Abstract
Objective
Among preterm infants, to examine tradeoffs between cognitive outcome and overweight/obesity at school age and in young adulthood in relation to infancy weight gain and linear growth.
Study design
We studied 945 participants in the Infant Health and Development Program, an 8-center study of preterm (≤37 weeks), low birth weight (≤2500 grams) infants from birth to 18 years. Adjusting for maternal and child factors in logistic regression, we estimated the odds of overweight/obesity (BMI ≥85th percentile at age 8 or ≥25 kg/m2 at age 18) and in separate models, low IQ (<85) per z-score change in infant length and BMI from term to 4 months, 4-12 months, and 12-18 months.
Results
More rapid linear growth from term to 4 months was associated with lower odds of IQ<85 at age 8 (OR 0.82, 95% CI 0.70, 0.96), but a higher odds of overweight/obesity (OR 1.27, 95% CI 1.05, 1.53). More rapid BMI gain in all 3 infant time intervals was also associated with a higher odds of overweight/obesity, and from 4-12 months with a lower odds of IQ <85 at age 8. Results at age 18 were similar.
Conclusions
In preterm, low birth weight infants born in the 1980’s, faster linear growth soon after term was associated with better cognition but also with a higher risk of overweight/obesity at 8 and 18 years of age. BMI gain over the entire 18 months after term was associated with later risk of overweight/obesity, with less evidence for a benefit to IQ.
In the United States, over 12% of births are preterm (<37 weeks gestation) (1). Despite intensive nutritional support, at the time of discharge home from the neonatal intensive care unit (NICU), most preterm infants are considerably lighter and shorter than their full term peers at birth (2, 3), but by school age, a majority reaches a similar weight and height (4, 5). Thus, on average, weight gain and linear growth are accelerated for preterm relative to full term infants.
Because brain and somatic growth are correlated, optimizing growth after NICU discharge is highly relevant to preterm infants, and more rapid weight gain during this time period appears to benefit neurodevelopment (6-8). Few studies, however, have distinguished linear growth from gain in weight relative to length, which is important because disproportionate weight gain may be harmful to other aspects of health. Specifically, in full term children, rapid weight gain (9, 10) and gain in weight-for-length (11) in infancy are strongly and consistently associated with later obesity, but only 2 studies (12, 13) have examined the impact of early weight gain on obesity-related outcomes in preterm infants. Although both found positive associations, neither distinguished weight gain proportional to linear growth from excess weight gain.
Differentiating linear growth from disproportionately rapid weight gain may be particularly important to guide nutritional support that will maximize neurodevelopment while minimizing the risk of later obesity in preterm infants. One relevant study (14) found that more rapid linear growth but not body mass index (BMI) gain from term (40 weeks postmenstrual age) to 4 months was associated with a better motor outcome at 18 months, with no follow-up beyond infancy. Another (15) found that both linear growth and excess gain in weight-for-length in the first year of life were associated with a higher IQ at school age, although differences were relatively small and clinically relevant categories (e.g. normal vs. low IQ) were not examined, nor was there follow-up beyond early school age.
Clues to optimizing preterm infant growth after NICU discharge may come from examining both neurodevelopmental and obesity-related outcomes in the same cohort. The aim of this study was to examine associations of infant gain in body mass index (BMI, kg/m2) and linear growth from term to 18 months with (1) overweight or obesity and (2) IQ <85 at ages 8 and 18 years in a cohort of children born preterm and low birth weight (<2500 grams). We hypothesized that linear growth but not BMI gain would positively impact IQ whereas more BMI gain would lead to greater overweight and obesity.
Methods
We performed an observational analysis of participants in the Infant Health and Development Program (IHDP), an 8-center longitudinal study of preterm (≤37 completed weeks gestation) and low birth weight (≤2500g) infants. In 1984 and 1985, IHDP recruited infants to participate in a randomized trial of an early child development intervention for which the primary outcomes were cognitive development, behavior, and health status. Details of recruitment and follow up and study results have been reported through age 18 years (16-19). Institutional review boards from all participating centers granted approval and caregivers gave written informed consent.
