Abstract
Notch signaling can regulate both hematopoietic progenitors and alloimmune T cells in the setting of allogeneic bone marrow or hematopoietic cell transplantation (allo-HCT). Ex vivo culture of multipotent blood progenitors with immobilized Delta-like ligands induces supraphysiological Notch signals and can markedly enhance progenitor expansion. Infusion of Notch-expanded progenitors shortened myelosuppression in preclinical and early clinical studies, while accelerating T cell reconstitution in preclinical models. Notch also plays an essential role in vivo to regulate pathogenic alloimmune T cells that mediate graft-versus-host disease (GVHD), the most severe complication of allo-HCT. In mouse allo-HCT models, Notch inhibition in donor-derived T cells or transient blockade of Delta-like ligands after transplantation profoundly decreased GVHD incidence and severity, without causing global immunosuppression. These findings identify Notch in T cells as an attractive therapeutic target to control GVHD. In this review, we discuss these contrasting functions of Notch signaling with high translational significance in allo-HCT patients.
Keywords: Notch, Notch ligands, bone marrow transplantation, hematopoietic stem cells, T cells, graft-versus-host disease
Introduction
Allogeneic bone marrow or hematopoietic cell transplantation (allo-HCT) is a critically important and potentially curative therapy for many patients with hematological diseases.1, 2 In the absence of underlying cancer, allo-HCT provides a source of healthy progenitors to replace failing or diseased cells (e.g. in bone marrow failure syndromes, congenital immunodeficiencies and hemoglobinopathies). However, the majority of allo-HCT procedures are performed for patients with leukemias, lymphomas and other clonal hematological disorders. In these cases, the allogeneic graft provides T cells and other immune cells that play major therapeutic roles through recognition and elimination of cancer cells in the host (graft-versus-tumor, or GVT, effect).3–5 Unfortunately, donor-derived T cells also lead to immune-mediated damage in normal host tissues, a life-threatening complication referred to as graft-versus-host disease (GVHD).6–8
Multiple shortcomings limit the success and broader applicability of allo-HCT: absence or insufficient numbers of adequately matched progenitors in some patients; prolonged myelosuppression and lymphopenia after transplantation; high morbidity and mortality associated with GVHD; and insufficient graft-versus-tumor effects leading to post-transplant relapse.2 Progress in the field requires creative new solutions to these problems. Interestingly, the Notch signaling pathway was recently identified as a target for intervention to mitigate several complications of allo-HCT. Both ex vivo and in vivo observations have been reported, reflecting diverse effects of Notch signaling, different target cells (hematopoietic progenitors vs. T cells) and contrasting interventions (induction vs. blockade of Notch signaling). In this paper, we review emerging work describing important effects of Notch signaling in allo-HCT with a focus on potential translational impact in patients.
Use and limitations of allogeneic hematopoietic cell transplantation
The devastating health effects of radiation exposure from nuclear warfare in World War II prompted pioneering studies and ultimately the first bone marrow transplantations, which were of limited benefit.1 Intense subsequent clinical and laboratory research improved success rates and made allo-HCT available to an expanding number of patients. Recent estimates indicate that ca. 25’000 allo-HCT procedures are being performed annually worldwide. Multiple advances contributed to this success, including progress in HLA matching and donor selection, improved donor registries, access to alternative sources of hematopoietic progenitors such as cord blood, better conditioning regimen and supportive care, as well as systematic use of prophylactic immunosuppression to control GVHD.
Despite advances in transplant care, several major problems limit the safety and effectiveness of allo-HCT. First, a sizable subset of patients lacks a related or unrelated donor with a sufficiently high degree of HLA matching.9, 10 This problem affects ethnic minorities to a disproportionate extent. In these cases, cord blood transplantation (CBT) can be considered as an alternative approach, as a higher degree of HLA mismatch can be tolerated with this source of hematopoietic progenitors and T cells.11 However, a significant limitation of CBT especially for adult recipients is the low progenitor content of cord blood grafts. Low progenitor numbers typically lead to delayed engraftment with prolonged myelosuppression and an increased risk of serious infections. Slow lymphoid reconstitution is also particularly prevalent and severe after CBT. The first historic use of Notch signaling in allo-HCT addresses these significant issues via enhanced ex vivo expansion of cord blood progenitors.12
As a second major problem, acute and chronic GVHD remains a source of high morbidity and mortality after allo-HCT.6, 7 Current strategies to prevent GVHD rely either on T cell depletion from the donor inoculum, or on global immunosuppression (typically with calcineurin inhibitors such as cyclosporin A or tacrolimus, plus other agents).13 However, severe acute GVHD still occurs in a high proportion of patients (up to 50% or even more depending on donor/recipient characteristics, conditioning and GVHD prophylaxis). Patients with severe acute GVHD are treated with steroids, but only about half demonstrate a sustained response. Allo-HCT recipients with steroid-refractory acute GVHD have unacceptably high mortality (>70%).14 Furthermore, T cell depletion and global immunosuppression increase the risk of opportunistic infections and also decrease the potency of graft-versus-tumor activity.4, 7 This problem is best illustrated by studies of T cell depletion as a preventative approach for GVHD: improved GVHD control was counterbalanced by a markedly increased risk of tumor relapse, so that overall patient outcome was not improved.15–17 Finally, chronic GVHD represents a major unmet clinical need, as all current treatment strategies perform poorly in this condition.18 Altogether, the field would benefit from novel interventions that control GVHD without causing global immunosuppression and without eliminating potent GVT activity. Notch inhibition in T cells is emerging as an attractive new strategy to achieve these goals.19–22
