Abstract
Objectives
The features and clinical applications of balanced-charge kilohertz frequency alternating currents (KHFAC) are reviewed. Preclinical studies of KHFAC block have demonstrated that it can produce an extremely rapid and reversible block of nerve conduction. Recent systematic analysis and experimentation utilizing KHFAC block has resulted in a significant increase in interest in KHFAC block, both scientifically and clinically.
Materials and Methods
We review the history and characteristics of KHFAC block, the methods used to investigate this type of block, the experimental evaluation of block, and the electrical parameters and electrode designs needed to achieve successful block. We then analyze the existing clinical applications of high frequency currents, comparing the early results with the known features of KHFAC block.
Results
Although many features of KHFAC block have been characterized, there is still much that is unknown regarding the response of neural structures to rapidly fluctuating electrical fields. The clinical reports to date do not provide sufficient information to properly evaluate the mechanisms that result in successful or unsuccessful treatment.
Conclusions
KHFAC nerve block has significant potential as a means of controlling nerve activity for the purpose of treating disease. However, early clinical studies in the use of high frequency currents for the treatment of pain have not been designed to elucidate mechanisms or allow direct comparisons to preclinical data. We strongly encourage the careful reporting of the parameters utilized in these clinical studies, as well as the development of outcome measures that could illuminate the mechanisms of this modality.
Keywords: Nerve Block, High Frequency, Kilo Hertz Frequency Nerve Block, Electrical Stimulation, Pain Block, Spasticity Block
INTRODUCTION
A primary goal of neuromodulation and neurostimulation devices is to achieve control over the nervous system in order to alleviate the effects of disease. Generation of action potentials through electrical stimulation is a well-understood mechanism, and this technology has been utilized in many common clinical devices. In contrast, the use of electrical currents to directly arrest or diminish nerve conduction has proven to be much more difficult to achieve. In general, inactivation of the nervous system has relied on indirect methods, such as neuromodulation, that presumably activate natural inhibitory mechanisms (1). The purpose of this review is to evaluate what is known about the ability of electrical currents to directly inhibit the conduction of action potentials. In this review, we focus specifically on the use of charge-balanced kilohertz frequency alternating current (KHFAC) (i.e. zero net charge delivery) because this method has been shown to produce an extremely rapid block of nerve conduction that is quickly reversible. This review only addresses electrical methods that have been shown to produce a conduction block that is rapidly reversible, and therefore does not include the use of alternating current (AC) for neurolysis, such as radio frequency ablation. We also do not discuss direct current (DC) block in detail in this review.
While electrical activation of nerve has become widely studied and well-understood, KHFAC electrical nerve block has been poorly understood and historically ignored by the scientific and medical community. The few reports in the literature presented extremely varied experimental conditions with no systematic analysis of the influence of the KHFAC waveform parameters on conduction block (2). These factors have made it difficult to discern the important characteristics that produce a repeatable and consistent nerve block using KHFAC. The past decade has experienced a significant increase in scientific interest in KHFAC block, with significantly improved analysis of this type of nerve block in both experimental and simulation conditions.
A variety of terms have been used in the literature to describe the use of AC to block nerves. Unfortunately, the most common term, “high frequency alternating current” (HFAC) is ambiguous and has resulted in some confusion in the literature. Frequencies as low as 130 Hz have been termed high frequency(3,4). It is important to properly distinguish the specific parameters used for KHFAC block because the characteristic effects on the nerve vary considerably as a function of frequency, amplitude and electrode design (and possibly other factors as well). In order to accurately distinguish between the different parameters used for electrical nerve block and the differing properties achieved, we propose a nomenclature summarized in Table 1. In particular, we suggest the use of the term KHFAC to refer to the use of continuous charge-balanced alternating current in the frequency range of ~1 kHz to 100 kHz. This particular range of frequencies has received the most study in the past few years and is the focus of this review. KHFAC block should also not be confused with the use of brief bursts of electrical stimulation in the kHz frequency range. These bursts, typically delivered at 50Hz or lower, are used in an attempt to activate tissue more effectively and are not a method of nerve block.
TABLE 1.
Types of Frequency-related Nerve Blocks
| Suggested Nomenclature | Typical Frequency Range | Charge- balanced? | Blocks Action Potential Conduction? [Direct Block] | Depletes Neurotransmitter at Synapse? | Immediate Reversibility | Example References |
|---|---|---|---|---|---|---|
| Direct Current (DC) Block | DC | No | Yes | No | Conditional1 | Pfluger, 1858; Bhadra and Kilgore, 2004 |
| Monophasic High Frequency | 100Hz–600Hz | No | Yes | Yes | Yes | Solomonow, et al., 1983 |
| Neurotransmitter Depletion | 100Hz–5000Hz | Yes | No | Yes | Yes | Wedensky, 1903; Dowden et al., 2010 |
| Kilohertz Frequency Alternating Current (KHFAC) | 1kHz–100kHz | Yes | Yes | No | Yes | Tanner, 1962; Bowman & McNeal, 1986 |
| Radio Frequency (RF) Ablation | 500kHz | Yes | Yes | No | No | Van Kleef et al., 1996 |
Reversibility from DC block depends on amplitude and duration of block.
It is also important to distinguish between neurotransmitter depletion block and true nerve conduction block(2,5,6). In true nerve conduction block, action potentials are arrested as they pass under the blocking electrode. In neurotransmitter depletion, action potentials are generated in the nerve at such a fast rate (typically above 100 Hz) that the neurotransmitter is transiently depleted at the synaptic or neuromuscular junction. Thus, in a neurotransmitter depletion “block”, the axon itself is still activated and not blocked.
In addition to defining the frequency range for electrical nerve block, it is also important to distinguish between alternating current (AC), direct current (DC), and monophasic high frequency current blocks. As generally applied in the field of electrical stimulation, alternating current implies a zero net charge (noting that a true zero net charge is not always achieved, but that is the goal). Direct current generally implies the delivery of a single polarity of current for a prolonged period of time, typically tens of seconds or longer (7). Monophasic high frequency stimulation typically describes the repeated delivery of electrical pulses that are of the same polarity. Monophasic stimulation usually consists of short pulses (~100 μS) with 10–100 ms of no current delivery in between each pulse. This distinction is important in the field of electrical nerve block, as it has been demonstrated that monophasic stimulation at frequencies above ~300 Hz has an effect on the nerve that is similar to DC (2). Further, monophasic stimulation, like DC, is damaging to both nerve and electrode and thus is not practical clinically (8). Thus, the work of Solomonow et al. (9), who explored monophasic stimulation for the purpose of nerve block, is not included in this review. Unfortunately, this work has resulted in some confusion in the literature regarding optimal frequencies for nerve block, which has been reviewed previously (2).
Although kilohertz frequencies are sometimes applied at the skin surface, the authors are not aware of any demonstration of direct nerve block using this method. Given the likely attenuation of high frequency through the tissue, and the depth of neural structures, it is unlikely that KHFAC delivered to the skin surface produces the same effect on neural structures as those described in this review. Further study in this area is warranted.
Interest in KHFAC has increased in the last decade due to the unique characteristics of KHFAC block and the many potential clinical applications where these characteristics might be efficacious. KHFAC could be used to block motor nerves for the treatment of spasticity in stroke, cerebral palsy and multiple sclerosis. In these applications, KHFAC could provide an alternative to neurolysis procedures, and possibly an alternative to repeated use of denervating agents, such as botulinum toxin. KHFAC block could be used to produce a relaxation of the urinary sphincter “on command”, providing micturition in spinal cord injury (10,11). KHFAC could be used to block sensory nerves for the treatment of peripheral nerve pain, and may provide a more effective alternative when compared to peripheral nerve stimulation. Early clinical results indicate KHFAC may be effective in providing pain relief through spinal cord stimulation (SCS) without the paresthesia that is typically associated with that modality (12,13). Finally, KHFAC could be used to block autonomic nerves for treatment of conditions such as hyperhydrosis or the vagal nerve for the treatment of obesity. Given the potential of KHFAC block, it is important to understand the specific characteristics of this method and identify the advantages and limitations of this approach.
We will first review the historical exploration of the inhibitory effects of electrical current on nerve conduction. However, with the exception of the work of Bowman and McNeal (14), no detailed, systematic study of high frequency nerve block was conducted until the past decade. Therefore, the bulk of this review summarizes the characteristics of KHFAC and related nerve block methods that have been described in the literature, reviewing the methods used to evaluate KHFAC block and the electrical parameters and electrode designs needed to achieve successful block. We describe the “onset response”, which is the transitory volley of activity produced in the nerve each time KHFAC is delivered, and we review the various approaches to reduce or eliminate the onset response. We review KHFAC block in different species, nerve diameters, and nerve fiber types. We summarize the study of the chronic effects of KHFAC and review the current use of KHFAC in clinical applications. We conclude with a discussion of future areas of research related to KHFAC.
HISTORY OF KHFAC NERVE BLOCK
The response of the nerve and muscle to trains of high frequency AC waveforms was first characterized by Wedensky(15). The rapid failure of neuromuscular junction transmission following stimulation at frequencies in excess of 100 Hz has been referred to as ‘Wedensky inhibition’ but was not a nerve conduction block. In 1935 Bugnard and Hill(16) published their observation on the diminished nerve responses to frequencies up to 2500 Hz in the frog sciatic nerve. In the same year, Cattel and Gerard(17) used KHFAC up to 2300 Hz and were the first to conclude that there was a local decrease in excitability of the nerve membrane as opposed to a neuromuscular junction block. Reboul et al. showed inhibition using KHFAC up to 40 kHz in the popliteal nerve of the cat(18), the first such demonstration in a mammalian species. Tanner(19) was the first to show a gradable block of nerve conduction in the frog sciatic nerve, using the nerve compound action potential (CAP). Alternating currents (presumably sinusoidal) at 20 kHz could be used selectively to block nerve fibers of different sizes by varying the amplitude of the signal. Large fibers were blocked at 4.9 root mean square volts (Vrms), and all activity was blocked at 10.2 Vrms. Woo and Campbell(20) confirmed the findings of Tanner in the frog sciatic and cat tibial nerves, using the CAP or single fiber recordings (SFR) with 20 kHz KHFAC. They showed that the response of the nerve varied as the stimulus amplitude was increased. At low amplitudes, axons rhythmically fired at approximately 100 Hz. The firing frequencies increased with increasing stimulation amplitudes, up to a maximum frequency of 400–700 Hz. As the AC amplitude was further increased, the axon firing became asynchronous. Eventually, all activity ceased at typically 1–2 V. At these levels, it was demonstrated that the KHFAC produced a region of the nerve membrane that blocked conduction of action potentials.
The most comprehensive early study on KHFAC nerve block was conducted by Bowman and McNeal in 1986(14). Responses to voltage-controlled biphasic rectangular pulses over the range of 100–10,000 Hz in the cat sciatic nerve were evaluated by measuring the firing frequencies of spinal ventral roots with SFR. The waveform used in these studies consisted of a balanced rectangular pulse of 50 μs duration for each phase. Therefore, at frequencies below 10 kHz there was an off-time between biphasic pulses. They found that a nerve conduction block could be achieved at 4 kHz with an amplitude of 7 V. The KHFAC produced an initial increase in firing that lasted 1–2 s, followed by a period of a few seconds where pulses could pass uninhibited through the electrode region, before a true conduction block was established. Once established, this block could be maintained at least 80 s, and conduction could be restored within 1 s after the cessation of block.
