 (n, %) | 32 (55.2%) | 3 (5.2%) | 0.135 |
| Var (n, %) | 17 (29.3%) | 6 (10.3%) | |
| AUC0→90 (min•ng/mL) | 0.006 | ||
| GM (95% CI) | 216 (182–257) | 401 (267–602) | 1.9 (1.2–2.9) c |
| AUC0→360 (min•ng/mL) | 0.007 | ||
| GM (95% CI) | 1714 (1544–1902) | 2535 (1752–3667) | 1.5 (1.1–2.0) c |
| Cmax (ng/mL)b | 0.007 | ||
| GM (95% CI) | 6.6 (6.0–7.3) | 9.5 (7.5–12.0) | 1.4 (1.1–1.9) c |
| Tmax (min) | |||
| median (IQ range) | 240 (120–420) | 120 (90–420) | 0.018 |
Notes: GM = geometric mean; IQ = interquartile range;
Fisher’s exact test for categorical variables, Wilcoxon two-sample test for age and Tmax, two-sample test for continuous variables;
genotype data for two subjects and Cmax for one subject were missing;
variable ratio (toxic/nontoxic group)
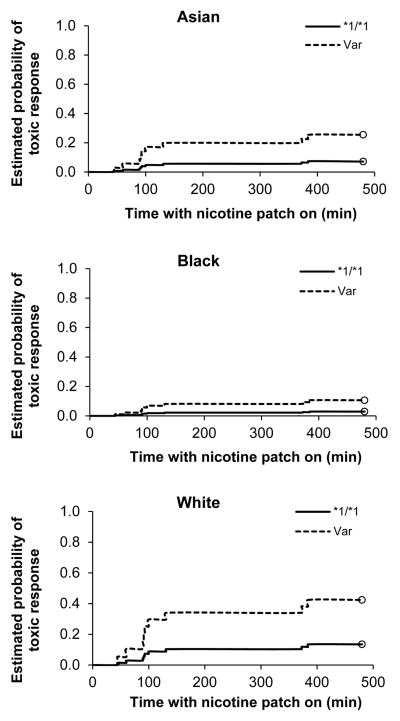
Time-to-event analysis (also known as survival analysis) was used to formally test the association between pharmacokinetic parameter or genotype and nicotine-induced toxicity. These results are presented in Table 4. After adjusting for the effects of age, BMI, gender, and race, the hazard ratio (HR) of nicotine-induced toxicity in those with a variant genotype compared to the *1/*1 group was 3.81 (0.84–17.2) (p = 0.083). While the probability of nicotine-induced toxicity following patch placement over an 8 h time-course among individuals with the variant genotype tended to be higher among whites compared to Asians followed by blacks, these differences were not statistically significant (p = 0.21) (Figure 3). In time to event analyses for pharmacokinetic parameters, the adjusted HR of toxicity among participants were significant for high compared to low categories based on median-split for AUC0→90, AUC0→360, and Cmax (Table 4). The unadjusted hazard ratios were not significant and are not presented.
Table 4.
Associations between CYP2A6 genotype, nicotine pharmacokinetics and nicotine-induced toxicity (patch removal) obtained using time-to-event analysis.
| Predictor a | Hazard Ratio (95% CI) | χ2 | p-value |
|---|---|---|---|
| Model 1: Genotype | |||
| variants vs. *1/*1 | 3.81 (0.84–17.2) | 3.01 | 0.08 |
| Model 2: AUC0→90 | |||
| high vs. low | 5.88 (1.07–32.2) | 4.16 | 0.042 |
| Model 3: AUC0→360 | |||
| high vs. low | 8.35 (1.40–49.6) | 5.44 | 0.020 |
| Model 4: Cmax | |||
| high vs. low | 6.91 (1.23–38.9) | 4.81 | 0.028 |
| Model 5: Tmax | |||
| long vs. short | 0.22 (0.04–1.15) | 3.24 | 0.07 |
NOTES:
Hazard ratios (HR) were adjusted for race, sex, BMI, and age; AUC0→90 (median = 249 min•ng/mL), AUC0→360 (1886 min•ng/mL), Cmax (7 ng/mL), and Tmax (180 min) were median-split into categories. Chi squared (χ2) and p-values are for overall test of each predictor.