Overview of Notch signaling
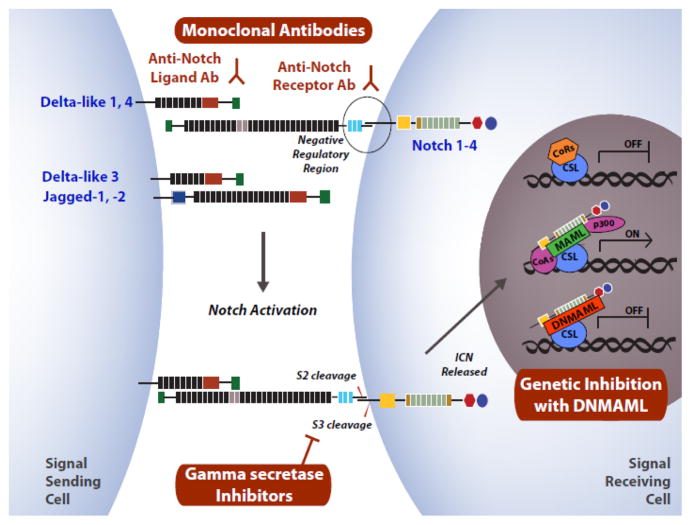
Notch is a highly conserved intercellular communication pathway with important functions in health and disease.23, 24 In mammalian organisms, four Notch receptor genes have been identified (Notch1-4) (Fig. 1). Notch1-4 receptors are expressed as transmembrane proteins after constitutive cleavage at the S1 site during transport through the Golgi complex. Notch receptors interact with ligands of the Delta-like (Dll1, 3, 4) or Jagged family (Jag1, 2) on adjacent cells. Ligand-receptor interaction generates a physical force that displaces a negative regulatory region of the Notch receptor and opens access to proteolysis at the S2 site by an ADAM-family metalloprotease.25–27 S2 cleavage generates an unstable intermediate that becomes a substrate for intramembrane proteolysis by the γ-secretase complex (S3 cleavage), releasing intracellular Notch (ICN).28 ICN migrates into the nucleus where it interacts with the CSL (CBF-1, Su(H), Lag-1) transcription factor.29 In turn, ICN and CSL recruit a key transcriptional coactivator of the Mastermind-like (MAML) family that nucleates assembly of a large transcriptional activation complex to mediate target gene activation.30–32 The Hairy/enhancer-of-split (Hes) gene family encodes recurrent direct transcriptional targets of Notch signaling, although many other targets have been reported or remain to be identified.23
Figure 1. Overview of Notch signaling.
Activation of Notch signaling is triggered by the interaction between one of five Notch ligands (Delta-like1, 3, 4; Jagged-1, 2) with one of four mammalian Notch receptors (Notch1-4). Ligand-receptor binding induces a mechanical change in the Notch receptor, displacing the Negative Regulatory Region to allow proteolytic cleavage at the S2 site by an ADAM family metalloprotease. S2 is rapidly followed by S3 cleavage mediated by the γ-secretase complex, releasing the intracellular portion of the Notch receptor (ICN) into the cytoplasm. ICN migrates into the nucleus to assemble a transcriptional activation complex together with the transcription factor CSL (CBF-1/Suppressor of Hairless/LAG-1) and a co-activator of the Mastermind-like family (MAML). During Notch activation, co-repressors (CoRs) are displaced and co-activators (CoAs) recruited, stimulating target gene transcription. Selected strategies of Notch inhibition are highlighted in red: neutralizing monoclonal antibodies against Notch ligands or receptors; pharmacologic inhibition of γ-secretase; and genetic blockade of the Notch transcription activation complex with dominant negative Mastermind-like (DNMAML). Other methods include gene inactivation of Notch1-4 or Rbpj (encoding CSL).
The biochemical features of Notch activation have been reviewed in detail elsewhere.23 Our increasing understanding of the pathway has set the stage for multiple interventions to activate or inhibit Notch signaling, both experimentally and in clinical studies (Fig. 1). Unlike soluble ligands, plate-bound or cell-bound Notch ligands can induce high levels of Notch activation in cultured cells (e.g. hematopoietic progenitors).33, 34 Neutralizing monoclonal antibodies were developed to target Delta-like Notch ligands and prevent their productive interaction with Notch receptors.20, 21, 35 Other antibodies block Notch activation by preventing S2 cleavage after ligand binding.20, 36 Originally developed for their activity in Alzheimer’s disease, γ-secretase inhibitors block the rate-limiting step of intramembrane proteolysis during Notch activation, leading to pan-Notch inhibition.37 Finally, genetic approaches have been instrumental to capture the effects of Notch signaling mediated by the ICN-CSL-MAML complex downstream of all Notch receptors and ligands. This can be achieved either by genetic inactivation of Rbpj (encoding CSL) or by expression of a dominant negative form of Mastermind-like1 (DNMAML) in specific cell types.38–42
Notch signaling is involved in multiple aspects of organ development, with additional functions during tissue homeostasis in adults. We will focus here on the effects of Notch in hematopoiesis and the immune system that are relevant to allo-HCT. Regarding the important role of Notch as an oncogene or tumor suppressor in an expanding range of malignancies, we refer the reader to several recent comprehensive reviews.43–45
Notch signaling in hematopoiesis and immunity
In the hematopoietic system, an essential role for Notch signaling was first recognized at early stages of T cell development in the thymus.46 Rare bone marrow-derived progenitors seeding the thymus experience a high intensity of Notch signaling after exposure to Dll4 Notch ligands expressed by the thymic epithelium.47, 48 In the absence of Notch signaling, T cell development is arrested at a very early stage, while cells differentiating along alternative lineages accumulate in the thymus.49–51 Notch is required continuously until T cell progenitors successfully clear the pre-T cell receptor or β selection checkpoint.42, 52–54 Multiple mechanisms then actively inhibit Notch signaling, so that CD4+CD8+ double positive (DP) thymocytes experience little, if any Notch signals during positive and negative selection. Due to this careful regulation of signaling intensity, Notch blockade in DP thymocytes does not interfere with T cell development.41,55, 56 In contrast to DP thymocytes, mature CD4+ and CD8+ T cells regain the ability to respond to Notch signaling during antigen-mediated immune responses in secondary lymphoid tissues. Emerging data highlight multiple context-dependent Notch functions in peripheral T cell immunity.57–59 These effects will be discussed in detail below in the regulation of T cell alloimmunity after allo-HCT.