In the 1980s, there were a group of publications purporting to be KHFAC nerve block. However, most of these were at much lower frequencies and closer examination show them to be neuromuscular junction fatigue blocks. Since these articles are still often cited and confused with KHFAC block, we will briefly mention them here. This type of nerve/endplate block was investigated by Solomonow and his colleagues(9,21,22). Studies were performed using the sciatic nerve of the cat, with muscle force as the outcome measure. A broad frequency range was evaluated, and 600 Hz was identified as the optimum block frequency. It should be noted that these studies utilized a very different waveform from that used by previous investigators, consisting of a monophasic current-controlled depolarizing pulse lasting 50–100 ms, delivered at the specified frequency (Table 1).
In the 1990s, investigators in Montreal focused on the 600 Hz conduction block produced by Solomonow, but using a biphasic rather than a monophasic waveform(23–27). Unlike the Solomonow waveform, the Sawan waveform has a zero net charge. Shaker et al.(23) reported the use of a 600 Hz block in the pudendal nerves of dogs to enable proper voiding by blocking unwanted sphincter activity. These investigators initially began their investigations utilizing a 200–300 Hz waveform to produce a fast fatigue of the sphincter muscles to achieve a ‘fatigue block’(24–26). They discovered, however, that if the frequency was increased to 600 Hz, they could achieve what appeared to be a true conduction block, rather than just a quickly fatiguing response. Another attempt to utilize the methods of Solomonow to produce a block of sphincter activity was performed by Ishigooka, et al. (28). A monophasic waveform was used, with a constant amplitude of 10 V and constant phase duration of 200 ms. The waveform was different from the Solomonow waveform because it was voltage-controlled rather than current controlled. This is likely to result in a biphasic current waveform being delivered to the nerve. Although blocking was achieved in this study, it was not more than 60% effective; and the blocking effectiveness was essentially equivalent above 200 Hz.
Beginning in 2004, a number of research groups began publishing research on KHFAC block(2,5,29). These reports represented a much more systematic analysis of the parameters necessary for KHFAC block, the key characteristics of KHFAC block, the mechanisms underlying KHFAC block, and established early exploration of potential clinical applications using this block. The remainder of this review summarizes what is known to date. Despite the recent explosion of interest, there still remain many unknown aspects of KHFAC on nerve conduction and there is significant room for additional exploration and application.
METHODOLOGY IN KHFAC RESEARCH
The methodology used to investigate KHFAC nerve block is not complex. The fundamental strategy is to induce a localized area of block in a whole nerve trunk using some type of encircling electrode (30,31). To test the degree and quality of block, a second electrode is placed at some distance from the blocking electrode and used to deliver supramaximal test pulses that activate all the fibers of interest (either motor or sensory). For motor block experiments, the most common outcome measure has been force measurements of one of the muscles supplied by that particular nerve. This gives summated information about the status of block of all the relevant motor fibers in the nerve. The typical experimental setup for these types of experiments is shown in Figure 1. For sensory block experiments, compound action potentials (CAPs) have been recorded to assess block. Unfortunately, although the use of CAP recordings would appear to be the most direct method for measuring the block effect, it can be difficult to accomplish during KHFAC. Specifically, the presence of the relatively high amplitude KHFAC waveform in close proximity to the CAP recording site produces significant background noise, thus reducing the signal to noise ratio(32). Signal averaging to reduce the noise results in misrepresentation of the onset response (described later). As a result, some experiments have been performed by recording the CAP immediately after the KHFAC is terminated(32,33), although this method does not directly confirm conduction block during KHFAC and probably underestimates the true percentage of block(32). In addition, KHFAC at some amplitudes produces asynchronous firing in the nerves as well as changes in axonal conduction velocity, which can result in an artificially lowered CAP (due to the diminished summation of action potentials), thus providing misleading information about the degree of nerve block(20). In a few studies, single fiber recordings (SFR) have been used to investigate the responses of motor or sensory fibers to block(14,32). This method gives accurate information about both the onset response and the block status of single fibers. However, it is experimentally very demanding and can only test a small percentage of fibers in the nerve trunk.
Figure 1.
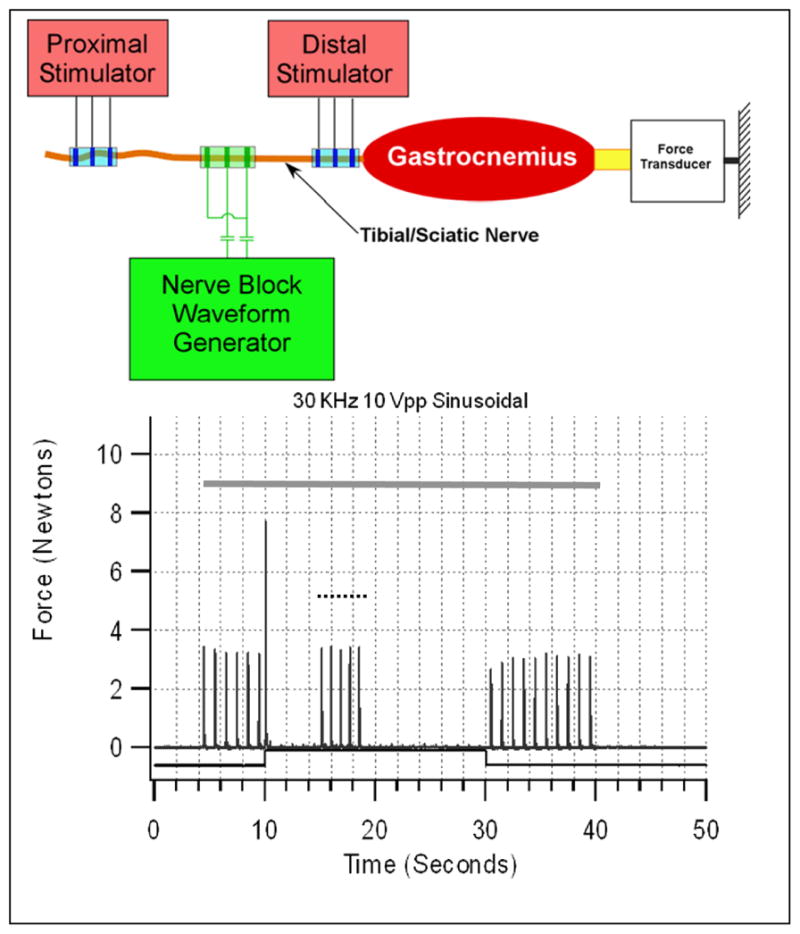
Above: Typical experimental setup. Below: Block of rat sciatic nerve using 30 kHz sinusoidal KHFAC at an amplitude of 10 volts peak-to-peak (Vpp). Grey solid bar shows duration of proximal stimulation at 1 Hz. Black bar (below data) shows timing of KHFAC delivery. Short dashed bar shows timing of distal stimulation. There is 99% motor block during the KHFAC delivery after the brief onset response. Response to distal stimulation shows that the neuromuscular junction is responsive during the block, proving this to be a true localized nerve conduction block.
When muscle force is utilized as the outcome measure for block, it is important to experimentally distinguish between nerve conduction block and neurotransmitter depletion block(2). This can be accomplished by stimulating the nerve between the blocked region and the synaptic junction (typically the neuromuscular junction in the case of muscle force measurement), as proposed by Bowman and McNeal(14). KHFAC conduction block is generally localized under the electrode and does not affect the neuromuscular junction, allowing full activation of the nerve just distal to the blocked region. Therefore, activation of this distal electrode should produce the same magnitude of muscle response as the test stimulus that is proximal to the blocking site (Figure 1). If distal activation fails to produce muscle force, it indicates neuromuscular junction depletion(2,5,34).
Our laboratory has introduced a number of measurements of KHFAC motor block that have facilitated comparison of results over a wide variety of experiments. One important measure is the “block threshold”, which is the lowest current or voltage of the delivered KHFAC that results in complete motor block of a whole peripheral nerve(34). This is a repeatable measure that can be evaluated over the duration of the experiment. It is measured by achieving complete block at high amplitude and then lowering the amplitude in a stepwise fashion until unblocked action potentials start producing small muscle twitches. The lowest amplitude at which complete block persists is defined as the block threshold. The other measures we developed have been used to quantify the onset response. The motor onset response produces a summated force similar to a tetanic response. This was quantified in three ways: measuring the peak force, measuring the duration of the onset response, and, the most important measure, the force-time integral of the onset response(34).
The use of stimulating electrodes on both sides of a blocked region provides an immediate measure of any prolonged effect of the KHFAC. Specifically, action potentials generated by the proximal stimulating electrode must travel through the region of nerve surrounded by the KHFAC blocking electrode prior to reaching the neuromuscular junction. Action potentials generated at the distal electrode do not pass the KHFAC region and only pass through a short region of untouched nerve prior to reaching the neuromuscular junction. Each electrode activates every motor fiber in the nerve, and therefore the peak forces should be identical. Thus, under normal conditions, the ratio of the peak twitch force generated by the proximal electrode to the peak twitch force generated by the distal electrode should be 1.0. This ratio, referred to as the PS/DS ratio (Proximal Stimulation/Distal Stimulation) can be used as a measure of nerve patency(34). Any temporary depression or damage to the transmission capabilities of the nerve caused by the KHFAC block will be immediately apparent. The PS/DS ratio is insensitive to naturally occurring fatigue or other muscle-based changes, and is therefore ideal as an outcome measure for longer-term KHFAC block studies.
It is important to note that the instrumentation used to generate and deliver KHFAC can have a significant impact on the results obtained. Unfortunately, the instrumentation is often poorly described in the literature, and verification of the output parameters is rarely presented. In our experience, for example, we have found that it is difficult to generate current-controlled AC waveforms above 5 kHz at the amplitudes necessary to produce effective nerve block. We have found that many instruments based on voltage-to-current convertors significantly attenuate and distort the outputted waveform above 5 kHz, despite supplied specifications to the contrary. In addition, nearly all waveform generators require specific efforts at electrical isolation in order to prevent DC leakage or imbalanced charge through the electrodes. A charge imbalance can give misleading block effects because DC is a very effective blocking agent. DC as low as 6 μA can, in some cases, produces a block of nerve conduction(35), which is below the typical isolation requirements even for medical instrumentation. Most researchers utilize capacitors placed in series on the output of the waveform generator to minimize DC delivery, yet even this practice may not guarantee a truly charge-balanced output, particularly when a current-controlled output stage is used. DC nerve damage can confound the experimental results.
CHARACTERISTICS OF KHFAC BLOCK
Proof of conduction block
Conduction block using KHFAC is produced by creating a finite region of axons through which action potentials cannot pass(34). This region is positioned directly under the KHFAC electrode and generally extends longitudinally a few millimeters. This characteristic was established experimentally through two distinct experiments. The first experiment utilized the three electrode configuration shown in Figure 1. The use of a distal stimulating electrode placed as close as 1 mm away from the KHFAC electrode can successfully activate the entire motor nerve, producing the same magnitude of muscle twitch as the proximal electrode and demonstrating that the conduction block is proximal to the distal stimulating electrode(5,34,36). The second experiment (unpublished) involved blocking one branch of a bifurcated nerve, where each branch innervates a separate muscle (tibial nerve supplying the gastrocnemius muscle and the common peroneal nerve innervating the tibialis anterior muscle). Stimulation was applied to the nerve proximal to the branching point, thus activating both muscles simultaneously. KHFAC was then delivered to one branch, producing complete block in one muscle while allowing full activation of the second muscle. This experiment verified that the blocking electrode does not produce any electrical interference with the proximal stimulating electrode. Thus, the blocking effect is isolated to the immediate vicinity of the blocking electrode, with no systemic effects.