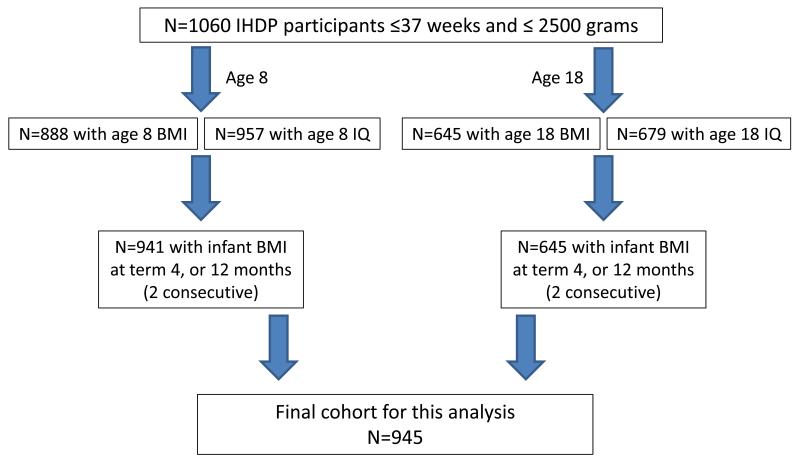
For this analysis, of the original 1060 IHDP participants, we included the 945 with data for IQ or BMI at 8 or 18 years and 2 consecutive infant BMI measurements (term and 4 months or 4 and 12 months or 12 and 18 months). In total, 941 participants were included in the age 8 analyses and 645 in the age 18 analyses. Details about participant flow are shown in Figure 1 (available at www.jpeds.com).
Figure 1.
Participant flow
IHDP study staff weighed and measured participants at term and at 4, 12, and 18 months corrected for prematurity (chronologic age in days minus the number of days by which the participant was born prior to 40 weeks) using a calibrated infant balance scale and recumbent length board (20). At age 8 years, study staff weighed children and measured them with a Ross stadiometer. At age 18 years, participants reported their own height and weight in a structured interview. To measure general intelligence, trained assessors administered the Weschler Intelligence Scale for Children-III (WISC-III) at age 8 and the Weschler Abbreviated Scale of Intelligence (WASI) at age 18.
Study staff collected data from the neonatal and maternal medical record and through interviews and questionnaires regarding parental and child demographic, social, economic, and health information. Maternal obesity was noted if the mother reported her pre-pregnancy weight as over 200 pounds. Gestational age was estimated using a modification of the Ballard assessment (21). Maternal intelligence was measured using the Peabody Picture Vocabulary Test-R when the child was 18 months old.
Statistical analyses
We converted all infant measurements to z-scores based on the World Health Organization growth standards (22) which were designed to reflect optimal growth in infancy. Primary predictors were BMI gain and linear growth from term to 4 months, 4 to 12 months, and 12 to 18 months corrected for prematurity, defined as the z-score change between time points.
All age 8 and 18 measurements were converted to percentiles based on Centers for Disease Control and Prevention growth charts (23). Primary outcomes were weight status (underweight, overweight/obesity) and low IQ. We defined underweight at age 8 as BMI percentile <5 and at age 18, BMI <18.5 kg/m2; and overweight/obesity at age 8 as BMI percentile of ≥85 and at age 18, BMI ≥25 kg/m2. We also examined short stature, defined as height <2.5th percentile. We defined low IQ as WISC-III or WASI score <85, which is 1 standard deviation below the population mean of 100.
Using multinomial logistic regression, we estimated the odds of underweight vs. normal weight, overweight/obesity vs. normal weight, and in separate models, short stature vs. not and low IQ vs. IQ ≥85, with 95% confidence intervals (CI’s), adjusting for child age, sex, and gestational age; and maternal age, education, smoking in pregnancy; and annual household income. As we have done previously (14), we additionally adjusted the 4 to 12 month growth analyses for term to 4 month growth and size at 4 months in the same measurement. Similarly, we adjusted the 12 to 18 month growth analyses for term to 4 month and 4 to 12 month growth, and size at 12 months. We adjusted all analyses for the IHDP study group (intervention vs. control) and the cognitive analyses for maternal IQ.
To examine possible confounding by neonatal complications, we repeated the same analyses after excluding participants with any of the following: 5 minute Apgar score <5; bronchopulmonary dysplasia; necrotizing enterocolitis; or grade 3 or 4 intraventricular hemorrhage and repeated the same analyses. To examine potential effect modification by fetal growth status, we performed analyses stratified by small for gestational age (SGA), defined as birth weight <10th percentile for gestational age based on a contemporary national reference (24) vs. non-SGA. Only 4 participants were large for gestational age (>90th percentile) and we included them in the non-SGA group. We used SAS version 9.1 (SAS Institute Inc., Cary, NC).