Besides the role of Notch1 in T cell development, Notch2-mediated signals control the homeostasis of splenic marginal zone B cells and ESAMhi myeloid dendritic cells.60, 61 Other developmental functions of Notch signaling continue to be reported, such as the requirement for Notch to generate subsets of innate lymphoid cells (ILCs).59 Given this multiplicity of functions, cell-specific Notch inhibition strategies have been essential to dissect the effects of Notch in the hematopoietic system.
In addition to lineage-specific effects of the pathway, much attention has been devoted to the putative role of Notch signaling in hematopoietic stem cells and multipotent progenitors. Work from several groups showed that in vitro exposure of mouse or human hematopoietic stem and progenitor cells to a high density of Notch ligands can vastly expand progenitor numbers, especially when Notch signaling intensity and concomitant cytokine use are optimized.12, 34, 62–70 Progenitor expansion was also reported upon coculture with immortalized endothelial cells expressing endogenous Notch ligands.69 These findings have been exploited to achieve expansion of cord blood progenitors in human patients and will be discussed in detail below for their high relevance to allo-HCT (Table 1).
Table 1. Preclinical and early clinical interventions based on ex vivo Notch ligand-mediated expansion of hematopoietic progenitors.
After the founding observation by Varnum-Finney et al. (1998), this table lists studies in which multipotent hematopoietic progenitors expanded in the presence of Notch ligands were evaluated functionally in vivo using transplantation assays. Additional studies not meeting these criteria are discussed in the text.
| Progenitor source | Notch ligand source | Key Observation(s) | Reference |
|---|---|---|---|
| Mouse BM LSKs | Jag-1 transfected NIH-3T3 fibroblasts hJag-1ext coated beads |
Mouse BM LSKs express Notch2>Notch1 BM and fetal liver stromal cells express Jag-1 Jag-1 expands BM HPCs |
Varnum-Finney et al., 1998 62 |
| Human UCB: CD34+CD38− cells | Soluble rhJag-1-IgG1 | Soluble Jag-1 expands HPCs | Karanu et al., 2000 63 |
| Human UCB: CD34+CD38− cells | Soluble rhDelta-1-IgG1, Soluble rhDelta-4-IgG1 | Soluble Delta-like1, but not soluble Delta-like4, expands HPCs | Karanu et al., 2001 64 |
| Human UCB: CD34+CD38− cells | Immobilized Delta-1ext-myc | Immobilization of Delta-like1 significantly improves HPC expansion | Ohishi et al., 2002 34 |
| Mouse BM LSKs | Immobilized Delta-1ext-IgG Soluble Delta-1ext-IgG |
Immobilized Delta-like1 and cytokines cooperate to expand HPCs and inhibit differentiation | Varnum-Finney et al., 2003 65 |
| Human UCB: CD34+CD38− cells | Immobilized Delta-1ext-IgG | Lower densities of Delta-like1 promote HPC expansion, higher densities promote T lineage development | Delaney, et al, 200566 |
| Human UCB: CD34+ or CD133+ cells | Immobilized Delta-1-Fc | Expanded CD133+ fraction has better engraftment potential than expanded CD34+ fraction | Suzuki et al., 2006 95 |
| Human UCB: CD34+ cells | Immobilized Delta-1ext-IgG OP9-Delta-like1 cells |
Synergistic effect of HoxB4 and Delta-like1 on CD34+ cell expansion and engraftment | Watts et al., 2010 96 |
| Mouse BM LSKs | Adenoviral E4ORF1- transduced HUVECs | Endothelial cells express Notch ligands and support HPC expansion | Butler et al., 2010 69 |
| Human UCB: CD34+CD38− cells | Immobilized Delta-1ext-IgG | Expanded HPCs are safe and shorten neutropenia after human double UCB allo-HCT | Delaney et al., 2010 12 |
BM, bone marrow; LSK, Lin-Sca-1+c-Kit+ hematopoietic stem and progenitor cells; Jag-1, Jagged-1; UCB, umbilical cord blood; rh, recombinant human; HPC, hematopoietic progenitor cells; Allo-HCT, allogeneic hematopoietic cell transplantation; HUVECs, Human umbilical vein endothelial cells.