Species and nerve diameter
KHFAC nerve block has been demonstrated in multiple species and over a range of peripheral nerve diameters. Animal models used have been the sea slug(37), frog(2,38), rat(5,34), cat(39), dog(40), goat(32), pig(41) and non-human primates(42). Nerve diameters have ranged from around 1 millimeter to approximately 6 mm (dog radial nerve)(40). Three separate human studies are using KHFAC on different components of the nervous system; vagus nerve(43), sciatic, tibial and common peroneal nerves(44) and the dorsal region of the thoracic spinal cord(13,45). Though larger nerve diameters require higher amplitudes to achieve block (since axons at the center of the nerve are farther from the electrode), to date there has not been a systematic study of the influence of nerve diameter on block thresholds.
Fiber Type and Fiber Size
It is possible to use KHFAC to block all sizes of nerve fibers, from the largest motor (34) and sensory fibers (46), to the smallest unmyelinated fibers (38). Tanner (19) was the first to describe fiber size selectivity using KHFAC. He reported that larger fibers were blocked at lower amplitudes using a 20 kHz waveform. This result has been confirmed for sensory nerves (46). Joseph and Butera (38) have demonstrated that larger motor fibers have lower thresholds for block than C-fibers at frequencies below 30 kHz. This relationship appears to be reversed at frequencies above 30 kHz, but it is not clear if this inversion relates to unmyelinated fibers only or to all fibers sizes in general.
Onset Response Characteristics
KHFAC always produces a transient neural activity when turned on, an effect termed the “onset response” (34). As shown in Figure 2, the onset response can take the form of a large “twitch” response, typically 2–3 times stronger than a single maximal twitch from a single action potential, or it can be a prolonged period of strong activity that takes many seconds to diminish and cease. This initial response has been observed in computer simulations (2,5,47,48) and in animal experiments using single fiber recording (14,20), muscle force (2,5,29,34) and urethral sphincter pressure (10,36). The onset response was first described by Woo and Campbell [1964]. Using 20 kHz, 1–5 volts and recording CAP, they found that this waveform initially produced “asynchronous firing followed by block.” They showed that the asynchronous firing tended to occur at lower to mid voltages, whereas complete block required higher voltages.
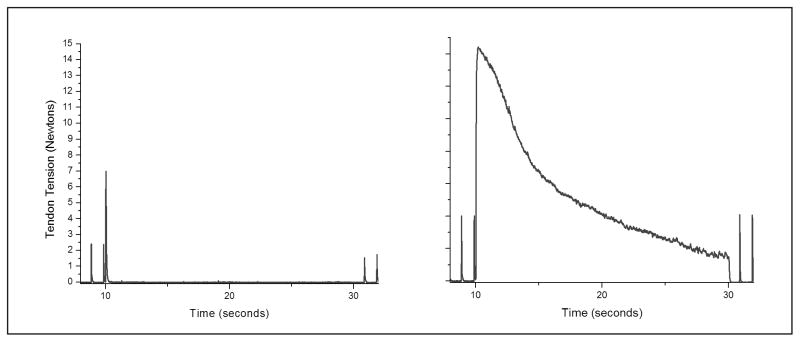
Figure 2.
Comparison of onset response characteristics in the same nerve (rat sciatic) using different frequency and amplitude. The scales are the same in each plot, and block is delivered from 10 seconds to 30 seconds. The plot on the right is with KHFAC of 10 kHz and 10 Vpp and shows onset activity for the whole period. The plot on the left is with a KHFAC of 30 kHz and 10 Vpp and has a very brief onset response of lower amplitude.
The motor onset response has been shown to consist of two sequential phases, as identified through the motor response to KHFAC block (34,48). The first phase consists of a single summated muscle twitch with a peak force equal to or larger than that of a normal supramaximally elicited muscle twitch. This was defined as the “Phase I onset” and it is always present when the KHFAC block is initiated. “Phase II onset” was defined as the variable period of repetitive firing (and the resulting summated tetanic muscle force) that follows immediately after Phase I and ends with complete or partial block (48–51).
The onset response is a significant impediment to many potential clinical uses of KHFAC block. If KHFAC block were applied to a mixed nerve, the onset response would produce a painful sensation coupled with muscle contractions. Therefore, research has been conducted to reduce or eliminate the onset response. Miles et al., (52) demonstrated that slowly ramping the KHFAC from zero to block threshold could not be used to reduce the onset response and, in fact, generally enhanced the onset response. However, several methods for shortening the onset response have been identified, and include: the use of large KHFAC amplitudes (53), higher frequencies (>20 kHz) (34,54), and optimal electrode geometry (49,50,55). However, the phase I portion of the onset response, lasting less than two seconds, is a fundamental component of KHFAC block that cannot be eliminated through modification of the waveform or electrode design alone (49). Efforts to further eliminate the onset response have centered around the use of charge-imbalanced waveforms, as reviewed later in this review.
The onset response occurs every time KHFAC is delivered to a nerve after any period during which the KHFAC has been turned off. However, once block has been established and the onset response has subsided, it is possible to modulate the KHFAC waveform without producing an additional onset response. In particular, Gerges et al., (53) showed that it was possible to transition both frequency and amplitude using linear transitions without producing further onset responses. In order to achieve transitions without onset, it was necessary to ramp frequency and amplitude over time. In some cases, it was possible to transition from 30 kHz to 10 kHz in as little as 30 ms without producing an onset response, whereas other preparations required more than ten seconds.
Bhadra et al.,(56) has shown that it is possible to transition KHFAC amplitude between block threshold down to 90%, 75%, and in some cases 50% of block threshold amplitude and then return to block threshold without producing an onset response. Specifically, the transition from 50% to 100% block could be achieved in less than 2.5 seconds without producing an onset response. The potential usefulness of this approach is that certain sub-block threshold amplitudes (typically around 70% block threshold) can allow normal nerve conduction(48,56). Thus, it may be possible, once block is established, to transition the KHFAC amplitude below block threshold, allow normal conduction as needed, and then return to complete block without inducing another onset response.
Electrical Parameters for KHFAC Block
The effect of KHFAC on nerve conduction is strongly influenced by the waveform parameters. The two most important parameters are frequency and amplitude. Other parameters have a lesser impact, such as the waveform shape, pulsed vs. continuous waveforms, and current-controlled vs. voltage-controlled output. As described in the introduction, a true nerve conduction block generally requires a frequency of 1 kHz or higher(36), and most researchers have typically used frequencies of 5 kHz or higher. Between 1 kHz and 5 kHz, the frequency at which nerve conduction block can be obtained appears to depend on the fibers to be blocked, as well as other factors as yet unknown. For example, Bhadra et al.(36) reported true conduction block as low as 1 kHz for the cat pudendal nerve. Williamson and Andrews(5) observed that frequencies between 5–10 kHz did not produce consistent block and frequently produced prolonged activation of the nerve. In contrast, there have been no reports of a “maximum” frequency that can be used to achieve KHFAC block, with the highest reported frequency being 50 kHz(32).
Nerve conduction block with KHFAC requires a minimum waveform amplitude, defined as the “block threshold”(34). Bhadra and Kilgore(34) demonstrated that block threshold is linearly related to frequency over the range of 5–30 kHz. This study was performed in the rat sciatic nerve, but the relationship has proven to be very robust and has been demonstrated in other experimental conditions, including cat pudendal(36,57), aplysia(58) and frog(38). More recently, Butera(38) has suggested that the linear relationship may not hold for frequencies above 30 kHz in C-fibers. They found that C-fiber block threshold decreases between 30 kHz and 50 kHz, with the result that C-fibers could be blocked at lower amplitudes than larger myelinated fibers. These results were obtained in aplysia and frog using micropipette electrodes, and it is unknown if the electrode design plays a role in these results.
Most recent studies of KHFAC block have utilized continuous waveforms (no off times between phases). Tai et al.,(59) compared 8 kHz continuous waveforms with 8 kHz pulsed waveforms (10 or 30 μs pulses). They found that the pulsed waveforms produced a longer onset response and required a higher amplitude to achieve block. No significant differences have been reported between sinusoidal(5,34), rectangular(59), or triangular wave shapes except that the block amplitudes are lowest for the square waves and highest for triangular waves (unpublished). Peng et al.,(60) evaluated asymmetric 6 kHz waves in which the first phase was shorter than the second (charge balanced maintained). They found more effective block with this waveform when compared to a purely symmetric waveform. It is likely that the block tested in these experiments was a neuromuscular junction block, but this was not reported.
Researchers have used both voltage-controlled and current-controlled waveforms with no significant difference in reported response(2). However, these waveforms have not been compared systematically in a consistent preparation with identical electrode designs, so there may be some effects as yet unknown. Based on the studies to date, however, it would appear that frequency and amplitude have much more significant effects than these other parameters.
Rapidity of Block
One of the common observations regarding KHFAC block is the fact that block appears to be nearly instant. However, as described in the section “Onset Response”, there is always at least a brief period of nerve activity associated with the start of the KHFAC delivery, and therefore most researchers reported block as occurring within a few seconds, but the exact start was unknown. A recent study by Foldes et al.(54) used a “counted cycles” method to explicitly measure how long it takes for an applied KHFAC waveform to induce complete motor block. The aim of the counted cycles method is to obtain temporal information about neural firing and subsequent nerve block based solely on muscle force measurements. This method uses trains of KHFAC applied to the nerve that consist of specific numbers of complete cycles of sinusoidal KHFAC, with cycle counts ranging from 1 (0.05 ms) to 25,000 (2.5 sec) for a 10 kHz waveform (as an example). These trains are tested at two amplitudes of KHFAC, one above the block threshold and the other below block threshold. The supra-block threshold trains produce an onset that is quickly damped due to the nerve reaching a blocked state. The sub-block threshold trains permit the full manifestation of the onset response. The area under the onset response curve is compared between the two, and the number of cycles at which the two areas start to diverge indicates the time to initiate block.
The counted cycle method was utilized in four adult Sprague-Dawley rats. Block initiation time was evaluated at three frequencies (10, 20 and 40kHz) using two amplitude levels (one above and one below block threshold). Thirteen cycle counts from 1 cycle to 25,000 cycles were randomly delivered through a nerve block electrode placed on the sciatic nerve, and the resulting gastrocnemius muscle contraction was recorded and the area under the curve calculated, as shown in Figure 3. The results indicated that complete block can be initiated in 7.5 to 14 ms. These results compare well with previous modeling data using a mammalian axon model(48), which predicted that the time to achieve block was 10 to 30 ms. In the conditions with the shortest onset, the onset appears to consist of seven or fewer summated twitches, which is immediately followed by complete block of the nerve. This indicates that, with KHFAC block, the nerve initially fires extremely rapidly for a very brief period, followed by continuous block that is maintained as long as the KHFAC continues to be delivered.
Figure 3.