Results
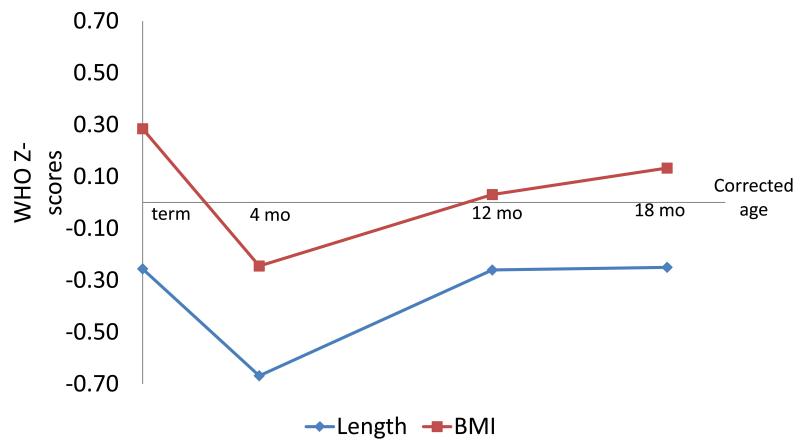
Participant characteristics are shown in Table 1 (available at www.jpeds.com). The mean (SD) gestational age was 33 (2.6) weeks and birth weight 1800 (455) grams. Just over one third were SGA and almost 20% had a neonatal complication. Figure 2 shows participants’ BMI and length z-scores at term, 4, 12, and 18 months. In our sample, BMI was higher than the reference population at term, but fell below by 4 months. At 12 months, BMI was similar to the reference population, and was slightly higher at 18 months. Length was below the reference population at term, even lower at 4 months, and increased somewhat by 12 months but remained below the reference population through 18 months.
Table 1.
Characteristics of 945 IHDP participants
| Maternal | Mean (SD) or number (%) |
|---|---|
| Age (years) | 25.0 (6.0) |
| Maternal IQ | 81 (22) |
| Preeclampsia or eclampsia | 150 (17%) |
| Obese (>200 lbs before pregnancy) | 33 (3.5%) |
| Smoked during pregnancy | 610 (32%) |
| Education | |
| < high school diploma | 342 (35%) |
| high school graduate | 261 (29%) |
| at least some college | 297 (33%) |
| Annual household income | |
| <$15,000 | 230 (25%) |
| $15,000 to <$35,000 | 286 (32%) |
| ≥$35,000 | 266 (30%) |
| missing | 118 (13%) |
| Race | |
| Black | 475 (53%) |
| White | 333 (37% |
| Hispanic | 92 (10%) |
|
| |
| Child/young adult | Mean (SD) or number (%) |
| Gestational age (weeks) | 33.0 (2.6) |
| Birth weight (g) | 1800 (455) |
| Male | 440 (49%) |
| <32 weeks gestation | 245 (27%) |
| SGA | 327 (36%) |
| NICU complications* | 170 (19%) |
chronic lung disease, necrotizing enterocolitis, intraventricular hemorrhage grade III or IV, or 5 minute Apgar score <5
Figure 2.
Participant body mass index (BMI) and length z-scores at term, 4, 12, and 18 months corrected age. A z-score of 0 represents the mean for the reference population.(45)
Table II (available at www.jpeds.com) shows Pearson correlations of the infant size and growth measures. BMI and length were weakly positively correlated at term and 4 months, and not correlated at 12 and 18 months. BMI gain and linear growth were not correlated from term to 4 months, but from 4-12 and 12-18 months, they were negatively correlated,
Table 2.
Correlations of infant size and growth variables
| BMI z-score | BMI z-score change | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Term | 4 months | 12 months | 18 months | Term-4 months | 4-12 months | 12-18 months | |
| Length z-score | Pearson correlation coefficients and p-values | ||||||
| Term | 0.19 | 0.12 | 0.08 | 0.08 | −0.10 | −0.04 | −0.00 |
| p<0.0001 | p=0.004 | p=0.02 | p=0.02 | p=0.003 | p=0.2 | p=1.0 | |
| 4 months | 0.16 | 0.13 | 0.17 | 0.15 | −0.06 | 0.04 | −0.02 |
| p<0.0001 | p<0.0001 | p<0.0001 | p<0.0.001 | p=0.08 | p=0.3 | p=0.5 | |
| 12 months | 0.02 | 0.25 | 0.04 | 0.16 | 0.16 | −0.22 | 0.16 |
| p=0.6 | p<0.0001 | p=0.2 | p<0.0001 | p<0.0001 | p<0.0001 | p<0.0001 | |
| 18 months | −0.02 | 0.23 | 0.21 | 0.00 | 0.19 | −0.04 | −0.28 |
| p=0.5 | p<0.0001 | p<0.0001 | p=0.9 | p<0.0001 | p=0.3 | p<0.0001 | |
| Length z-score change | |||||||
| Term to 4 months | −0.07 | −0.02 | 0.07 | 0.05 | 0.06 | 0.09 | −0.02 |
| p=0.03 | P=0.6 | p=0.04 | p=0.10 | p=0.09 | p=0.005 | p=0.6 | |
| 4-12 months | −0.18 | 0.13 | −0.18 | −0.00 | 0.25 | −0.32 | 0.23 |
| p<0.0001 | p=0.0001 | p<0.0001 | p=1.0 | p<0.0001 | p<0.0001 | p<0.0001 | |
| 12-18 months | −0.07 | −0.03 | 0.25 | −0.23 | 0.04 | 0.28 | −0.64 |
| p=0.03 | p=0.3 | p<0.0001 | p<0.0001 | p=0.21 | p<0.0001 | p<0.0001 | |
Tables III and IV show the primary outcomes at 8 and 18 years. At 8 years, 20.4% of participants were overweight or obese and at 18 years, 30.6% were overweight or obese. At 8 years, 35.9% had a full scale IQ score <85 and the proportion was similar (31.7%) at 18 years.