FIGURE 3.
Probability of patch removal over time by race by CYP2A6 genotype group (wildtype, *1/*1, versus those with at least one variant allele, Var).
DISCUSSION
The aim of our study was to examine factors that determine pharmacologic response to nicotine in nonsmokers. We studied nonsmokers because we wanted to look at nicotine effects in the absence of tolerance, which is considerable in regular tobacco users. Individual differences in nicotine sensitivity among nonsmokers are thought to influence vulnerability to tobacco addiction10–15. We used nicotine patches to probe sensitivity because nicotine delivery from patches is controlled as opposed to smoking cigarettes, where individuals can alter levels of nicotine delivery through difference in frequency, duration and depth of inhalation. We hypothesized that variability in the rate of nicotine metabolism, determined largely by CYP2A6 activity, would strongly influence responses to nicotine patches, including the risk of toxicity.
We found that pharmacokinetic factors were the strongest predictors of development of nicotine toxicity, which occurred in 15% of our subjects. The peak plasma nicotine, plasma nicotine AUC from 0–90 minutes (reflecting rate of rise) and the plasma nicotine AUC from 0–360 min were all strong predictors of toxicity (hazard ratios 6.9, 5.9 and 8.4, respectively). Rapid rise of blood concentrations is known to be associated with greater effects of many psychoactive drugs, presumably because higher brain levels are achieved with less time to develop receptor-based tolerance20,21. Thus finding that Cmax and rate of rise of plasma nicotine concentration were associated with nicotine toxicity is not surprising. It is remarkable that AUC0→360 is also associated with nicotine toxicity since most of the subjects with toxicity had their patches removed prior to about 2 hours. Persistently high nicotine levels despite patch removal indicate slow metabolism, either due to intrinsic metabolic differences or perhaps due to effects of nicotine toxicity on its own clearance (such as by reducing liver blood flow)22.
The basis for more rapid absorption of nicotine in some subjects is not entirely clear. Genetic differences in nicotine metabolism appear to play some role, as discussed below, but do not fully explain the phenomenon. Possibly differences in rate of absorption across the epidermis or differences in binding of nicotine to dermal tissues play a role. Individual differences in volume of distribution would be expected to influence peak levels for any given rate of drug delivery. However we saw no effect of body mass index or gender on nicotine levels, arguing against a role of distribution volume in determining rate of rise of nicotine levels.
We hypothesized that differences in CYP2A6 activity would influence response to transdermal nicotine. There is considerable genetic polymorphism in the CYP2A6 gene, including known racial/ethnic differences in allele frequencies 23. As expected we found that the presence of reduced function variants was higher in Asian and Black compared to White subjects. We did not however find a significant effect of CYP2A6 genotype on plasma nicotine Cmax or AUC0→90, perhaps because there is relatively little nicotine metabolism, relative to absorption, in the first 90 minutes of patch application.
Among the nine subjects who developed nicotine toxicity, 67% had reduced function CYP2A6 variants, compared to 35% reduced function variants among those who did not develop toxicity. This difference was not statistically significant, but the lack of significance may be a power problem, given relatively few cases of toxicity. Time-to-event analysis indicated a borderline significant effects of genotype (p=0.083). There was no association between race, gender or age and nicotine toxicity. In addition to toxicity we studied subjective and cardiovascular effects of transdermal nicotine in relation to CYP2A6 genotype. Increases in anxiousness, lightheadedness, nausea and palpitations and decreases in alertness, concentration and calmness at 120 minutes, and heart rate acceleration from 30 to 180 minutes were significantly greater in subjects with CYP2A6 reduced function variants. A limitation of our study is that we did not examine genetic variation in other pathways of nicotine metabolism – glucuronidation and N-oxidation.7 These are generally minor metabolic pathways, but could influence nicotine clearance, particularly when metabolism via CYP2A6 is genetically slow.