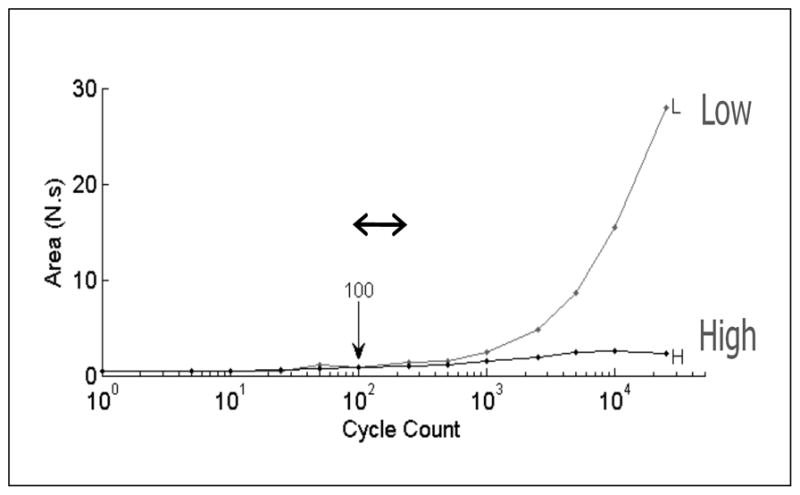
The counted cycles method to determine the time to achieve block. Specific numbers of KHFAC cycles (from 1 to 50,000 cycles at 20 kHz) are randomly applied at two different KHFAC amplitudes and the resulting onset response (area under the force curve = to the force-time integral in Newton·seconds) is compared in the plot. “Low” uses an amplitude below the block threshold. With the low amplitude, increasing cycle counts always result in increasing force-time integrals, since there is no nerve block effect. “High” uses an amplitude above the block threshold. Since the high amplitude produces a complete block after a specific number of cycles, the resulting force-time integral reaches a plateau. The point of bifurcation of the two curves defines the lower bound of the number of cycles needed to achieve complete block (100 cycles in the figure). The next data point on the right is therefore chosen as a conservative estimate of the block time (after conversion of the cycle count to absolute time). In this example, the block time is 12.5 milliseconds. The fastest time across multiple trials was 7.5 ms.
Reversibility
A unique characteristic of KHFAC block is the rapid reversibility of the block when the KHFAC is terminated(2,5,14,29). This reversibility is most clearly shown in experiments in peripheral nerve, as shown in Figure 4. Electrical stimulation is used to generate action potentials that are blocked at the blocking electrode. By delivering the electrical stimulation as the block is turned off, it is then possible to observe the reversibility by measuring how quickly the twitch force returns to the pre-block height. When KHFAC is delivered at, or just above, the block amplitude, the reversibility is nearly instantaneous once the KHFAC block is stopped. Experiments have been performed in multiple species, demonstrating that the entire nerve completely recovers the ability to conduct action potentials through the blocked region within one second of the cessation of the KHFAC(34,38).
Figure 4.
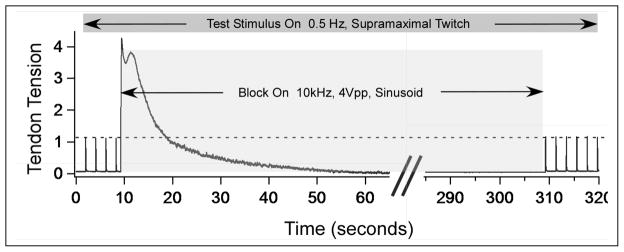
KHFAC block at 10 kHz. Gastrocnemius force is shown during proximal stimulation and while block is on (block begins at 10 seconds). After 300 seconds of KHFAC delivery, the block is turned off. The peak force due to the proximal stimulation is identical immediately after the cessation of block when compared to prior to block, showing the instantaneous reversibility of this method.
Under certain conditions, KHFAC block has been shown to produce a “carry-over” effect, in which nerve conduction is depressed for a period of time even after the cessation of KHFAC delivery. Waataja et al.,(33) showed this effect after only one minute on KHFAC delivery in the rat vagal nerve. The appearance of a carry-over effect after only one minute is in contrast to experiments in rat and cat sciatic, where immediately reversibility can be maintained for periods of at least 40 minutes(61). These differences may be due to differing responses between motor and autonomic nerve fibers, or they may be due to different methods and waveform parameters.
The carry-over effect described by Waataja(33) and Bhadra et al.,(61) is a temporary loss of conduction in the nerve. Over the course of seconds to hours, full nerve conduction returns. Cuellar et al.,(32) also noted a carry-over effect immediately after the cessation of KHFAC in the block of dorsal nerve roots in rats and goats, but recognized that the block during carry-over appeared to underestimate the percentage of block during KHFAC. They noted that complete recovery of nerve response typically occurred within 2–3 minutes, but that some neurons remained suppressed for as long as 10 minutes. They suggested that time to recovery was a function of KHFAC duration. Liu et al. (62) evaluated 5 kHz AC on frog sciatic nerve using Ag/AgCl electrodes and noted slowed conduction through the blocked region after 60 seconds. They observed that the amplitude of the recorded CAP recovered within 150 s, but the conduction velocity did not recover in that time.
Bhadra et al.(61) identified three distinct categories of recovery and carry-over effect from KHFAC block. These were: 1) “instantaneous recovery”, where muscle force recovered within 0–3 seconds after cessation of the block when block was delivered for up to 15 minutes; 2) “fast recovery”, which occurred when block was delivered for longer than 15 minutes (either continuously or cumulatively), and was characterized by a complete recovery of muscle force after cessation of block over a period of no more than three minutes; and “slow recovery”, which was identified when block was applied for more than approximately 40 minutes. During slow recovery, the muscle force was found to take as long as two hours to fully recover to pre-block levels. They also found that cycling the block on and off could delay the emergence of the slow recovery response. The underlying mechanism of the carry-over effect is unknown, but it is likely due to a local depletion of metabolic products critical for action potential initiation and conduction. The reversibility and carry-over effects of KHFAC are areas that have yet to be fully explored.
Partial Block
The percentage of a whole nerve that is blocked by KHFAC can be modulated by adjusting the waveform amplitude in a manner similar to spatial modulation in electrical activation of nerve (2,5,6,34,38). Block effectiveness is defined as the percent reduction in the peak twitch force measured during block compared to that obtained without block. Nerve fibers closest to the electrode experience the highest current, and therefore are blocked at the lowest amplitudes, whereas nerve fibers furthest from the electrode are only blocked at the highest current. In addition, it has been shown that larger fibers are blocked at lower thresholds than smaller fibers(19,38), and therefore it is likely that the first fibers blocked at the lowest amplitude are the largest motor fibers near the electrode. Note that block threshold is defined as the amplitude that blocks all motor fibers in a specific nerve, and thus is equivalent to 100% block effectiveness.
Modulation in the block effect could be extremely useful in the clinical setting, where it is frequently desirable to partially block muscle activity, but retain some voluntary or spastic muscle function. For example co-contraction of voluntary agonist against a spastic antagonist muscle prevents functional use of a joint or extremity. Blocking a portion, but not all, of the spastic muscle, could be sufficient to allow the natural control of agonists to predominate, allowing function to be restored.
Electrode Design
Effective nerve block using KHFAC requires control over the delivery of current to the nerve to be blocked. As a result, electrode design plays an important role in achieving successful nerve block. Historically, this has been achieved using electrodes that make intimate contact with the nerve to be blocked. Nerve cuff electrodes, which typically consist of an insulating outer layer and metal electrode contacts inside the cuff(30,31), have been utilized(2,5,31,34,57), although block has been also achieved using intrafascicular electrodes(6,51) and even glass suction electrodes(38). In some cases, it has been possible to achieve block using electrodes placed alongside nerves with no outer insulating portion (unpublished results), but the resulting block requires higher current levels and results in a significantly longer onset response when compared to cuff electrodes.
Cuellar(32) attempted to utilize a variety of electrode styles to block dorsal roots in rat and goats. They were able to achieve block with: 1) a paddle electrode (spinal cord stimulation style), 2) a bipolar hemi-cuff, 3) bipolar hook electrodes, and 4) a percutaneous cylindrical electrode placed next to the nerve. Although the different styles were not systematically compared, they reported that the bipolar hook electrodes generally required the lowest current for block.
Dowden et al.(6) evaluated the use of a multi-contact penetrating electrode array to determine if it was possible to selectively block individual fascicles in a nerve. Selective block was evaluated in the sciatic nerve in five cats. Activation of targeted muscles could be selectively blocked by KHFAC in all five animals. In most cases, however, the block achieved was due to a neuromuscular junction block (2–8 kHz), although conduction block was achieved at 16 kHz in three electrodes in one preparation.
The effect of the number and spacing of cuff electrode contacts on KHFAC block was evaluated by Ackermann et al.(50,55). It was possible to achieve block using monopolar, bipolar and tripolar cuff electrodes, although monopolar configurations typically demonstrated longer onset responses. Gaunt and Prochazka(57) found that bipolar electrodes typically produced a more effective block than monopolar electrodes. Minimal differences in blocking effectiveness were determined between bipolar and tripolar electrodes. The longitudinal spacing between contacts in a bipolar electrode was found to affect both the block threshold and onset response(55). For the rat sciatic nerve, which is ~1mm in diameter, the optimum separation between contact spacing was found to be 0.5 – 1.0mm. This provided the optimal combination of lowest block threshold and minimal onset response. It is likely, however, that the optimum spacing is dependent on the nerve diameter, with larger nerves requiring larger spacing between contacts. Gaunt and Prochazka(57) used an interelectrode spacing of 2 mm to achieve block of the cat pudendal nerve. Contact width may also play a role in the nerve block effectiveness(55). Typically, contact width, when described, is 1–2 mm, and the optimum contact width has not been fully explored.
Charge-imbalanced KHFAC for the Elimination of the Onset Response
Research into methods of eliminating the onset response associated with KHFAC has centered around the use of charge-imbalanced waveforms, which can then be transitioned to a charge-balanced waveform for maintaining block. This approach is based on the fact that direct current (DC) block can be generated without producing an onset response by simply performing a gradual increase in the DC amplitude up to the level that produces a conduction block(63,64). Unfortunately, DC block cannot be utilized for long periods of time due to the damaging electrochemical effects at the electrode(65). Ackermann et al(66) proposed combining the features of DC and KHFAC block by utilizing the DC block only during the transient KHFAC onset response and then turning the DC block off once the onset response was complete and utilizing the KHFAC to maintain block as needed. This approach was evaluated in a rat sciatic nerve preparation(66). The proposed “no-onset” nerve block system consists of two DC electrodes placed on either side of a KHFAC electrode. The DC electrodes block the onset firing which is generated by the central KHFAC electrode. It was demonstrated that this configuration is capable of producing a no-onset nerve conduction block that is rapidly reversible. In these animals, successful DC block of the onset response was achieved using an average of 2.2 ± 0.7 mA of DC with an average duration of 5.1 ± 1.7 sec. An example of successful no-onset KHFAC block is shown in Figure 5. Although the results with the combined DC + KHFAC approach were promising and demonstrated the potential utility of the approach, it was found that even the short duration and low amplitude of DC required for establishing a conduction block resulted in damage to the nerve when using platinum DC electrodes.
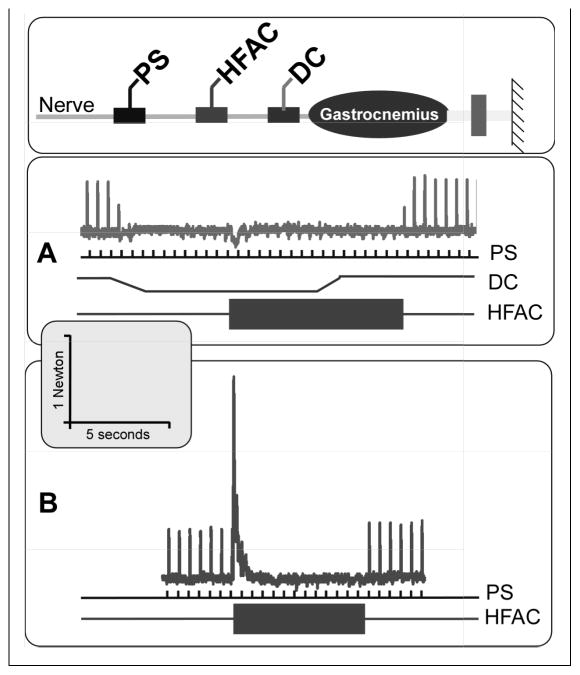
Figure 5.