Table 3.
Participant size and weight and height status at 8 and 18 years
| 8 years (n=871) | 18 years (n=633) | |||
|---|---|---|---|---|
|
|
||||
| Native unit | Percentile* | Native unit | Percentile* | |
|
|
||||
| Mean (SD) | ||||
| BMI | 16.7 (2.7) kg/m2 | 53.5 (29.9) | 24.0 (4.9)kg/m2 | 59.8 (28.8) |
| Height | 127.1 (5.9) cm | 44.3 (28.9) | 168.0 (10.8) cm | 45.2 (31.5) |
|
|
||||
| Weight status† | Number (%) | |||
| Underweight | 49 (5.6%) | 42 (6.6%) | ||
| Healthy weight | 644 (73.9%) | 397 (62.7%) | ||
| Overweight | 104 (11.9%) | 130 (20.5%) | ||
| Obese | 74 (8.5%) | 64 (10.1%) | ||
| Short stature‡ | 42 (4.4%) | 33 (3.5%) | ||
based on CDC growth charts
based on percentile cut points at age 8 and BMI cut points at age 18
height <2.5th percentile for age and sex
Table 4.
Participant IQ at 8 and 18 years
| 8 years (n=936) | 18 years (n=669) | |
|---|---|---|
|
|
||
| Mean (SD) or number (%) | ||
| Full scale IQ | 90.6 (18.0) | 91.8 (16.4) |
| Low full scale IQ (<85) | 336 (35.9%) | 212 (31.7%) |
| Performance IQ | 89.9 (17.4) | 92.4 (15.8) |
| Low performance IQ (<85) | 379 (40.3%) | 195 (29.1%) |
| Verbal IQ | 92.7 (17.9) | 92.5 (16.6) |
| Low verbal IQ (<85) | 293 (31.3%) | 198 (29.6%) |
Table V shows the adjusted odds of underweight, overweight/obesity, short stature, and full scale IQ <85 at 8 and 18 years. More rapid linear growth from term to 4 months was associated with lower odds of IQ<85 [odds ratio (OR) 0.82, 95% CI 0.70, 0.96], but higher odds of overweight/obesity (OR 1.27, 95% CI 1.05, 1.53). Infant BMI gain in all 3 time intervals was associated with higher odds of overweight/obesity at age 8 [term to 4 months odds ratio (OR) 1.36 per additional z-score BMI gain, 95% CI 1.14, 1.62; 4 to 12 months OR 1.66, 95% CI 1.33, 2.06; and 12 to 18 months OR 2.00, 95% CI 1.53, 2.61]; and from 4 to 12 months with somewhat lower odds of IQ<85 (OR 0.81, 95% CI 0.68, 0.96). Associations with age 18 outcomes were similar in magnitude and direction. Linear growth from term to 4 months and 4-12 months and BMI gain from 4-12 months were associated with lower odds of short stature at age 8 but not 18 years.
Table 5.