In summary, nicotine toxicity (patch removal) in never-smokers is most strongly influenced by rate of rise and peak levels of plasma nicotine. There was a significant association between subjective pharmacological responses and the presence of CYP2A6 reduced function alleles, presumably related to the slow rate of nicotine metabolism. One implication of our research relates to the administration of nicotine as a medication. Our study of non-smokers suggests that nicotine toxicity would be more likely to occur in genetically slow metabolizers who are receiving usual therapeutic doses of transdermal nicotine to aid smoking cessation, although the previous development of tolerance in many smokers is likely to mitigate the problem of toxicity, even among slow metabolizers. Nicotine medications have also been proposed for the treatment of ulcerative colitis, Parkinson’s disease and other disorders 24–26. If these patients are non-smokers prior to nicotine therapy, the CYP2A6 genotype might be a useful predictor of toxicity and need for smaller doses at initiation of treatment.
Another implication relates to vulnerability to developing nicotine dependence. Several studies have reported that initial sensitivity to pleasurable effects of nicotine predicts progression to dependent smoking, and some report that aversive responses predict a greater level of dependence 10–15. These studies suggest that increased global sensitivity to nicotine is an important determinant of who becomes a regular smoker. Our data indicate that determinants of initial sensitivity to nicotine are rate of rise of plasma nicotine levels and reduced activity CYP2A6 genotype. It is unclear how our data with nicotine rate of rise from patch use would translate to smoking the first cigarette, from which nicotine absorption is much more rapid. We considered studying more rapid nicotine delivery systems, such as nicotine gum, lozenge or inhaler, but there is large individual variability in systemic nicotine delivery from such formulations, making comparisons of nicotine effects across subjects difficult. The importance of the rate of nicotine metabolism as a determinant of sensitivity is supported by cohort studies among adolescents reporting that having reduced function CYP2A6 gene variants is a risk factor for acquisition of dependence, for persistent smoking and a cross-sectional study of adolescents indicating that phenotypically slow nicotine metabolism is a risk factor for a higher level of dependence 18,19,27. Our data directly link slow nicotine metabolism with a greater likelihood of experiencing subjective, cardiovascular and toxic effects of nicotine. The mechanisms by which slow nicotine metabolism and greater sensitivity to nicotine facilitate development of dependence have not been established. We speculate that slower metabolism results in longer persistence of nicotine in the brain, resulting in greater neuroadaptive changes and therefore faster development of dependence
METHODS
Subjects
The subjects were 20 whites (11 males, 9 females), 20 Blacks (10 males, 10 females) and 20 Asians (10 males, 10 females) who were never regular smokers and had smoked fewer than 100 cigarettes lifetime. In all subjects the screening plasma cotinine level measured by gas chromatography was below the limit of quantitation (10 ng/ml).
Twenty eight (46.7%) of subjects never smoked even one cigarette, 23 (38.3%) of subjects smoked 1–5 cigarettes, and nine (15%) of subjects smoked more than 5 but less than 100 cigarettes lifetime. The time interval between when a subject had last tried a cigarette and study enrollment was not specifically recorded, but the intervals were in years.
Subjects were healthy based on questionnaire, screening blood chemistries and electrocardiogram, and taking no medications. The criterion for belonging to a particular racial group was having four grandparents of the same race. Subjects were recruited by flyer advertisements at local colleges, cafes, restaurants and laundromats, by newspaper advertisements, and by a notice on a local website. Subjects were compensated financially for their participation. The study was approved by the UCSF Committee on Human Research and the Research Ethics Board for the University of Toronto, and subjects provided signed consent before entering the study.
Procedures
Subjects were admitted to the Clinical Research Center (CRC) at the San Francisco General Hospital Medical Center on the evening before the day of the study. They were asked not to consume any alcoholic beverages for 48 hours prior to admission. Subjects did not eat any food or drink any alcoholic or caffeinated beverages after midnight prior to the study. A light breakfast was served at 7:30 A.M. At about 8:00 A.M. a catheter was placed in a forearm vein for blood drawing. Baseline questionnaires were administered at 8:30 A.M.
At 9:00 AM a 7 mg Nicoderm patch (GlaxoSmithKline) was placed over the deltoid muscle. This dose of nicotine is lower than the typical (14–21 mg) doses used to aid smoking cessation 28. The patch was kept in place for eight hours unless there was a clinically significant adverse event or if requested by the subject, in which case the patch was removed. In the event of patch removal, subjects were asked to complete the study (sample collection, subjective responses, vital signs, etc.), although four subjects who experienced toxicity left the study early. Subjects remained supine from the time of patch placement until after lunch (approximately 5 hours).