DC+KHFAC no-onset blocking system. Diagram shows schematic electrode configuration on the nerve, with a proximal stimulating electrode (PS), a KHFAC electrode, and a distal direct current (DC) electrode. “A” shows the no-onset block. Top trace shows tendon tension during trial. Proximal stimulation (PS) at 2 Hz is delivered throughout the trial (middle trace). DC (middle trace) ramps down (cathodic block) and plateaus at 4.5s, producing complete block (note partial block during ramp). DC block allows KHFAC (lowest trace) to be turned on without producing an onset response (7.5s). DC is turned off and block is maintained by KHFAC. KHFAC is turned off at 17.5s and normal conduction is restored. “B” shows the normal KHFAC onset (when DC block is not used). Scale is the same for both graphs.
Ackermann et al.(67) proposed the use of a “separated interface nerve electrode” (SINE) for the purpose of achieving safe DC block for the time durations required for the no-onset nerve block system (typically 2–10 seconds). The SINE concept is based on the theory that stimulation-induced damage is due to deleterious electrochemical reaction products generated from the reduction/oxidation reactions at the site of the electrode(65,68), acknowledging that there may be other mechanisms of damage as well(69,70). The SINE is designed to physically separate the electrode-electrolyte interface from the nerve interface, in a manner similar to that achieved by a pipette, suction, cotton and felt electrodes(70).
A prototype SINE was fabricated using a saline-filled syringe, which was connected to a polymer nerve cuff via a silicone tube. The neural interface of the SINE was a ~1.0 mm2 window in the nerve cuff. A metal conductor was placed in the barrel of the syringe to deliver the DC. Experiments on rat sciatic nerve demonstrated that DC could be delivered through the prototype SINE without evidence of acute nerve damage for approximately ten times longer than with standard platinum electrode (200 s compared to 20 s). By combining the DC-SINE with a KHFAC electrode, successful no-onset block was obtained.
Vrabec et al(71) has suggested the use of high charge capacity DC electrodes as part of a no-onset nerve block system. The use of high charge capacity electrodes has been explored for other electrical stimulation purposes(72). The advantage of these electrodes, such as Pt-black and Iridium oxide, is the capacity of these electrodes to deliver significant charge without undergoing irreversible reactions. This would allow the DC block to be delivered longer in order to accommodate longer onset responses.
Evaluation of Chronic Application of KHFAC
To date, only a few chronic studies of KHFAC have been reported in the literature. Lin et al.,(40) reported on a study of five adult dogs implanted with tripolar nerve cuffs on the peroneal and radial nerves. KHFAC was delivered to the left deep radial nerve for 15 minutes two times per week for four weeks. The KHFAC was 10–20 kHz and of sufficient amplitude to block nerve conduction and produce temporary foot drop, typically 10–14 Vpp. At the completion of the five week test, the animals showed no visible signs of nerve damage. There was no gross histological evidence of damage to the nerve fascicles.
Gaunt and Prochazka(57) performed a study of a chronically implanted KHFAC system for pudendal nerve block in the cat. A nerve cuff electrode with three platinum contacts was implanted on the pudendal nerve. Low frequency stimulation was applied to one of the contacts, and KHFAC at frequencies ranging from 1 to 10 kHz was applied to the two distal contacts in a bipolar configuration. The system was implanted for 6.5 months, with six evaluation sessions performed over that time period. Successful pudendal nerve block (measured as decreased evoked bladder pressure) was obtained in each session. There was no evidence of tissue reaction around the electrode, based on stable stimulation thresholds on each contact taken at each session. One of the six sessions was performed with the animal awake. During delivery of KHFAC at 6 kHz, there was mild aversive response of the animal with no vocalization or attempts to move away. A ten-second long KHFAC train produced the same brief response as a one-second long train.
The use of KHFAC to block sphincter contractions for bladder voiding was evaluated in three chronic cats(11). The animals were implanted with a system consisting of bilateral nerve cuff electrodes on the sacral roots for activation of the bladder and bilateral nerve cuff electrodes on the distal pudendal nerve for blocking pudendal nerve activity to the external urethral sphincter. The animals were maintained for up to 140 days. Each animal was spinalized after approximately two months at the T10–T12 level, resulting in loss of voluntary bladder function. Spinalized animals were maintained for 40, 55 and 90 days using either manual bladder expression (control) or stimulated bladder contraction with KHFAC nerve block. KHFAC block thresholds remained stable throughout the entire period. Voiding was achieved using the implanted system for up to 44 consecutive weekdays. Voiding volumes with KHFAC block were equivalent or better than volumes achieved with manual expression.
Tweden et al.(41) reported on a chronic animal study to evaluate the safety of a 5 kHz waveform for treatment of obesity. Electrodes were placed on the vagal nerve of the pig and the 5 kHz waveform delivered intermittently for 55 days. There was no histological evidence of Wallerian degeneration or demyelination of the vagus nerve.
KHFAC was applied to the spinal cord of six goats for 24 hrs/day for ten days (240 continuous hours)(73). Blinded comparison of histological spinal cord samples from the KHFAC animals and six control animals indicated no morphological differences between the two groups. Although the specific details of the KHFAC applied were not described, the implanted device used for the study generates a 10 kHz waveform up to at least 5 mA(13).
Mathematical Modeling of KHFAC Block
Computer simulations have been used to investigate features of KHFAC block(48). Simulation runs are constructed in a manner that mimics in vivo experimental design(2). A mathematical model of an axon is chosen and a single axon constructed. A point source delivers the KHFAC to the middle of the axon. A test pulse is delivered to one end of the axon and the opposite end monitored to determine if nerve block occurs. Similar to experiments, the block threshold is determined, usually by performing a binary search while changing the KHFAC amplitude(48). Different parameters (e.g. axon diameter, electrode to axon distance, frequency, etc.) can be varied over a range and the onset response and block thresholds measured.
Models of varying accuracy and complexity have been used. The first known modeling of KHFAC was performed by Bromm in 1975 using a Frankenhauser-Huxley model of frog axons(74). Rattay(75,76) showed an example of KHFAC nerve conduction block at 2 kHz in an unmyelinated Hodgkin-Huxley model. Tai et al. (47,59,77–79) used a variety of unmyelinated and frog models. Williamson and Andrews(5) performed simulations in three mammalian axon models, using sinusoidal waveforms and also performed mammalian block experiments.
Our laboratory has been using a topologically detailed mammalian model, named the McIntyre-Richardson-Grill (MRG) model(80), based on rat, cat and human data. We have used the model extensively to look at many features of KHFAC block, including block thresholds, KHFAC frequency ranges and amplitudes, effects of electrode distances from the axon, axon size and the onset response(48). We have used the model predictively to design experiments(2,49,52–55,81) and have had success in relating model outputs to real experimental data.
There are some cautions about KHFAC modeling. None of the existing nerve membrane models have been validated for KHFAC frequency ranges and the relationship of membrane capacitance to the input frequency could cause some unknown effects(37). Axon length is important in the models as a short axon length causes spurious onset activity with failure of block. Current models do not accurately demonstrate features like reversibility or carry-over. The field of electrical nerve block could benefit from more accurate models.
Mechanisms of KHFAC Nerve Block
Despite the increasing pace of research in KHFAC block over the last decade, there have been no concerted efforts to identify the mechanism of block. Three biophysical explanations have been reported. One early explanation was the accumulation of extracellular potassium (14). While this has been identified in the block of CNS neurons, it is unlikely to be the major element in KHFAC block due to the speed at which KHFAC block takes effect(54). The second more recent proposal has been that outward potassium currents overwhelm the inward sodium currents at the nodes or axon section (in unmyelinated axons) influenced by the KHFAC and produce block. This theory has been based on a number of modeling studies using the Hodgkin-Huxley (squid axon model) and Frankenhauser-Huxley (frog axon model), that note the correlation between elevated fast and slow potassium currents and the degree of block(47,78,79,82). However, there has been no experimental confirmation of these postulates and is unlikely to be the primary mechanism in mammals, since there are few fast potassium channels in mammalian nodes(83).
The third hypothesis focuses on sodium channel inactivation as the cause of KHFAC block and has been put forward by a number of researchers(2,5,19,81,84,85). We have supported this postulate based on initial modeling studies using the MRG axon model (described in the model section), which showed that the KHFAC resulted in an increased inward sodium current compared to the outward potassium current, leading to a dynamic membrane depolarization of a number of nodes under the electrode(2,48,81). This depolarization led to the inactivation of about 90% of the sodium channels in the node directly under the electrode (81). The depolarization in the MRG model was on the order of 32 mV (positive to the resting potential) for all tested axons at the block threshold.
The sodium channel inactivation hypothesis is supported by experimental evidence. Bromm(74) performed both modeling and experimental studies of 4 kHz to 20 kHz waveforms. His modeling showed a 33 mV depolarization of the node. He injected a 10 kHz waveform in frog nodes and measured the transmembrane voltages, finding a similar depolarization of 30 mV. This depolarization disappeared when the sodium channels were blocked with tetrodotoxin. The second piece of experimental evidence resulted from our modeling studies with the MRG model. The results of the modeling study show that both depolarizing and hyperpolarizing currents play an important role in conduction block and that the conductance to each of three ionic currents increases relative to resting values during high frequency stimulation. However, depolarizing currents were found to promote the blocking effect, and hyperpolarizing currents were found to diminish the blocking effect. The MRG model includes both fast and persistent sodium channels, both of which contributed to KHFAC block. If the persistent sodium channels were removed from the model, block thresholds increased by approximately 18%. This was experimentally investigated by the intra-peritoneal administration of the persistent sodium channel blocker Ranolazine in a randomized, controlled in-vivo rat study. It resulted in an approximately 20% increase in the block threshold of KHFAC required to produce conduction block in rats, confirming that depolarizing currents promote the conduction block phenomenon.
Clinical Applications of KHFAC
The first recorded test of KHFAC in humans was by Bowman(86). Block of the musculocutaneous nerve in three humans was performed intraoperatively to attempt to block biceps activation via stimulation on the proximal musculocutaneous nerve. A 4 kHz waveform at 15 mA was delivered through a bipolar platinum electrode for block. In one of the three subjects, block was successful in producing complete relaxation of the biceps. During block, stimulation on the nerve distal to block produced biceps activation, indicating that a true conduction block was achieved.
An obesity control system, developed by Enteromedics [St. Paul. MN], called the “VBLOC” system, uses a 5 kHz waveform applied to the vagal nerve to produce appetite suppression and subsequent weight loss(87,88). Nerve cuff electrodes are placed on each vagal nerve trunk and connected to an implanted pulse generator. The 5 kHz waveform is delivered for 5 min on and 5 min off throughout the day, with amplitudes ranging from 1–6 mA. This system has now been tested in over 200 patients, some for as long as five years. A randomized, double-blind controlled trial was conducted with 294 subjects(43). Subjects were followed for one year and weight loss was found to be linearly related to hours of device use. There were no significant adverse events related to device use. Further study of this system is ongoing.