Associations of infant growth with weight and height status and IQ category at 8 and 18 years
| Weight status (vs. normal weight)† | Height status | Full scale IQ | ||||||
|---|---|---|---|---|---|---|---|---|
| Age 8 | Age 18 | Age 8 | Age 18 | Age 8 | Age 18 | |||
| Adjusted odds ratios (95% confidence intervals) | ||||||||
| BMI z-score change* |
Underweight | Overweight/obese | Underweight | Overweight/obese | Short† (vs. not short) | <85 vs. ≥85 | ||
| Term-4 months | 0.44 | 1.36 | 0.66 | 1.40 | 1.05 | 1.05 | 0.92 | 1.11 |
| (0.33, 0.59) | (1.14, 1.62) | (0.50, 0.89) | (1.16, 1.69) | (0.85, 1.30) | (0.92, 1.21) | (0.80, 1.06) | (0.92, 1.33) | |
| 4-12 months | 0.44 | 1.66 | 0.70 | 1.28 | 0.74 | 0.90 | 0.81 | 0.85 |
| (0.30, 0.63) | (1.33, 2.06) | (0.48, 1.01) | (1.02, 1.60) | (0.57, 0.96) | (0.76, 1.05) | (0.68, 0.96) | (0.69, 1.06) | |
| 12-18 months | 0.73 | 2.00 | 0.75 | 1.67 | 0.74 | 1.03 | 1.02 | 0.86 |
| (0.47, 1.13) | (1.53, 2.61) | (0.48, 1.17) | (1.28, 2.18) | (0.53, 1.03) | (0.85, 1.26) | (0.82, 1.26) | (0.67, 1.11) | |
| Length z-score change* | ||||||||
| Term-4 months | 0.73 | 1.27 | 0.67 | 1.19 | 0.65 | 0.98 | 0.82 | 0.78 |
| (0.54, 0.98) | (1.05, 1.53) | (0.48, 0.93) | (0.98, 1.43) | (0.52, 0.82) | (0.85, 1.14) | (0.70, 0.96) | (0.65, 0.95) | |
| 4-12 months | 0.62 | 1.10 | 0.68 | 1.09 | 0.65 | 0.93 | 1.07 | 1.15 |
| (0.42, 0.90) | (0.89, 1.37) | (0.45, 1.02) | (0.88, 1.36) | (0.50, 0.86) | (0.79, 1.11) | (0.89, 1.29) | (0.92, 1.44) | |
| 12-18 months | 0.60 | 0.94 | 0.88 | 0.90 | 0.77 | 0.91 | 0.84 | 1.07 |
| (0.41, 0.88) | (0.73, 1.20) | (0.57, 1.37) | (0.70, 1.16) | (0.55, 1.06) | (0.75, 1.11) | (0.68, 1.03) | (0.83, 1.38) | |
based on WHO growth standards
Estimates adjusted for child age, sex, and gestational age; and maternal age, education, smoking in pregnancy; and annual household income.
based on percentile cut points at age 8 and BMI cut points at age 18
height <2.5th percentile for age and sex
After excluding infants with neonatal complications, associations of infant BMI gain and linear growth with age 8 and 18 outcomes were similar to the full cohort in magnitude and direction, although CI’s were somewhat wider. Associations were also similar for SGA and non-SGA participants, although CI’s were wide (data not shown).
Discussion
In this study, we found that more rapid BMI gain and linear growth in infancy were associated with better cognition, but also with a greater risk of overweight/obesity at age 8, with similar effects seen at age 18. These results suggest important trade-offs to consider with respect to optimal growth targets for preterm, low birth weight children after NICU discharge.
Our study extends prior work linking early rapid weight gain with later obesity and cognition in two ways. First, although most researchers have examined full term populations, we studied a preterm, low birth weight cohort. We found some similarities to studies of full term children, but also important differences. Consistent with studies of full term children(9, 10), our results suggest that preterm infants are also vulnerable to the obesogenic effects of early rapid weight gain out of proportion to linear growth. This finding is notable in the context of epidemic child obesity (25), with an obesity prevalence that is similar among preterm and full term children (13, 26).
In terms of cognition, however, preterm infants appear to be different from their full term counterparts. Other studies including ours (27) and a subsequent meta-analysis (28) found that in healthy, full term populations, rapid weight gain after birth was not associated with better cognition later in life, although few studies have separated weight gain from linear growth. In contrast, in our preterm, low birth weight cohort, we found that more rapid linear growth in the months after term – developmentally equivalent to the months after a full term birth – may be of substantial benefit to later cognition. It is possible that for the preterm, low birth weight infant, the months after term represent a sensitive period for that may be particularly important for neurodevelopment after a period of prenatal (29) and/or postnatal (2, 3) growth restraint.
A second way that our study extends prior work is in differentiating linear growth from BMI gain after NICU discharge. In our study, even though early (term to 4 months) linear growth was associated with substantially lower odds of IQ<85 at 8 and 18 years, in contrast, BMI gain during the same time period did not appear to benefit cognition, but was associated with higher odds of overweight/obesity. These findings are consistent with another study (14) of preterm infants <33 weeks’ gestation, which found an association of linear growth but not BMI gain from term to 4 months with the Bayley motor score at 18 months. Thus, even though early linear growth appears to be important for later cognition, our results suggest that excess early weight gain out of proportion to linear growth may contribute to later obesity, without cognitive benefits.
In our study, early (term to 4 months) linear growth appeared to benefit later cognition, but notably it was also associated with modestly higher odds of overweight/obesity. This finding raises the possibility that supporting optimal brain growth may come at a cost with respect to later cardio-metabolic health. A similar tradeoff was apparent with greater BMI gain from 4 to 12 months, which was associated with higher odds of overweight/obesity, but also with lower odds of IQ<85 at 8 years.
Defining optimal linear growth and BMI gain will require balancing the magnitude of effects on cardio-metabolic health and cognition, as well as the value that clinicians and families place on the different outcomes. In addition to obesity and cognition, an important consideration with respect to early linear growth is its impact on adult height. In our study, more rapid linear growth in infancy appeared protective against short stature at age 8 but not at age 18, suggesting that additional catch-up in linear growth occurred after age 8. Other relevant outcomes to consider include risk for re-hospitalization, which may be greater for low birth weight children with slow weight gain (30, 31), and asthma which in one study (32) was more prevalent in low birth weight children with high vs. low BMI in adolescence.