In contrast to these gain-of-function studies, the overall physiological impact of Notch signaling at the apex of the hematopoietic hierarchy in vivo remains controversial. During fetal life, Notch1 is essential for the emergence of definitive hematopoietic stem cells from specialized hemogenic endothelium.71 However, most studies using well-validated methods of Notch inhibition failed to identify a role for canonical Notch signaling in the maintenance of adult hematopoietic stem cells in vivo.70, 72–74 This was true in steady-state conditions and upon transplantation, although recent data from the Bernstein group revealed an effect of Notch2 on the initial speed of reconstitution after transplantation.70 Other laboratories have reported an inhibitory function for Notch signaling on myeloid cell fate in multipotent progenitors, downstream of hematopoietic stem cells.75 The nature of the Notch ligands mediating these effects is not known. However, as compared to Notch activity in early T cell progenitors, the overall intensity of Notch signaling remains low in multipotent bone marrow progenitors.73, 76 Expression of the Dll4 ligand is actively suppressed in the bone marrow environment, suggesting that other ligands might be involved.77 Open questions include how findings in the mouse apply to human progenitors and whether non-canonical Notch signals that do not require CSL and MAML could be important.
Notch ligand-mediated expansion of multipotent hematopoietic progenitors
Pioneering studies from Bernstein’s group first described Notch1/2 receptor expression in human and mouse hematopoietic progenitors.62, 78 These observations triggered a string of studies exploring the functional effects of engaging Notch receptors in ex vivo cultures, followed by in vitro and in vivo readouts of progenitor function (Fig. 2, Table 1). Co-culture of murine Lin− Sca-1hic-Kithi (LSK) hematopoietic stem and progenitor cells with cytokines and 3T3 cells expressing the Notch ligand Jagged1 led to a 4–8-fold expansion in clonogenic progenitors over cells cultured with cytokines and parental 3T3 cells.62 Although the degree of expansion remained modest in these conditions, this was the first demonstration that inducing Notch signals could increase the numbers of primitive progenitors. Bhatia’s group exposed human cord blood CD34+ cells to a soluble recombinant Jagged1-IgG1 fusion protein. Modest in vitro CD34+ cells expansion was observed, but importantly leading to enhanced engraftment in NOD/SCID mice.63 Similar findings were reported with Dll1 but not Dll4 fusion proteins.64
Figure 2. Notch-mediated ex vivo progenitor expansion promotes hematopoietic recovery or T cell reconstitution after hematopoietic cell transplantation.
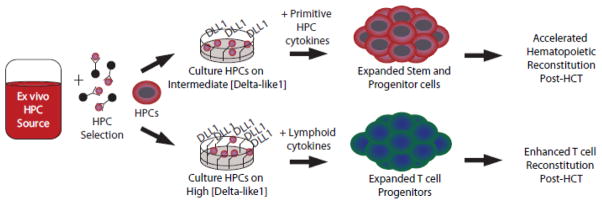
Culture of isolated hematopoietic progenitor cells (HPC) with cytokines and the Notch ligand Delta-like1 (DLL1) results in a multi-log expansion of hematopoietic progenitor cells (intermediate concentration of DLL1, primitive HPC cytokines) or differentiated T cell progenitors (high concentration of DLL1, lymphoid cytokines). Expanded progenitors can then be infused to enhance hematopoietic or lymphoid reconstitution after hematopoietic cell transplantation. To be effective, DLL1 must be provided as an immobilized plate-bound or cell-bound ligand. See Table 1 and 2 for applications in mouse models, xenograft systems and early clinical trials.
Notch-mediated progenitor expansion was perfected by the demonstration that immobilization of Dll1 ligands was necessary to efficiently induce Notch signaling.33, 34 When combined with optimized cytokine cocktails, Notch induction led to multi-log expansion of progenitors capable of long-term multilineage reconstitution in irradiated recipients.12, 34, 65 Genetic studies showed that Notch2 but not Notch1 was essential to mediate the effects of Notch ligands in multipotent hematopoietic progenitors.70 Another interesting lesson learned was the dose-dependent effects of Notch signals: intermediate doses enhanced expansion of multipotent progenitors, while high Notch signaling intensity promoted T lineage development.66–68 These considerations are important to target the desired clinical outcome, i.e. accelerated myeloid and overall hematopoietic reconstitution vs. enhanced T cell recovery. Rafii’s group described an interesting system using primary human endothelial cells transduced with adenoviral E4ORF1.69 Coculture of mouse LSK progenitors with these cells induced potent Notch-dependent expansion of progenitors capable of long-term multilineage engraftment. Thus, provision of cell-bound Notch ligands by endothelial cells in this system achieved comparable expansion of primitive hematopoietic progenitors as plate-bound ligands.