The specific parameters utilized in the Enteromedics system were tested in an acute rat preparation(33). A 5 kHz waveform at amplitudes up to 8 mA was applied to the vagus nerve and the compound action potential (CAP) was recorded to verify lack of nerve conduction. However, it was not possible to record a clean CAP signal during the delivery of the 5 kHz waveform and therefore, the experiment involved delivering the 5 kHz for one minute, then recording the recovery of nerve function once the 5 kHz wave was terminated. Immediately after cessation of delivery of the 5 kHz, it was found that nerve conduction was partially blocked for periods up to five or more minutes, depending on the amplitude of the 5 kHz waveform. Higher amplitudes resulted in longer block. Thus, in contrast to the instant reversibility observed in similar experiments, this application relies on a “carry-over” effect to produce nerve block. It is not clear if block is achieved while the 5 kHz is delivered, although it is likely that there is a brief onset response that occurs during this time.
A 5 to 10 kHz waveform is being used for amputee pain relief [Neuros Medical, Inc., Willoughby, OH]. Pain relief was achieved in a preliminary sample of five lower extremity amputees through the application of 10–20 kHz waveform to the sciatic nerve stump proximal to a distal neuroma (44). A spiral nerve cuff electrode (30) was surgically placed on the sciatic or tibial nerve with percutaneous leads connected to the waveform generator. Pain relief was obtained with a ten minute application of the KHFAC, and the relief lasted for many hours in some subjects.
Similarly, back pain relief [Nevro Corp., Menlo Park, CA] has been obtained using a current-controlled 10 kHz waveform delivered to the thoracic spinal cord in 83 subjects with significant back pain(12). The electrodes utilized in this system are the same as those used for spinal cord stimulation (SCS) and are placed epidurally over the dorsal aspect of the thoracic spinal cord and connected to an implanted pulse generator. Significant pain relief has been reported in 72 of 82 subjects (88%) at six months. An early US trial (13) reported similarly positive results in 24 patients using an external stimulator that delivered 10 kHz, 30 μS pulses at a current range of 0.5 to 5.0 mA. A key feature of this system is that it does not appear to produce the paresthesia typically associated with SCS at lower frequencies. The lack of paresthesia is an unexpected result, since it may indicate that the onset response, if present, does not produce a conscious effect when delivered to the spinal cord with the parameters utilized in this trial. However, analysis of this response (or lack thereof) is certainly complicated by the fact that the electrodes are separated from the neural structures by cerebral spinal fluid, the spinal cord is composed of multiple neural structures with a variety of projections, by the possibility that parasthesia may be difficult to specifically evaluate, etc. These factors combine to make it extremely difficult to dissect the possible causes and effects of KHFAC on the spinal cord, which surely is an area ready for significant scientific investigation.
A recent study by Perruchoud et al. (45) illustrates the difficulty in evaluating the effect of KHFAC on the spinal cord. This study utilized a stimulator that delivered a voltage-controlled 5kHz, 60 μS pulse waveform. By setting the maximum KHFAC stimulation amplitude below the level of parasthesia for each patient, they were able to conduct a successful double-blinded and placebo-controlled trial of SCS. The results showed that KHFAC was equivalent to sham for the primary and secondary outcome measures, in contrast to previous uncontrolled studies. This result serves to underscore the complexity in applying KHFAC to the region of the spinal cord. In addition to the varied physiological environment of the spinal cord (as mentioned previously), it must be appreciated that the technical aspects of high frequency electrical current delivery are critical as well. As we have presented in this review, neural structure response varies directly as a function of frequency, amplitude, electrode geometry, waveform shape, etc. In this case, for example, it is not possible to directly compare a voltage-controlled 5kHz waveform (45) to a current-controlled 10kHz waveform (12). Ignoring the obvious difference in frequency, the actual waveform shape that is directly experienced by the neural structures under a current-controlled square wave is very different from that experienced under a voltage-controlled square wave (2). Further, as we have reviewed, introducing off times in the waveform (as opposed to a continuous wave) tend to be less effective in producing a block effect (59). The Perruchoud waveform has an 80 μS off time, whereas the Van Buyten waveform has a 40 μS off time. Whether these factors have any influence on the outcome cannot be determined without further study. Further complicating analysis of these results is the fact that one of the most important parameters of KHFAC is the amplitude, which is not reported in either study. This is a significant oversight, as it may be that the effect of KHFAC is more directly related to the amplitude of the waveform than to any parasthesia or lack of parasthesia. Therefore, it is critical that investigators carefully track and report the parameters used in these studies if scientific understanding is to be further advanced in this field.
The use of “high frequency” burst SCS, as reported by De Ridder (89), has been shown to produce pain relief without requiring the presence of parasthesia. However, this waveform, which uses 500 Hz, 10ms long bursts delivered at 40 Hz, is unlikely to operate through the same mechanisms as KHFAC. As we have discussed, KHFAC block typically requires at least 1000 Hz, and is generally more effective at 5 kHz or higher. In addition, since the 500 Hz burst is monophasic (recharge phase follows in the intervening 15 ms), it may function more like the “monophasic high frequency” waveform (Table 1). It is, of course, premature to conclude that the mechanisms are distinct, since both are unknown, but the similarity in producing pain relief without parasthesia is not sufficient to directly link burst SCS and KHFAC SCS.
The use of kilohertz waveforms in these systems may provide pain relief through direct conduction block of pain signals, or they may provide relief through more indirect means. At present, it has not been verified that the parameters utilized in these systems are sufficient to produce a direct conduction block, and direct sensory or motor testing sufficient to verify block has not been performed in these clinical studies. There may be additional effects of KHFAC related to the complex structure of the CNS. For example, it cannot be discounted that KHFAC, or the resulting depolarization field, might have a direct effect on cell bodies and synaptic transmission. In addition, time-dependent effects of KHFAC, such as the carry-over effect described above (33,61), are poorly understood at present and could play a significant role in the effects described. These considerations clearly represent a rich field for future research and exploration.
Finally, it is important to note that the generation of a truly charge-balanced high frequency waveform is difficult in the physiological environment (see Methodology). As far as these authors have been able to ascertain, there have been no reports of any attempt to carefully characterize the true in-vivo waveform output of the high frequency generators used in the studies reported. In particular, the elimination of stray DC is extremely difficult as frequency is increased, particularly for continuous waveforms. Since DC is a very effective nerve blocking agent (35), this should be included in the discussion regarding etiology in these studies until it has been experimentally ruled out. Such verification of in-vivo output would be an important addition to the scientific understanding of the responses observed.
CONCLUSION
The use of charge-balanced AC waveforms in the kilohertz range has been shown to have a unique blocking effect on nerve conduction. The block produced by KHFAC can be established within a few milliseconds and, under specific conditions, can be rapidly reversible so that the nerve returns to normal conduction within less than one second. This block is characterized by a block threshold, which is the lowest amplitude that produces a complete conduction block. Block threshold has been shown to increase with increasing frequency. KHFAC block can be achieved from ~2 KHz to at least 50 KHz, and can be achieved in a variety of species and nerve diameters. KHFAC block is very localized to a few millimeters along the nerve.
The primary disadvantage of KHFAC block is the onset response that occurs each time the block is initiated. The onset response can be reduced, but not eliminated, by proper choice of KHFAC parameters and electrode design. Elimination of the onset response can be achieved only with the use of transiently charge-imbalanced waveforms. The practicality of this approach has yet to be demonstrated chronically or in the clinical setting.
Chronic safety of KHFAC has been demonstrated in a few animal studies and the use of KHFAC has now progressed to human application in clinical trials. These clinical studies show promise in the treatment of obesity, chronic back pain, and amputee pain. It is anticipated that further clinical studies will be forthcoming.
The understanding of KHFAC is still in its infancy. Although the mechanism of conduction block has been studied, only clues exist about possible mechanisms. The depolarization hypothesis has some experimental evidence behind it. Features such as the onset response and carry-over effect are poorly understood at present.
It would be a significant advantage from a clinical standpoint if KHFAC block could be achieved using electrodes that were less invasive than nerve cuff electrodes. Although some success has been obtained using electrodes placed alongside nerves, the characteristics are generally poorer (high threshold, prolonged onset response) (13,90). Further study in electrode design could allow for less invasive use of KHFAC block.
In general, KHFAC block is likely to require high levels of power, since the frequency and amplitudes needed for block are relatively high when compared to electrical stimulation modalities. Thus, implanted systems generating KHFAC block will probably require the use of inductive powering or, at best, rechargeable power sources, in order to achieve practical device lifetimes. Research into waveforms that are more energy efficient is warranted.
We have reviewed the status of the field of KHFAC nerve conduction block. We have tried to stress in this review the critical importance that the electrical parameters, electrode design, and electrode location play in KHFAC. We strongly encourage investigators to pay careful attention to these details in their studies and reports. This field holds significant promise in the treatment of pain, muscle spasticity, and other nervous system disorders. We anticipate a continued exponential increase in the knowledge and use of this promising technique.
Acknowledgments
Supported by: NIBIB R01-EB-002091 and NINDS R01-NS-074149.
ABBREVIATIONS
- AC
Alternating Current
- CAP
Compound Action Potential
- DC
Direct Current
- DS
Distal Stimulation
- KHFAC
Kilo Hertz Frequency Alternating Current
- MRG Model
McIntyre-Richardson-Grill Model
- PS
Proximal Stimulation
- SFR
Single Fiber Recording
Footnotes
Conflict of interest statement:
Dr. Kilgore and Dr. Bhadra have equity ownership in Neuros Medical, Inc and have received consulting fees from Neuros Medical, Inc.
Case Western Reserve University (Drs. Kilgore and Bhadra’s institution) has patents related to the work described in this manuscript.
Authorship statemtent: Both authors contributed to preparing and editing this manuscript.