Children in our study had a higher BMI and were shorter at term than the WHO reference population of full term children at birth. This finding is consistent with studies that directly measured body composition at term using either air displacement plethysmography (33, 34) or DXA(35) and found a greater fat mass and lower fat free mass in preterm children vs. full term children. This difference may reflect excess non-protein energy that is stored as fat in growing preterm infants (36). In one study that followed children after NICU discharge (33) by 3 to 6 months of age, fat mass and fat free mass were similar between preterm and full term children, whereas in our cohort at 4 months, both BMI and length were substantially below the reference population. This difference may reflect the fact that anthropometric measurements only partially account for differences in direct measures of body composition (37). Also, our cohort was born in the 1980’s when nutritional practices were different, for example nutrient enriched postdischarge formulas were not in routine use. Specific determinants of early fat accumulation and lean body mass growth, such as composition and fortification of formula and expressed milk (38), energy expenditure (39), and oral-motor feeding skills (40) represent potential targets for intervention during this developmentally sensitive period.
Strengths of our study include a multi-center cohort with availability of detailed growth data at several time points after term, and later IQ and BMI measures in the same cohort. IQ and BMI measured at age 8 and 18 years are strongly correlated with adult outcomes (41, 42). One limitation is reliance on self-report of weight and height at age 18 years. Although highly correlated with direct measures (43), self-reported weight and height may underestimate the prevalence of overweight and obesity (44). We were reassured that in our study, exposure-outcome relationships were similar to those using direct measurements at age 8. We controlled for a number of relevant covariates, but as in any observational study, there may be residual confounding by unmeasured factors. In particular, both slow early weight gain and poor cognitive outcomes could occur as a result of neonatal brain injury or other complications of preterm birth, although excluding children with such complications did not materially change our results. Finally, IHDP included a large proportion of infants from poor households, and mothers of low educational status and of minority race and ethnicity, possibly limiting generalizability to more affluent, educated, and non-minority populations.
In conclusion, we found in a cohort of preterm, low birth weight infants born in the 1980’s that early linear growth after term was associated with a better cognitive outcome, but also with a higher risk for overweight/obesity later in life. Excess weight gain out of proportion to linear growth from term through 18 months was associated with later overweight/obesity, with less evidence for a substantial cognitive benefit. These tradeoffs represent important considerations for pediatricians and other clinicians who monitor growth and provide nutritional care for preterm infants.
Acknowledgments
Supported by the National Institutes of Health (K23 DK083817, K24 HL68041, R01 HD27344), Robert Wood Johnson Foundation, Maternal and Child Health Bureau (039543, MCJ-060515, MCJ-360593), and Pew Charitable Trust (91-01142).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Mathews TJ, Minino AM, Osterman MJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2008. Pediatrics. 2011;127(1):146–57. doi: 10.1542/peds.2010-3175. [DOI] [PubMed] [Google Scholar]
- 2.Ehrenkranz RA, Younes N, Lemons JA, Fanaroff AA, Donovan EF, Wright LL, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics. 1999;104(2 Pt 1):280–9. doi: 10.1542/peds.104.2.280. [DOI] [PubMed] [Google Scholar]
- 3.Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics. 2003;111(5 Pt 1):986–90. doi: 10.1542/peds.111.5.986. [DOI] [PubMed] [Google Scholar]
- 4.Hack M, Schluchter M, Cartar L, Rahman M, Cuttler L, Borawski E. Growth of very low birth weight infants to age 20 years. Pediatrics. 2003;112(1 Pt 1):e30–8. doi: 10.1542/peds.112.1.e30. [DOI] [PubMed] [Google Scholar]
- 5.Saigal S, Stoskopf B, Streiner D, Paneth N, Pinelli J, Boyle M. Growth trajectories of extremely low birth weight infants from birth to young adulthood: a longitudinal, population-based study. Pediatr Res. 2006;60(6):751–8. doi: 10.1203/01.pdr.0000246201.93662.8e. [DOI] [PubMed] [Google Scholar]