Subjective responses, blood sampling and cardiovascular measures
Subjective responses, blood samples, heart rate, and blood pressure were recorded or obtained before patch placement (baseline) and at 30, 60, 90, 120, 240, 300,360, 420, and 480 minutes post-dosing. The visual analog nicotine effect scale (VANES) included 11 subjective ratings: I feel content; I feel alert and awake; I feel calm and relaxed; I am able to concentrate; the strength of the dose is …; I feel lightheaded or dizzy; I feel high; I feel nauseated; I feel anxious or tense; I feel stimulated; my heart is beating fast (palpitations). Each response had a 10 cm line marked off in 0.5 cm intervals. Subjects marked the line to describe how much they rated the particular effect at the moment. The subjective response for the strength of the dose was scored as a 0 at baseline.
Analytical chemistry and genotyping
Plasma was analyzed for nicotine and its metabolites cotinine. Nicotine and cotinine analyses were performed by gas chromatography with nitrogen phosphorus detection.29
CYP2A6 genotyping was performed at the University of Toronto using methods previously described.30,31 The following alleles were genotyped in study subjects: *1, *2, *4, *7, *8, *9; *10, *12, *14,*17, *20, *23, *24, *25, *26, *27 and *35. Because the number of people with any one particular variant genotype was small (Table 1), data analysis was performed comparing subjects with normal activity (*1/*1) to those with one or two reduced function variants (Var).
Data analysis
Plasma nicotine concentrations were analyzed as the peak concentrations and area under the plasma concentration-time curve (AUC). To examine the rate of absorption of nicotine as a predictor of toxicity, we computed the partial AUC from time zero to 90 minutes. Overall exposure to nicotine was estimated by the AUC from zero to 360 minutes. AUC0→90 and AUC0→360 were computed using the trapezoidal rule. VANES scores for each time point were analyzed as the change from baseline (before application of the nicotine patch). We used two-sample t-test to test for differences in log-AUC, log-Cmax, and BMI, the Wilcoxon two-sample test to test for differences in age and Tmax between individuals with toxicity and those without, and Fisher’s exact test to test for univariate associations between categorical variables. To investigate the associations between CYP2A6 genotype, and median-split nicotine AUC, Cmax, and Tmax on nicotine-induced toxicity, we performed a time-to-event analysis (also known as survival analysis) in which the event modeled was toxicity-induced patch removal. Genotype was entered as a categorical predictor with levels for *1/*1 genotype vs. all variants alleles (Var). All models were adjusted for covariates age and BMI (as continuous variables) and gender and race. Interactions between race and main predictors were non-significant and were omitted from the final models. Based on the model with genotype as a predictor, Kaplan-Meier curves of the probabilities of nicotine-induced toxicity by genotype and race were generated. Statistical analyses were carried out using SAS v. 9.3 (SAS Institute, Inc., Cary, NC, USA). All statistical tests were considered significant at α = 0.05.
Study Highlights.
What is the current knowledge on the topic?
Differences in pharmacologic response to early nicotine exposure appears to be an important determinant of vulnerability to developing tobacco addiction.
What question this study addressed?
Pharmacokinetic and genetic factors underlying individual differences in response to transdermal nicotine in never smokers were characterized.
What this study adds to our knowledge?
Subjective, cardiovascular and toxic effects of transdermal nicotine in never smokers are associated with the rate of rise and peak plasma concentrations of nicotine and the presence of reduction function CYP2A6 gene variants.
How this might change clinical pharmacology and therapeutics?
Our data, in conjunction with other published research, support the idea that global sensitivity to nicotine, mediated in part by genetically slow metabolism of nicotine, is an important determinant of addiction vulnerability in non-smokers. Our data also suggest that CYP2A6 genotype may be a predictor of nicotine toxicity and need for dose modification in nonsmokers treated with transdermal nicotine for ulcerative colitis and other disorders.
Acknowledgments
This study was supported by US Public Health Service grants DA02277, DA12393 and DA020830 awarded by the National Institute on Drug Abuse, and carried out in part at the General Clinical Research Center at San Francisco General Hospital with support of the NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. The genetics work was funded in part by a Canadian Institutes for Health Research grant MOP86471.