References
- 1.Linderoth B, Foreman RD. Physiology of spinal cord stimulation: review and update. Neuromodulation: journal of the International Neuromodulation Society. 1999 Jul;2(3):150–164. doi: 10.1046/j.1525-1403.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- 2.Kilgore KL, Bhadra N. Nerve conduction block utilising high-frequency alternating current. Medical & biological engineering & computing. 2004 May;42(3):394–406. doi: 10.1007/BF02344716. [DOI] [PubMed] [Google Scholar]
- 3.Jensen AL, Durand DM. High frequency stimulation can block axonal conduction. Exp Neurol. 2009 Nov;220(1):57–70. doi: 10.1016/j.expneurol.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wodlinger B, Rashid S, Durand DM. Block of Peripheral Pain Response by High-Frequency Sinusoidal Stimulation. Neuromodulation: journal of the International Neuromodulation Society. 2013 Jan 7; doi: 10.1111/ner.12011. [DOI] [PubMed] [Google Scholar]
- 5.Williamson RP, Andrews BJ. Localized electrical nerve blocking. IEEE transactions on bio-medical engineering. 2005 Mar;52(3):362–370. doi: 10.1109/TBME.2004.842790. [DOI] [PubMed] [Google Scholar]
- 6.Dowden BR, Wark HA, Normann RA. Muscle-selective block using intrafascicular high-frequency alternating current. Muscle & nerve. 2010 Sep;42(3):339–347. doi: 10.1002/mus.21678. [DOI] [PubMed] [Google Scholar]
- 7.Whitwam JG, Kidd C. The use of direct current to cause selective block of large fibres in peripheral nerves. British journal of anaesthesia. 1975;47(11):1123–1133. doi: 10.1093/bja/47.11.1123-b. [DOI] [PubMed] [Google Scholar]
- 8.Merrill DR, Bikson M, Jefferys JG. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. Journal of neuroscience methods. 2005 Feb 15;141(2):171–198. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Solomonow M, Eldred E, Lyman J, Foster J. Fatigue considerations of muscle contractile force during high-frequency stimulation. American journal of physical medicine. 1983 Jun;62(3):117–122. [PubMed] [Google Scholar]
- 10.Boger A, Bhadra N, Gustafson KJ. Bladder voiding by combined high frequency electrical pudendal nerve block and sacral root stimulation. Neurourology and urodynamics. 2008;27(5):435–439. doi: 10.1002/nau.20538. [DOI] [PubMed] [Google Scholar]
- 11.Franke M, Bhadra N, Gustafson KJ. Chronic bladder voiding after SCI using electric HFAC pudendal nerve block. Paper presented at: Neural Interfaces Conference; June 2012; Salt Lake CIty, Utah, USA. 2012. [Google Scholar]
- 12.Van Buyten JP, Al-Kaisy A, Smet I, Palmisani S, Smith T. High-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical study. Neuromodulation: journal of the International Neuromodulation Society. 2013 Jan;16(1):59–66. doi: 10.1111/ner.12006. [DOI] [PubMed] [Google Scholar]
- 13.Tiede J, Brown L, Gekht G, Vallejo R, Yearwood T, Morgan D. Novel Spinal Cord Stimulation Parameters in Patients with Predominant Back Pain. Neuromodulation: journal of the International Neuromodulation Society. 2013 Feb 21; doi: 10.1111/ner.12032. [DOI] [PubMed] [Google Scholar]
- 14.Bowman BR, McNeal DR. Response of single alpha motoneurons to high-frequency pulse trains. Firing behavior and conduction block phenomenon. Applied neurophysiology. 1986;49(3):121–138. doi: 10.1159/000100137. [DOI] [PubMed] [Google Scholar]
- 15.Wedensky NE. Die Erregung, Hemmung und Narkose. Pflugers Arch. 1903;100:1. [Google Scholar]
- 16.Bugnard L, Hill AV. Electric excitation of the fin nerve of sepia. The Journal of physiology. 1935 Mar 15;83(4):425–438. doi: 10.1113/jphysiol.1935.sp003240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cattel M, Gerard RW. The inhibitory effect of high-frequency stimulation and the excitation state of nerve. J Physiology. 1935;83:407–415. doi: 10.1113/jphysiol.1935.sp003238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reboul J, Rosenblueth A. The blocking and deblocking effects of alternating currents on nerve. The American journal of physiology. 1939;125:251– 264. [Google Scholar]
- 19.Tanner JA. Reversible Blocking of Nerve Conduction by Alternating Current Excitation. Nature. 1962;195:712–713. doi: 10.1038/195712b0. [DOI] [PubMed] [Google Scholar]
- 20.Woo MY, Campbell B. Asynchronous Firing And Block Of Peripheral Nerve Conduction By 20 Kc Alternating Current. Bull Los Angel Neuro Soc. 1964 Jun;29:87–94. [PubMed] [Google Scholar]
- 21.Zhou BH, Baratta R, Solomonow M. Manipulation of muscle force with various firing rate and recruitment control strategies. IEEE transactions on bio-medical engineering. 1987 Feb;34(2):128–139. doi: 10.1109/tbme.1987.326037. [DOI] [PubMed] [Google Scholar]
- 22.Baratta R, Ichie M, Hwang SK, Solomonow M. Orderly stimulation of skeletal muscle motor units with tripolar nerve cuff electrode. IEEE transactions on bio-medical engineering. 1989 Aug;36(8):836–843. doi: 10.1109/10.30809. [DOI] [PubMed] [Google Scholar]
- 23.Shaker HS, Tu LM, Robin S, et al. Reduction of bladder outlet resistance by selective sacral root stimulation using high-frequency blockade in dogs: an acute study. The Journal of urology. 1998 Sep;160(3 Pt 1):901–907. doi: 10.1016/S0022-5347(01)62830-1. [DOI] [PubMed] [Google Scholar]
- 24.Hassouna M, Li JS, Sawan M, Duval F, Latt R, Elhilali MM. Effect of early bladder stimulation on spinal shock: experimental approach. Urology. 1992 Dec;40(6):563–573. doi: 10.1016/0090-4295(92)90418-v. [DOI] [PubMed] [Google Scholar]
- 25.Li JS, Hassouna M, Sawan M, Duval F, Elhilali MM. Long-term effect of sphincteric fatigue during bladder neurostimulation. The Journal of urology. 1995 Jan;153(1):238–242. doi: 10.1097/00005392-199501000-00084. [DOI] [PubMed] [Google Scholar]
- 26.Sawan M, Hassouna MM, Li JS, Duval F, Elhilali MM. Stimulator design and subsequent stimulation parameter optimization for controlling micturition and reducing urethral resistance. IEEE transactions on rehabilitation engineering: a publication of the IEEE Engineering in Medicine and Biology Society. 1996 Mar;4(1):39–46. doi: 10.1109/86.486056. [DOI] [PubMed] [Google Scholar]
- 27.Abdel-Gawad M, Boyer S, Sawan M, Elhilali MM. Reduction of bladder outlet resistance by selective stimulation of the ventral sacral root using high frequency blockade: a chronic study in spinal cord transected dogs. The Journal of urology. 2001 Aug;166(2):728–733. [PubMed] [Google Scholar]
- 28.Ishigooka M, Hashimoto T, Sasagawa I, Izumiya K, Nakada T. Modulation of the urethral pressure by high-frequency block stimulus in dogs. European urology. 1994;25(4):334–337. doi: 10.1159/000475313. [DOI] [PubMed] [Google Scholar]
- 29.Tai C, Roppolo JR, de Groat WC. Block of external urethral sphincter contraction by high frequency electrical stimulation of pudendal nerve. The Journal of urology. 2004 Nov;172(5 Pt 1):2069–2072. doi: 10.1097/01.ju.0000140709.71932.f0. [DOI] [PubMed] [Google Scholar]
- 30.Naples GG, Mortimer JT, Scheiner A, Sweeney JD. A spiral nerve cuff electrode for peripheral nerve stimulation. IEEE transactions on bio-medical engineering. 1988 Nov;35(11):905–916. doi: 10.1109/10.8670. [DOI] [PubMed] [Google Scholar]
- 31.Foldes EL, Ackermann DM, Bhadra N, Kilgore KL. Design, fabrication and evaluation of a conforming circumpolar peripheral nerve cuff electrode for acute experimental use. Journal of neuroscience methods. 2011 Mar 15;196(1):31–37. doi: 10.1016/j.jneumeth.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuellar JM, Alataris K, Walker A, Yeomans DC, Antognini JF. Effect of High-Frequency Alternating Current on Spinal Afferent Nociceptive Transmission. Neuromodulation: journal of the International Neuromodulation Society. 2012 Dec 17; doi: 10.1111/ner.12015. [DOI] [PubMed] [Google Scholar]
- 33.Waataja JJ, Tweden KS, Honda CN. Effects of high-frequency alternating current on axonal conduction through the vagus nerve. Journal of neural engineering. 2011 Oct;8(5):056013. doi: 10.1088/1741-2560/8/5/056013. [DOI] [PubMed] [Google Scholar]
- 34.Bhadra N, Kilgore KL. High-frequency electrical conduction block of mammalian peripheral motor nerve. Muscle & nerve. 2005 Dec;32(6):782–790. doi: 10.1002/mus.20428. [DOI] [PubMed] [Google Scholar]
- 35.Bhadra N, Kilgore KL. Direct current electrical conduction block of peripheral nerve. IEEE transactions on neural systems and rehabilitation engineering: a publication of the IEEE Engineering in Medicine and Biology Society. 2004 Sep;12(3):313–324. doi: 10.1109/TNSRE.2004.834205. [DOI] [PubMed] [Google Scholar]
- 36.Bhadra N, Bhadra N, Kilgore K, Gustafson KJ. High frequency electrical conduction block of the pudendal nerve. Journal of neural engineering. 2006 Jun;3(2):180–187. doi: 10.1088/1741-2560/3/2/012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joseph L, Haeffele BD, Butera RJ. Conduction block induced by high frequency AC stimulation in unmyelinated nerves. Conference proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society Conference. 2007;2007:1719–1722. doi: 10.1109/IEMBS.2007.4352641. [DOI] [PubMed] [Google Scholar]
- 38.Joseph L, Butera RJ. High-frequency stimulation selectively blocks different types of fibers in frog sciatic nerve. IEEE transactions on neural systems and rehabilitation engineering: a publication of the IEEE Engineering in Medicine and Biology Society. 2011 Oct;19(5):550–557. doi: 10.1109/TNSRE.2011.2163082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhadra N, Kilgore K, Gustafson KJ. High frequency electrical conduction block of the pudendal nerve. Journal of neural engineering. 2006 Jun;3(2):180–187. doi: 10.1088/1741-2560/3/2/012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin E, Kilgore KL, Bhadra N, Lahowetz EA. Chronic High-Frequency Nerve Block with an Implanted Waveform Generator. Paper presented at: International Functional Electrical Stimulation Society Conference; 2007; Philadelphia, Pennsylvania, USA. [Google Scholar]
- 41.Tweden KS, Sarr MG, Camilleri M, et al. 46: Vagal Blocking for Obesity Control (VBLOC): Studies of pancreatic and gastric function and safety in a porcine model. Surgery for Obesity and Related Diseases. 2006;2(3):301–302. [Google Scholar]
- 42.Ackermann DM, Jr, Ethier C, Foldes EL, et al. Electrical conduction block in large nerves: high-frequency current delivery in the nonhuman primate. Muscle & nerve. 2011 Jun;43(6):897–899. doi: 10.1002/mus.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarr MG, Billington CJ, Brancatisano R, et al. The EMPOWER study: randomized, prospective, double-blind, multicenter trial of vagal blockade to induce weight loss in morbid obesity. Obesity surgery. 2012 Nov;22(11):1771–1782. doi: 10.1007/s11695-012-0751-8. [DOI] [PubMed] [Google Scholar]
- 44.Soin A. Long-Term Human Testing of High-Frequency Nerve Block for Amputation Pain. Paper presented at: 16th Annual Meeting North American Neuromodulation Society; December 6 to 9, 2012; Las Vegas, Nevada, USA. [Google Scholar]
- 45.Perruchoud C, Eldabe S, Batterham AM, et al. Analgesic Efficacy of High-Frequency Spinal Cord Stimulation: A Randomized Double-Blind Placebo-Controlled Study. Neuromodulation: journal of the International Neuromodulation Society. 2013 Feb 20; doi: 10.1111/ner.12027. [DOI] [PubMed] [Google Scholar]
- 46.Lahowetz ELBNKKL. High frequency conduction block of sensory nerves. 12 th Annual conference of the International FUnctional Electical Stimulation Society; November 2007; Philadelphia, PA, USA. 2007. [Google Scholar]
- 47.Tai C, de Groat WC, Roppolo JR. Simulation of nerve block by high-frequency sinusoidal electrical current based on the Hodgkin-Huxley model. IEEE transactions on neural systems and rehabilitation engineering: a publication of the IEEE Engineering in Medicine and Biology Society. 2005 Sep;13(3):415–422. doi: 10.1109/TNSRE.2005.847356. [DOI] [PubMed] [Google Scholar]