- 6.Kan E, Roberts G, Anderson PJ, Doyle LW. The association of growth impairment with neurodevelopmental outcome at eight years of age in very preterm children. Early Hum Dev. 2008;84(6):409–16. doi: 10.1016/j.earlhumdev.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Casey PH, Whiteside-Mansell L, Barrett K, Bradley RH, Gargus R. Impact of prenatal and/or postnatal growth problems in low birth weight preterm infants on school-age outcomes: an 8-year longitudinal evaluation. Pediatrics. 2006;118(3):1078–86. doi: 10.1542/peds.2006-0361. [DOI] [PubMed] [Google Scholar]
- 8.Powers GC, Ramamurthy R, Schoolfield J, Matula K. Postdischarge growth and development in a predominantly Hispanic, very low birth weight population. Pediatrics. 2008;122(6):1258–65. doi: 10.1542/peds.2007-3453. [DOI] [PubMed] [Google Scholar]
- 9.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95(8):904–8. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 10.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. Bmj. 2005;331(7522):929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123(4):1177–83. doi: 10.1542/peds.2008-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Euser AM, Finken MJ, Keijzer-Veen MG, Hille ET, Wit JM, Dekker FW. Associations between prenatal and infancy weight gain and BMI, fat mass, and fat distribution in young adulthood: a prospective cohort study in males and females born very preterm. Am J Clin Nutr. 2005;81(2):480–7. doi: 10.1093/ajcn.81.2.480. [DOI] [PubMed] [Google Scholar]
- 13.Casey PH, Bradley RH, Whiteside-Mansell L, Barrett K, Gossett JM, Simpson PM. Evolution of obesity in a low birth weight cohort. J Perinatol. 2011 doi: 10.1038/jp.2011.75. [DOI] [PubMed] [Google Scholar]
- 14.Belfort MB, Rifas-Shiman SL, Sullivan T, Collins CT, McPhee AJ, Ryan P, et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics. 2011;128(4):e899–906. doi: 10.1542/peds.2011-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belfort MB, Martin CR, Smith VC, Gillman MW, McCormick MC. Infant weight gain and school-age blood pressure and cognition in former preterm infants. Pediatrics. 2010;125(6):e1419–26. doi: 10.1542/peds.2009-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enhancing the outcomes of low-birth-weight, premature infants. A multisite, randomized trial. The Infant Health and Development Program. Jama. 1990;263(22):3035–42. doi: 10.1001/jama.1990.03440220059030. [DOI] [PubMed] [Google Scholar]
- 17.McCormick MC, McCarton C, Tonascia J, Brooks-Gunn J. Early educational intervention for very low birth weight infants: results from the Infant Health and Development Program. J Pediatr. 1993;123(4):527–33. doi: 10.1016/s0022-3476(05)80945-x. [DOI] [PubMed] [Google Scholar]
- 18.McCarton CM, Brooks-Gunn J, Wallace IF, Bauer CR, Bennett FC, Bernbaum JC, et al. Results at age 8 years of early intervention for low-birth-weight premature infants. The Infant Health and Development Program. Jama. 1997;277(2):126–32. [PubMed] [Google Scholar]
- 19.McCormick MC, Brooks-Gunn J, Buka SL, Goldman J, Yu J, Salganik M, et al. Early intervention in low birth weight premature infants: results at 18 years of age for the Infant Health and Development Program. Pediatrics. 2006;117(3):771–80. doi: 10.1542/peds.2005-1316. [DOI] [PubMed] [Google Scholar]
- 20.Guo SS, Roche AF, Chumlea WC, Casey PH, Moore WM. Growth in weight, recumbent length, and head circumference for preterm low-birthweight infants during the first three years of life using gestation-adjusted ages. Early Hum Dev. 1997;47(3):305–25. doi: 10.1016/s0378-3782(96)01793-8. [DOI] [PubMed] [Google Scholar]
- 21.Ballard JL, Novak KK, Driver M. A simplified score for assessment of fetal maturation of newly born infants. J Pediatr. 1979;95(5 Pt 1):769–74. doi: 10.1016/s0022-3476(79)80734-9. [DOI] [PubMed] [Google Scholar]
- 22.de Onis M, Garza C, Onyango AW, Rolland-Cachera MF. [WHO growth standards for infants and young children] Arch Pediatr. 2009;16(1):47–53. doi: 10.1016/j.arcped.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 24.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. Jama. 2012;307(5):483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hack M, Schluchter M, Andreias L, Margevicius S, Taylor HG, Drotar D, et al. Change in prevalence of chronic conditions between childhood and adolescence among extremely low-birth-weight children. Jama. 2011;306(4):394–401. doi: 10.1001/jama.2011.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belfort MB, Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Infant growth and child cognition at 3 years of age. Pediatrics. 2008;122(3):e689–95. doi: 10.1542/peds.2008-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beyerlein A, Ness AR, Streuling I, Hadders-Algra M, von Kries R. Early rapid growth: no association with later cognitive functions in children born not small for gestational age. Am J Clin Nutr. 2010;92(3):585–93. doi: 10.3945/ajcn.2009.29116. [DOI] [PubMed] [Google Scholar]