We thank Faith Allen for protocol and data management, Sandra Tinetti for assistance in conducting the clinical study, Lita Ramos and Fredysha McDaniel for performing the analytical chemistry, Drs Bo Xu, Kerri Schoedel and Eva Hoffmann for performing the CYP2A6 genotyping and Marc Olmsted and Scott Rostler for editorial assistance.
Benowitz, St. Helen, Dempsey, Jacob III, and Tyndale Wrote Manuscript
Footnotes
Author contributions
Benowitz, St. Helen, Dempsey, Jacob III, and Tyndale wrote the manuscript
Conflict of interest statements
Dr. Tyndale has consulted for Novartis and McNeil. As an Associate Editor for CPT, Dr. Tyndale was not involved in the review or decision process for this paper. Dr Benowitz has served on smoking cessation advisory boards for Pfizer and has been an occasional consultant to McNeil and GlaxoSmithKline. He has provided paid expert testimony concerning nicotine addiction in litigation against tobacco companies. The other authors have no conflicts of interest.
References
- 1.Carmelli D, Swan GE, Robinette D, Fabsitz R. Genetic influence on smoking--a study of male twins. N Engl J Med. 1992;327:829–833. doi: 10.1056/NEJM199209173271201. [DOI] [PubMed] [Google Scholar]
- 2.Al Koudsi N, Tyndale RF. Genetic influences on smoking: a brief review. Ther Drug Monit. 2005;27:704–709. doi: 10.1097/01.ftd.0000179842.63515.c6. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal A, Verweij KJ, Gillespie NA, et al. The genetics of addiction-a translational perspective. Transl Psychiatry. 2012;2:e140. doi: 10.1038/tp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benowitz NL, Perez-Stable EJ, Herrera B, Jacob P., 3rd Slower metabolism and reduced intake of nicotine from cigarette smoking in Chinese-Americans. J Natl Cancer Inst. 2002;94:108–115. doi: 10.1093/jnci/94.2.108. [DOI] [PubMed] [Google Scholar]
- 5.Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354:333–342. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 6.Trinidad DR, Perez-Stable EJ, Emery SL, White MM, Grana RA, Messer KS. Intermittent and light daily smoking across racial/ethnic groups in the United States. Nicotine Tob Res. 2009;11:203–210. doi: 10.1093/ntr/ntn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 8.McDonagh EM, Wassenaar C, David SP, et al. PharmGKB summary: very important pharmacogene information for cytochrome P-450, family 2, subfamily A, polypeptide 6. Pharmacogenet Genomics. 2012;22:695–708. doi: 10.1097/FPC.0b013e3283540217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malaiyandi V, Sellers EM, Tyndale RF. Implications of CYP2A6 genetic variation for smoking behaviors and nicotine dependence. Clin Pharmacol Ther. 2005;77:145–158. doi: 10.1016/j.clpt.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Pomerleau OF, Pomerleau CS, Namenek RJ. Early experiences with tobacco among women smokers, ex-smokers, and never-smokers. Addiction. 1998;93:595–599. doi: 10.1046/j.1360-0443.1998.93459515.x. [DOI] [PubMed] [Google Scholar]
- 11.DiFranza JR, Savageau JA, Fletcher K, et al. Recollections and repercussions of the first inhaled cigarette. Addict Behav. 2004;29:261–272. doi: 10.1016/j.addbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Kandel DB, Hu MC, Griesler PC, Schaffran C. On the development of nicotine dependence in adolescence. Drug Alcohol Depend. 2007;91:26–39. doi: 10.1016/j.drugalcdep.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Stacy A, Zheng H, et al. Sensations from initial exposure to nicotine predicting adolescent smoking in China: a potential measure of vulnerability to nicotine. Nicotine Tob Res. 2003;5:455–463. doi: 10.1080/14622200307239. [DOI] [PubMed] [Google Scholar]