Based on these preclinical observations, Delaney and colleagues initiated human clinical trials using Notch-expanded progenitors in allo-HCT recipients.12 Cord blood transplantation involve administration of two cord blood units to mitigate the impact of low progenitor numbers.11 Building on this practice, investigators subjected one unit to Dll1-based ex vivo expansion, before reinfusing these cells in tandem with an unmanipulated second unit.12 Clinical-grade CD34+ cells expanded on average 140-fold in culture. In comparison to historical controls receiving two unmanipulated units, the duration of profound neutropenia was reduced from 26 to 16 days in study subjects. The expanded cord generated mature myeloid cells within weeks after transplantation, before being replaced in most patients by cells derived from the unmanipulated cord. These results met the endpoint of abbreviating the period of myelosuppression, likely from short-term progenitors. It remains more difficult to ascertain if the Notch-expanded product also contained cells capable of long-term reconstitution in patients, as seen in preclinical mouse models. Indeed, other work suggests that the dominance of one cord blood unit over the other is immunologically mediated (cord-versus-cord reactivity).79 As mature lymphocytes do not survive ex vivo culture, Notch-expanded progenitors could have ultimately been rejected by T cells or NK cells from the unmanipulated cord in this study.12 In any case, the findings provided a landmark observation that the procedure was safe and effective to improve early recovery of allo-HCT patients. Additional work is currently being performed to assess the utility of this approach in an expanding range of clinical allo-HCT situations.
Notch ligand-mediated expansion of T cell progenitors
Notch-based ex vivo culture systems were explored in preclinical models to mitigate the slow recovery of de novo T cell production after transplantation, a major problem following HCT in human patients (Fig. 2, Table 2).80 In these studies, a high intensity of Delta-like-mediated signals and provision of lymphoid cytokines (e.g. IL-7) led to preferential development and expansion of T lineage cells as opposed to multipotent progenitors. Either plate-bound Notch ligands or cocultures with stromal cells expressing Delta-like ligands were used successfully.34, 66–68, 81–84 As an overall approach, hematopoietic stem and progenitor cells were allowed to differentiate into the T cell lineage in culture, before being administered to transplant recipients. The bulk of mouse progenitors were infused after reaching the DN2-DN3 stage of T. cell development.68, 81 These in vitro specified T cell progenitors could complete their T cell development program in vivo, generating functional CD4+ and CD8+ T cells early after transplantation. Interestingly, expanded T lineage progenitors enhanced thymic reconstitution, but also led to extrathymic T cell development, in particular within mesenteric lymph nodes.81, 84 In aging recipients with compromised thymic function, the contribution of extrathymic development tended to be higher. The existence of extrathymic sites supporting T cell development from primitive progenitors after transplantation had previously been reported.85–87 Genetic studies showed that extrathymic T cell development was Notch-dependent, although the nature of the Notch ligand(s) involved is unknown.87 For ex vivo expanded T cell progenitors, it remains to be determined whether and how long they require Notch signaling to complete T cell development in vivo after reinfusion. Moreover, it is currently unknown if extrathymic T cell development happens in humans. This pathway could be relevant to older HCT recipients in whom integrity and function of the thymic epithelium are compromised.80
Table 2. Preclinical observations based on ex vivo Notch ligand-mediated expansion of T cell progenitors.
This table highlights studies in which Notch-expanded T cell progenitors were infused in vivo to improve immune reconstitution after transplantation. Additional studies not meeting these criteria are discussed in the text.
| Cell source | Notch ligand source | Key Observation(s) | Reference |
|---|---|---|---|
| Mouse BM LSKs | OP9-Delta-like1 cells | Delta-like1 expands DN2 and DN3 pre-T cells Expanded pre-T cells increase thymic engraftment, bacterial clearance, and have NK-independent GVT effects |
Zakrzewski et al., 2006 81 |
| Mouse BM LSKs | Immobilized Delta-1ext-IgG | Expansion of DN2 pre-T cells correlates with Delta-like1 density Expanded pre-T cells engraft the thymus and produce mature T cells |
Dallas et al., 2007 68 |
| Mouse BM LSKs | OP9-Delta-like1 cells | Expanded pre-T cells induce GVT, but not GVHD, and have potential for anti-tumor engineering | Zakrzewski et al., 2008 82 |
| Human UCB: CD34+ cells | OP9-Delta-like1 cells | Expanded pre-T cells engraft the thymus and produce mature T cells | Awong et al., 2009 83 |
| Mouse BM LSKs | OP9-Delta-like1 cells | Expanded pre-T cells engraft extra-thymically, producing mature T cells | Holland et al., 2012 84 |
BM, bone marrow; LSK, Lin-Sca-1+c-Kit+ hematopoietic stem and progenitor cells; GVT, graft-versus-tumor; GVHD, graft-versus-host disease; Ag, antigen; UCB, umbilical cord blood.
Besides work with mouse cells, human Notch-expanded T cell progenitors have been studied for their capacity to enhance T cell reconstitution in vivo (Table 2).34, 83 Subsets of human pro-T cells with a CD34+CD7++ phenotype were most efficient at thymic reconstitution in immunodeficient mice.83 The full clinical potential of this strategy remains to be investigated. Mouse studies have highlighted possible future applications. Infusion of T cell progenitors showed additive effects on T cell reconstitution over administration of keratinocyte growth factor as a trophic factor for the thymic epithelium.81 Thus, combined interventions could be considered. As compared to administration of mature T cells, pre-T cell infusion had the advantage of ensuring enhanced tolerance to host alloantigens, as final stages of T cell development including negative selection happened in vivo. This was associated with the absence of severe GVHD in allo-HCT models. Nevertheless, anti-tumor activity was still observed for reasons that remain to be fully clarified, perhaps because negative selection to host alloantigens was not fully enforced in transferred T cell progenitors.81, 82 Alloantigen-based anti-cancer effects remained relatively weak, but could be potentiated by genetic engineering of the T cell progenitors to express a chimeric antigen receptor against CD19. This could represent an interesting platform for treatment with engineered T cells, as off-the-shelf allogeneic progenitors could in principle be used with limited risks of inducing GVHD. It remains to be established whether using allogeneic pre-T cells will have advantages over engineering autologous mature T cells.