- 48.Bhadra N, Lahowetz EA, Foldes ST, Kilgore KL. Simulation of high-frequency sinusoidal electrical block of mammalian myelinated axons. Journal of computational neuroscience. 2007 Jun;22(3):313–326. doi: 10.1007/s10827-006-0015-5. [DOI] [PubMed] [Google Scholar]
- 49.Ackermann D, Foldes EL, Bhadra N, Kilgore KL. Electrode design for high frequency block: effect of bipolar separation on block thresholds and the onset response. Conference proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference. 2009;2009:654–657. doi: 10.1109/IEMBS.2009.5332738. [DOI] [PubMed] [Google Scholar]
- 50.Ackermann DM, Jr, Foldes EL, Bhadra N, Kilgore KL. Effect of bipolar cuff electrode design on block thresholds in high-frequency electrical neural conduction block. IEEE transactions on neural systems and rehabilitation engineering: a publication of the IEEE Engineering in Medicine and Biology Society. 2009 Oct;17(5):469–477. doi: 10.1109/TNSRE.2009.2034069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ackermann DM, Jr, Foldes EL, Bhadra N, Kilgore KL. Conduction block of peripheral nerve using high-frequency alternating currents delivered through an intrafascicular electrode. Muscle & nerve. 2010 Jan;41(1):117–119. doi: 10.1002/mus.21496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miles JD, Kilgore KL, Bhadra N, Lahowetz EA. Effects of ramped amplitude waveforms on the onset response of high-frequency mammalian nerve block. Journal of neural engineering. 2007 Dec;4(4):390–398. doi: 10.1088/1741-2560/4/4/005. [DOI] [PubMed] [Google Scholar]
- 53.Gerges M, Foldes EL, Ackermann DM, Bhadra N, Bhadra N, Kilgore KL. Frequency- and amplitude-transitioned waveforms mitigate the onset response in high-frequency nerve block. Journal of neural engineering. 2010 Dec;7(6):066003. doi: 10.1088/1741-2560/7/6/066003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foldes EL, Ackermann D, Bhadra N, Kilgore KL. Counted cycles method to quantify the onset response in high-frequency peripheral nerve block. Conference proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference. 2009;2009:614–617. doi: 10.1109/IEMBS.2009.5332758. [DOI] [PubMed] [Google Scholar]
- 55.Ackermann DM, Jr, Bhadra N, Foldes EL, Wang XF, Kilgore KL. Effect of nerve cuff electrode geometry on onset response firing in high-frequency nerve conduction block. IEEE transactions on neural systems and rehabilitation engineering: a publication of the IEEE Engineering in Medicine and Biology Society. 2010 Dec;18(6):658–665. doi: 10.1109/TNSRE.2010.2071882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhadra N, Foldes EL, Ackermann D, Kilgore KL. Reduction of the onset response in high frequency nerve block with amplitude ramps from non-zero amplitudes. Conference proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference. 2009;2009:650–653. doi: 10.1109/IEMBS.2009.5332735. [DOI] [PubMed] [Google Scholar]
- 57.Gaunt RA, Prochazka A. Transcutaneously coupled, high-frequency electrical stimulation of the pudendal nerve blocks external urethral sphincter contractions. Neurorehabilitation and neural repair. 2009 Jul-Aug;23(6):615–626. doi: 10.1177/1545968308328723. [DOI] [PubMed] [Google Scholar]
- 58.Joseph L, Butera RJ. Unmyelinated Aplysia nerves exhibit a nonmonotonic blocking response to high-frequency stimulation. IEEE transactions on neural systems and rehabilitation engineering: a publication of the IEEE Engineering in Medicine and Biology Society. 2009 Dec;17(6):537–544. doi: 10.1109/TNSRE.2009.2029490. [DOI] [PubMed] [Google Scholar]
- 59.Tai C, de Groat WC, Roppolo JR. Simulation analysis of conduction block in unmyelinated axons induced by high-frequency biphasic electrical currents. IEEE transactions on bio-medical engineering. 2005 Jul;52(7):1323–1332. doi: 10.1109/tbme.2005.847561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peng CW, Chen JJ, Lin CC, Poon PW, Liang CK, Lin KP. High frequency block of selected axons using an implantable microstimulator. Journal of neuroscience methods. 2004 Mar 15;134(1):81–90. doi: 10.1016/j.jneumeth.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Bhadra N, Vrabec T, Bhadra N, Kilgore KL. Response of peripheral nerve to high frequency alternating current (HFAC) nerve block applied for long durations. Paper presented at: Neural Interface Conference; 2012; Salt Lake City, UTAH, USA. [Google Scholar]
- 62.Liu H, Zhu L, Tang H, Qiu T. Effects of high frequency electrical stimulation on nerve’s conduction of action potentials. Paper presented at: Biomedical Engineering and Informatics (BMEI), 2011 4th International Conference; 2011. [Google Scholar]
- 63.Accornero N, Bini G, Lenzi GL, Manfredi M. Selective activation of peripheral nerve fibre groups of different diameter by triangular shaped stimulus pulses. J Physiol (Lond) 1977;273(3):539–560. doi: 10.1113/jphysiol.1977.sp012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petruska JC, Hubscher CH, Johnson RD. Anodally focused polarization of peripheral nerve allows discrimination of myelinated and unmyelinated fiber input to brainstem nuclei. Exp Brain Res. 1998 Aug;121(4):379–390. doi: 10.1007/s002210050472. [DOI] [PubMed] [Google Scholar]
- 65.Merrill D. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. Journal of Neuroscience Methods. 2005;141(2):171. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 66.Ackermann DM, Jr, Bhadra N, Foldes EL, Kilgore KL. Conduction block of whole nerve without onset firing using combined high frequency and direct current. Medical & biological engineering & computing. 2011 Feb;49(2):241–251. doi: 10.1007/s11517-010-0679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ackermann DM, Jr, Bhadra N, Foldes EL, Kilgore KL. Separated interface nerve electrode prevents direct current induced nerve damage. Journal of neuroscience methods. 2011 Sep 30;201(1):173–176. doi: 10.1016/j.jneumeth.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lilly JC. Brief, noninjurious electric waveform for stimulation of the brain. Science. 1955;121(3144):468. doi: 10.1126/science.121.3144.468. [DOI] [PubMed] [Google Scholar]
- 69.McCreery DB, Agnew WF, Yuen TG, Bullara LA. Comparison of neural damage induced by electrical stimulation with faradaic and capacitor electrodes. Annals of biomedical engineering. 1988;16(5):463–481. doi: 10.1007/BF02368010. [DOI] [PubMed] [Google Scholar]
- 70.Butterwick A, Vankov A, Huie P, Freyvert Y, Palanker D. Tissue damage by pulsed electrical stimulation. IEEE transactions on bio-medical engineering. 2007 Dec;54(12):2261–2267. doi: 10.1109/tbme.2007.908310. [DOI] [PubMed] [Google Scholar]
- 71.Vrabec T, Wainright J, Bhadra N, Bhadra N, Kilgore K. Use of High Surface Area Electrodes for Safe Delivery of Direct Current for Nerve Conduction Block. Paper presented at: Meeting Abstracts; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cogan SF, Ehrlich J, Plante TD, et al. Sputtered iridium oxide films for neural stimulation electrodes. Journal of biomedical materials research. Part B, Applied biomaterials. 2009 May;89(2):353–361. doi: 10.1002/jbm.b.31223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Butt M, Alataris K, Walker A, Tiede A. Histological findings using novel stimulation parameters in a caprine model. Paper presented at: European Journal of Pain Supplements; p. 52011. [Google Scholar]
- 74.Bromm B. Spike frequency of the nodal membrane generated by high-frequency alternating current. Pflügers Archiv European Journal of Physiology. 1975;353(1):1–19. doi: 10.1007/BF00584507. [DOI] [PubMed] [Google Scholar]
- 75.Rattay F. Electrical nerve stimulation: theory, experiments and applications. Springer Verlag; 1990. [Google Scholar]
- 76.Rattay F. High frequency electrostimulation of excitable cells. Journal of theoretical biology. 1986 Nov 7;123(1):45–54. doi: 10.1016/s0022-5193(86)80234-x. [DOI] [PubMed] [Google Scholar]
- 77.Tai C, Guo D, Wang J, Roppolo JR, de Groat WC. Mechanism of conduction block in amphibian myelinated axon induced by biphasic electrical current at ultra-high frequency. Journal of computational neuroscience. 2011 Nov;31(3):615–623. doi: 10.1007/s10827-011-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang X, Roppolo J, de Groat W, Tai C. Simulation analysis of nerve block by high frequency biphasic electrical current based on frankenhaeuser-huxley model. Conference proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference. 2005;4:4247–4250. doi: 10.1109/IEMBS.2005.1615402. [DOI] [PubMed] [Google Scholar]
- 79.Zhang X, Roppolo JR, de Groat WC, Tai C. Mechanism of nerve conduction block induced by high-frequency biphasic electrical currents. IEEE transactions on bio-medical engineering. 2006 Dec;53(12 Pt 1):2445–2454. doi: 10.1109/TBME.2006.884640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McIntyre CC, Richardson AG, Grill WM. Modeling the excitability of mammalian nerve fibers: influence of afterpotentials on the recovery cycle. J Neurophysiol. 2002 Feb;87(2):995–1006. doi: 10.1152/jn.00353.2001. [DOI] [PubMed] [Google Scholar]
- 81.Ackermann DM, Bhadra N, Gerges M, Thomas PJ. Dynamics and sensitivity analysis of high-frequency conduction block. Journal of neural engineering. 2011 Dec;8(6):065007. doi: 10.1088/1741-2560/8/6/065007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang X, Roppolo JR, de Groat WC, Tai C. Simulation analysis of conduction block in myelinated axons induced by high-frequency biphasic rectangular pulses. IEEE transactions on bio-medical engineering. 2006 Jul;53(7):1433–1436. doi: 10.1109/tbme.2006.873689. [DOI] [PubMed] [Google Scholar]
- 83.Schwarz JR, Reid G, Bostock H. Action potentials and membrane currents in the human node of Ranvier. Pflugers Arch. 1995 Jun;430(2):283–292. doi: 10.1007/BF00374660. [DOI] [PubMed] [Google Scholar]
- 84.Elbasiouny SM, Mushahwar VK. Modulation of motoneuronal firing behavior after spinal cord injury using intraspinal microstimulation current pulses: a modeling study. Journal of applied physiology. 2007 Jul;103(1):276–286. doi: 10.1152/japplphysiol.01222.2006. [DOI] [PubMed] [Google Scholar]
- 85.Tandri H, Weinberg SH, Chang KC, et al. Reversible cardiac conduction block and defibrillation with high-frequency electric field. Science translational medicine. 2011 Sep 28;3(102):102ra196. doi: 10.1126/scitranslmed.3002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bowman BR. Electrical block of peripheral motor activity. Yugoslavia: University of Ljubljana; 1981. [Google Scholar]
- 87.Camilleri M, Toouli J, Herrera MF, et al. Selection of electrical algorithms to treat obesity with intermittent vagal block using an implantable medical device. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2009 Mar-Apr;5(2):224–229. doi: 10.1016/j.soard.2008.09.006. discussion 229–230. [DOI] [PubMed] [Google Scholar]
- 88.Camilleri M, Toouli J, Herrera MF, et al. Intra-abdominal vagal blocking (VBLOC therapy): clinical results with a new implantable medical device. Surgery. 2008 Jun;143(6):723–731. doi: 10.1016/j.surg.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 89.De Ridder D, Plazier M, Kamerling N, Menovsky T, Vanneste S. Burst Spinal Cord Stimulation for Limb and Back Pain. World neurosurgery. 2013 Jan 12; doi: 10.1016/j.wneu.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 90.Boger AS. Application of high frequency electrical nerve block on the efferent nerves to the lower urinary tract for bladder voiding. Cleveland: Biomedical Engineering, Case Western Reserve University; 2009. [Google Scholar]