- 29.Bukowski R, Gahn D, Denning J, Saade G. Impairment of growth in fetuses destined to deliver preterm. Am J Obstet Gynecol. 2001;185(2):463–7. doi: 10.1067/mob.2001.115865. [DOI] [PubMed] [Google Scholar]
- 30.Victora CG, Barros FC, Horta BL, Martorell R. Short-term benefits of catch-up growth for small-for-gestational-age infants. Int J Epidemiol. 2001;30(6):1325–30. doi: 10.1093/ije/30.6.1325. [DOI] [PubMed] [Google Scholar]
- 31.Hui LL, Schooling CM, Wong MY, Ho LM, Lam TH, Leung GM. Infant growth during the first year of life and subsequent hospitalization to 8 years of age. Epidemiology. 2010;21(3):332–9. doi: 10.1097/EDE.0b013e3181cd709e. [DOI] [PubMed] [Google Scholar]
- 32.Lu FL, Hsieh CJ, Caffrey JL, Lin MH, Lin YS, Lin CC, et al. Body mass index may modify asthma prevalence among low-birth-weight children. Am J Epidemiol. 2012;176(1):32–42. doi: 10.1093/aje/kwr484. [DOI] [PubMed] [Google Scholar]
- 33.Ramel SE, Gray HL, Ode KL, Younge N, Georgieff MK, Demerath EW. Body composition changes in preterm infants following hospital discharge: comparison with term infants. J Pediatr Gastroenterol Nutr. 2011;53(3):333–8. doi: 10.1097/MPG.0b013e3182243aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roggero P, Gianni ML, Amato O, Orsi A, Piemontese P, Morlacchi L, et al. Is term newborn body composition being achieved postnatally in preterm infants? Early Hum Dev. 2009;85(6):349–52. doi: 10.1016/j.earlhumdev.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Cooke RJ, Griffin I. Altered body composition in preterm infants at hospital discharge. Acta Paediatr. 2009;98(8):1269–73. doi: 10.1111/j.1651-2227.2009.01354.x. [DOI] [PubMed] [Google Scholar]
- 36.Romera G, Figueras J, Rodriguez-Miguelez JM, Ortega J, Jimenez R. Energy intake, metabolic balance and growth in preterm infants fed formulas with different nonprotein energy supplements. J Pediatr Gastroenterol Nutr. 2004;38(4):407–13. doi: 10.1097/00005176-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Kiger J, Taylor S, Wagner C, Finch C, Ebeling M, Katikaneni L. Pediatric Academic Societies Annual Meeting. Boston, MA: 2012. Body measurements cannot predict body composition in preterm infants. [Google Scholar]
- 38.O’Connor DL, Khan S, Weishuhn K, Vaughan J, Jefferies A, Campbell DM, et al. Growth and nutrient intakes of human milk-fed preterm infants provided with extra energy and nutrients after hospital discharge. Pediatrics. 2008;121(4):766–76. doi: 10.1542/peds.2007-0054. [DOI] [PubMed] [Google Scholar]
- 39.Denne SC. Energy expenditure in infants with pulmonary insufficiency: is there evidence for increased energy needs? J Nutr. 2001;131(3):935S–7S. doi: 10.1093/jn/131.3.935S. [DOI] [PubMed] [Google Scholar]
- 40.DeMauro SB, Patel PR, Medoff-Cooper B, Posencheg M, Abbasi S. Postdischarge feeding patterns in early-and late-preterm infants. Clin Pediatr (Phila) 2011;50(10):957–62. doi: 10.1177/0009922811409028. [DOI] [PubMed] [Google Scholar]
- 41.Sattler JM. Assessment of Children: Cognitive Applications. 4th ed Jerome M. Sattler, Inc.; San Diego: 2001. [Google Scholar]
- 42.Deshmukh-Taskar P, Nicklas TA, Morales M, Yang SJ, Zakeri I, Berenson GS. Tracking of overweight status from childhood to young adulthood: the Bogalusa Heart Study. Eur J Clin Nutr. 2006;60(1):48–57. doi: 10.1038/sj.ejcn.1602266. [DOI] [PubMed] [Google Scholar]
- 43.Elgar FJ, Roberts C, Tudor-Smith C, Moore L. Validity of self-reported height and weight and predictors of bias in adolescents. J Adolesc Health. 2005;37(5):371–5. doi: 10.1016/j.jadohealth.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Sherry B, Jefferds ME, Grummer-Strawn LM. Accuracy of adolescent self-report of height and weight in assessing overweight status: a literature review. Arch Pediatr Adolesc Med. 2007;161(12):1154–61. doi: 10.1001/archpedi.161.12.1154. [DOI] [PubMed] [Google Scholar]
- 45.Group WMGRS . WHO Child Growth Standards: Growth velocity based on weight, length, and head circumference: Methods and development. World Health Organization; Geneva: 2009. [Google Scholar]