Notch and T cell alloimmunity
In addition to its involvement in T cell development, the Notch pathway is increasingly recognized for its context-dependent effects in the regulation of mature T cell function.57, 58, 88 A new major role for Notch signaling was recently reported in alloreactive T cells mediating GVHD (Table 3).19–22 To study the effects of Notch in T cell alloimmunity during GVHD, we took genetic and biochemical approaches to block all Notch signals specifically in donor-derived alloreactive T cells after allo-HCT in mice.19, 20 We observed efficient protection from acute GVHD in allo-HCT recipients of Notch-deprived T cells, as these mice survived as well as mice receiving only T cell-depleted bone marrow. Protection was observed in several models of major and minor histocompatibility antigen mismatched transplantation. Mechanistically, Notch inhibition blocked the production of multiple inflammatory cytokines by alloreactive T cells, including IFNγ, TNFα, IL-17 and IL-2, while enhancing the accumulation of regulatory T cells. Despite these potent effects on cytokine production, Notch blockade did not cause global immunosuppression, as the expansion of alloreactive T cells was preserved or even enhanced in vivo. Moreover, Notch-deprived T cells retained potent cytotoxic effects against allogeneic targets and were able to eliminate host-type leukemic cells, leading to long-term survival free of leukemia and severe GVHD. Thus, Notch inhibition in T cells had different effects on their individual effector functions and induced a unique pattern of beneficial immunomodulation in mouse allo-HCT models (Fig. 3).
Table 3. In vivo Notch inhibition in alloreactive T cells controls GVHD and immune-mediated bone marrow failure.
This table lists studies that investigated the effects of Notch inhibition in mature donor-derived T cells after allo-HCT, using GVHD or immune-mediated bone marrow failure as readouts of target organ damage.
| Mouse model | Method of Notch blockade | Proposed cellular mechanisms | Reference |
|---|---|---|---|
| B6→BALB/c B6→B6 x DBA/2 F1 B6→BALB/b |
ROSA26DNMAMLf x Cd4-Cre Rbpff/f x Cd4-Cre |
Decreased pro-inflammatory cytokines Increased Tregs Decreased alloreactive T cells in the gut |
Zhang et al., 2011 19 |
| B6→BALB/c |
ROSA26DNMAMLf x Cd4-Cre Notch1f/fNotch2f/f x Cd4-Cre γ-secretase inhibitor Anti-Notch1 Ab Anti-Notch2 Ab Anti-Dll1 Ab Anti-Dll4 Ab |
Decreased pro-inflammatory cytokines Increased Tregs |
Tran et al., 2013 20 |
| B6→BALB/c B6→B6 x DBA/2 F1 |
Anti-Dll1 Ab Anti-Dll4 Ab |
Decreased pro-inflammatory cytokines | Mochizuki et al., 2013 21 |
| B6→BALB/c B6→BALB/b |
ROSA26DNMAMLf x Cd4-Cre Rbpjf/f x Cd4-Cre |
Acquisition of T cell hyporesponsiveness Decreased pro-inflammatory cytokines Increased Tregs |
Sandy et al., 2013 22 |
| B6→B6 x BALB/c F1 | γ-secretase inhibitor Notch1f/f x Mx-Cre |
Decreased pro-inflammatory cytokines Decreased T-bet and Granzyme B |
Roderick et al., in press 90 |
Ab, antibody; Tregs, regulatory T cells.
Figure 3. Notch inhibition in donor-derived T cells provides long-lasting protection from graft-versus-host disease.
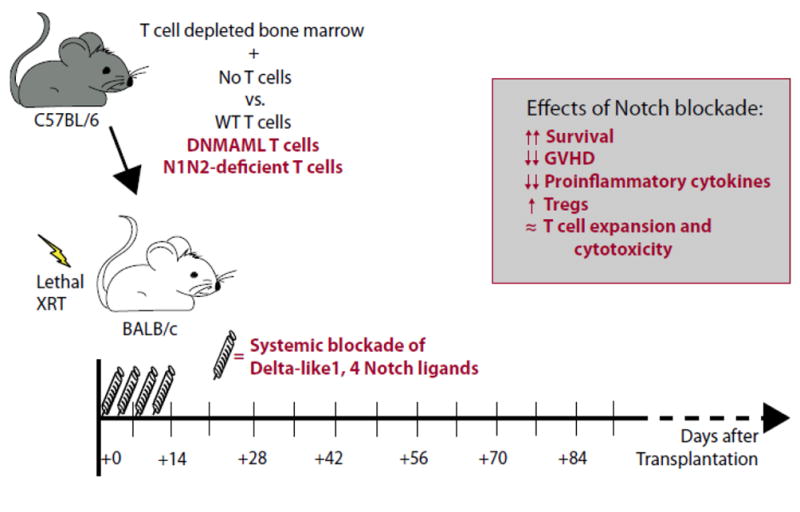
Schematic representation of a MHC-mismatched mouse model of allo-HCT used to identify a major function for Notch signaling in alloreactive T cells during GVHD. Lethally irradiated (8.5–10 Gy) BALB/c mice (H-2d) are transplanted with C57BL/6 (H-2b) T cell-depleted bone marrow, with or without C57BL/6 wild-type (WT) T cells, Notch-deprived DNMAML T cells or Notch1/Notch2 (N1/N2) double-deficient T cells. Alternatively, neutralizing monoclonal antibodies against Notch ligands Delta-like1 and Delta-like4 were administered for a short course (day 0–10 post-transplantation). Genetic inhibition of Notch signaling in T cells or transient Dll1/4 blockade had similar effects, increasing survival and preventing GVHD (box). Protective effects of Notch inhibition were also observed in other GVHD models (see Table 3).