- 14.Pomerleau OF. Individual differences in sensitivity to nicotine: implications for genetic research on nicotine dependence. Behav Genet. 1995;25:161–177. doi: 10.1007/BF02196925. [DOI] [PubMed] [Google Scholar]
- 15.Pomerleau OF, Collins AC, Shiffman S, Pomerleau CS. Why some people smoke and others do not: new perspectives. J Consult Clin Psychol. 1993;61:723–731. doi: 10.1037//0022-006x.61.5.723. [DOI] [PubMed] [Google Scholar]
- 16.Fattinger K, Verotta D, Benowitz NL. Pharmacodynamics of acute tolerance to multiple nicotinic effects in humans. J Pharmacol Exp Ther. 1997;281:1238–1246. [PubMed] [Google Scholar]
- 17.Audrain-McGovern J, Al Koudsi N, Rodriguez D, Wileyto EP, Shields PG, Tyndale RF. The role of CYP2A6 in the emergence of nicotine dependence in adolescents. Pediatrics. 2007;119:e264–274. doi: 10.1542/peds.2006-1583. [DOI] [PubMed] [Google Scholar]
- 18.O’Loughlin J, Paradis G, Kim W, et al. Genetically decreased CYP2A6 and the risk of tobacco dependence: a prospective study of novice smokers. Tob Control. 2004;13:422–428. doi: 10.1136/tc.2003.007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al Koudsi N, O’Loughlin J, Rodriguez D, Audrain-McGovern J, Tyndale RF. The genetic aspects of nicotine metabolism and thier impact on adolescent dependence. J Pediatr Biochem. 2010;1:105–123. [Google Scholar]
- 20.de Wit H, Bodker B, Ambre J. Rate of increase of plasma drug level influences subjective response in humans. Psychopharmacology (Berl) 1992;107:352–358. doi: 10.1007/BF02245161. [DOI] [PubMed] [Google Scholar]
- 21.Berridge MS, Apana SM, Nagano KK, Berridge CE, Leisure GP, Boswell MV. Smoking produces rapid rise of [11C]nicotine in human brain. Psychopharmacology (Berl) 2010;209:383–394. doi: 10.1007/s00213-010-1809-8. [DOI] [PubMed] [Google Scholar]
- 22.Gries JM, Benowitz N, Verotta D. Chronopharmacokinetics of nicotine. Clin Pharmacol Ther. 1996;60:385–395. doi: 10.1016/S0009-9236(96)90195-2. [DOI] [PubMed] [Google Scholar]
- 23.Mwenifumbo JC, Tyndale RF. Genetic variability in CYP2A6 and the pharmacokinetics of nicotine. Pharmacogenomics. 2007;8:1385–1402. doi: 10.2217/14622416.8.10.1385. [DOI] [PubMed] [Google Scholar]
- 24.Lunney PC, Leong RW. Review article: Ulcerative colitis, smoking and nicotine therapy. Aliment Pharmacol Ther. 2012;36:997–1008. doi: 10.1111/apt.12086. [DOI] [PubMed] [Google Scholar]
- 25.Thiriez C, Villafane G, Grapin F, Fenelon G, Remy P, Cesaro P. Can nicotine be used medicinally in Parkinson’s disease? Expert Rev Clin Pharmacol. 2011;4:429–436. doi: 10.1586/ecp.11.27. [DOI] [PubMed] [Google Scholar]
- 26.Silver AA, Shytle RD, Philipp MK, Wilkinson BJ, McConville B, Sanberg PR. Transdermal nicotine and haloperidol in Tourette’s disorder: a double-blind placebo-controlled study. J Clin Psychiatry. 2001;62:707–714. doi: 10.4088/jcp.v62n0908. [DOI] [PubMed] [Google Scholar]
- 27.Rubinstein ML, Shiffman S, Moscicki AB, Rait MA, Sen S, Benowitz NL. Nicotine metabolism and addiction among adolescent smokers. Addiction. 2013;108:406–412. doi: 10.1111/j.1360-0443.2012.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD000146. doi: 10.1002/14651858.CD000146.pub4. [DOI] [PubMed] [Google Scholar]
- 29.Jacob P, 3rd, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr. 1981;222:61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- 30.Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14:615–626. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Mwenifumbo JC, Myers MG, Wall TL, Lin SK, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6*7, CYP2A6*8 and CYP2A6*10 as assessed with a novel haplotyping method. Pharmacogenet Genomics. 2005;15:189–192. doi: 10.1097/01213011-200503000-00008. [DOI] [PubMed] [Google Scholar]