A remarkable consequence of Notch inhibition in alloreactive T cells was its broad impact on multiple T helper CD4+ subsets, suggesting that Notch did not merely control lineage-specific differentiation decisions during GVHD.19, 22 In addition, Notch blockade induced parallel effects in CD8+ alloreactive T cells, including markedly decreased production of the cytokine IFN γ.22 Decreased IFNγ production occurred despite preserved expression of the master transcription factors T-bet and Eomesodermin, which regulate Th1 CD4+ and CD8+ effector T cell differentiation. In contrast, Notch-deprived alloreactive T cells acquired a decreased capacity to activate Ras/MAPK and NF-kB signaling after stimulation through the T cell receptor, as well as increased expression of multiple negative regulators of T cell activation. Altogether, Notch blockade induced a hyporesponsive phenotype with features reminiscent of T cell anergy. Interestingly, downstream effector pathways were influenced to a very variable extent by these changes, with profoundly decreased cytokine production but preserved in vivo expansion and cellular cytotoxicity. More work is necessary to identify the direct transcriptional targets of Notch signaling that are ultimately responsible for these effects. Finally, Notch was also reported to play a pathogenic role in the dendritic cell compartment of Ikaros-deficient mice during GVHD.89 Thus, effects of Notch signaling beyond the T cell compartment must be considered.
Based on its importance in alloimmunity, it is tempting to speculate that Notch might also play an important role in other T cell-mediated disorders, including autoimmunity. Minter’s group recently provided an interesting observation that Notch signaling regulates T cell function in immune-mediated aplastic anemia (Table 3).90 Induction of aplastic anemia in this report was based on a major alloantigen mismatch using parent to F1 bone marrow transplantation. Pharmacological or genetic Notch blockade blunted immune-mediated damage to host hematopoietic progenitors and slowed progression to bone marrow failure. Given the use of alloantigens, these findings share characteristics with findings in GVHD models, with the main target organ being the bone marrow instead of epithelial organs. However, T cells isolated from human patients with aplastic anemia also showed evidence of Notch activation driving expression of Tbx21, Ifng and GzmB.90 These findings suggest that Notch inhibition could ameliorate bone marrow damage by dampening autoreactive T cells in immune-mediated aplastic anemia, in the presence of autoantigens rather than alloantigens as drivers of the immune response.
Blockade of individual Notch ligands and receptors controls graft-versus-host disease
Initial observations about the role of Notch in T cell alloimmunity were based on genetic strategies, but pharmacological approaches are needed to harness the therapeutic potential of Notch inhibition in GVHD. To this end, we first studied the effects of γ-secretase inhibitors (GSIs) after mouse allo-HCT. GSIs were effective at targeting Notch signaling in T cells, however systemic Notch blockade was poorly tolerated immediately after total body irradiation and allo-HCT due to major intestinal side effects.20 Notch was previously reported to regulate the differentiation of intestinal progenitors into absorptive vs. secretory lineages.91, 92 In addition to these effects, pan-Notch blockade after allo-HCT revealed a role of Notch in intestinal regeneration.20 GSIs might be useful in other contexts, as shown for example by Minter’s group in their model of aplastic anemia.90 However, more selective targeting of Notch signaling appears a better option immediately after HCT.
To overcome the limitations of systemic Notch blockade, we investigated the role of individual Notch receptors and ligands in T cell alloimmunity. Among the four mammalian Notch receptors and five ligands, Notch1/2 and Delta-like1/4 mediated all the effects of Notch signaling in alloreactive T cells, with a predominant role for Notch1 and Delta-like4.20 Notch1/Notch2 loss had a similar effect as blockade of CSL/MAML-dependent signaling in the nucleus, indicating that in this context at least the effects of the Notch pathway do not involve “non-canonical” pathway mediated by the Notch receptors independently of CSL/RBP-Jk and MAML. Regarding the role of Notch ligands, Mochizuki et al. also reported a dominant role for Dll4 using a different set of monoclonal antibodies, as well as a potential cellular source of Dll4 in host inflammatory dendritic cells.21 More work is required to establish how Notch ligand expression is regulated after allo-HCT and to identify all the cellular partners involved in the delivery of Notch signals to incoming alloreactive T cells.
From a therapeutic perspective, the winning strategy to control GVHD turned out to be blockade of Delta-like1/4 ligands in vivo at early time points after allo-BMT (Fig. 3).20 In contrast to GSIs and systemic Notch1/2 blockade, inhibition of Delta-like ligands preserved intestinal regeneration after allo-HCT, thus opening a therapeutic window in vivo. Interestingly, transient Dll1/4 blockade provided similar protection from GVHD as long-term or even permanent inhibition of Notch signaling in T cells. These observations suggest that incoming alloreactive T cells are rapidly exposed to a pulse of Notch signaling after transplantation, and that blockade of Notch signaling during this critical period can reprogram alloreactive T cells to a less pathogenic phenotype. At least in part, this could be related to the acquisition of permanent or long-lasting epigenetic changes. The effectiveness of short-term inhibition in GVHD has fundamental importance to understand the immunobiological effects of Notch signaling and its interaction with other signaling pathways that regulate alloreactive T cell function (e.g. costimulatory receptors). In addition, it has translational importance, as the use of short-term Notch blockade would mitigate safety concerns related to prolonged Notch inhibition in patients.43, 75, 93 Additional work is needed to define optimal schedules and intervention strategies to prevent and/or to treat GVHD via Notch-based therapeutics.
Conclusions and perspectives
When reflecting on the initial description of Notch activity in flies by Thomas Hunt Morgan nearly a century ago, it is fascinating to consider how far we have traveled while continuing to discover major functions of Notch in physiology and disease, and while now considering Notch as a target for therapeutic intervention in humans.94 Our increasingly sophisticated understanding of Notch genetics and biochemistry was derived from basic science investigations, but it is now providing potent experimental tools and potential therapeutic agents in patients.
In allo-HCT, Notch exerts very different effects on hematopoietic progenitors ex vivo and on alloreactive T cells in vivo. Notch-based expansion of multipotent hematopoietic progenitors primarily relies on ex vivo activation of Notch signaling above physiological levels to achieve the desired outcome. In contrast, GVHD prevention would involve transient inhibition of Notch signaling mediated by Delta-like ligands in vivo shortly after transplantation. Thus, these interventions are distinct and not mutually exclusive. Notch ligand-based expansion of hematopoietic progenitors has already progressed into clinical testing with promising early results. So far, pharmacological Notch inhibitors including neutralizing monoclonal antibodies have mostly been considered for therapeutic interventions targeting cancer cells or the tumor microenvironment, and some have been tested in early human clinical trials. Recent results highlight the potential of Notch-based therapeutics in immune diseases and also identify targeting of individual Notch receptors or ligands as a strategy to increase the therapeutic index of Notch inhibition. Although more preclinical studies are warranted, we hope that these findings will lead to carefully designed clinical trials that test the potential of Notch inhibitors in GVHD and in other T cell-mediated disorders.
Acknowledgments
Work on Notch signaling in the Maillard laboratory has been or is supported by a Damon Runyon-Rachleff Innovation award (DRR-05A-09), a Scholar Award of the American Society of Hematology, a Scholar Award from the Leukemia and Lymphoma Society and the National Institutes of Health (RO1-AI091627). C.E. is supported by a T32 training grant from NHLBI (HL00762-26).
Footnotes
Conflict of interest statement
There is no significant conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Appelbaum FR. Hematopoietic-cell transplantation at 50. N Engl J Med. 2007;357:1472–5. doi: 10.1056/NEJMp078166. [DOI] [PubMed] [Google Scholar]
- 2.Gyurkocza B, Rezvani A, Storb RF. Allogeneic hematopoietic cell transplantation: the state of the art. Expert Rev Hematol. 2010;3:285–99. doi: 10.1586/ehm.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu CJ, Ritz J. Induction of tumor immunity following allogeneic stem cell transplantation. Adv Immunol. 2006;90:133–73. doi: 10.1016/S0065-2776(06)90004-2. [DOI] [PubMed] [Google Scholar]
- 4.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–70. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 5.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–83. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 6.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–52. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12:443–58. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeiser R, Beilhack A, Negrin RS. Acute graft-versus-host disease-challenge for a broader application of allogeneic hematopoietic cell transplantation. Curr Stem Cell Res Ther. 2006;1:203–12. doi: 10.2174/157488806776956896. [DOI] [PubMed] [Google Scholar]
- 10.Anasetti C, Aversa F, Brunstein CG. Back to the future: mismatched unrelated donor, haploidentical related donor, or unrelated umbilical cord blood transplantation? Biol Blood Marrow Transplant. 2012;18:S161–5. doi: 10.1016/j.bbmt.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Wagner JE, Gluckman E. Umbilical cord blood transplantation: the first 20 years. Semin Hematol. 2010;47:3–12. doi: 10.1053/j.seminhematol.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–6. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao NJ, Chen BJ. Prophylaxis and treatment of acute graft-versus-host disease. Semin Hematol. 2006;43:32–41. doi: 10.1053/j.seminhematol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Antin JH, Chen AR, Couriel DR, Ho VT, Nash RA, Weisdorf D. Novel approaches to the therapy of steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2004;10:655–68. doi: 10.1016/j.bbmt.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Apperley JF, Mauro FR, Goldman JM, Gregory W, Arthur CK, Hows J, et al. Bone marrow transplantation for chronic myeloid leukaemia in first chronic phase: importance of a graft-versus-leukaemia effect. Br J Haematol. 1988;69:239–45. doi: 10.1111/j.1365-2141.1988.tb07628.x. [DOI] [PubMed] [Google Scholar]
- 16.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–62. [PubMed] [Google Scholar]
- 17.Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. 2001;98:3192–204. doi: 10.1182/blood.v98.12.3192. [DOI] [PubMed] [Google Scholar]
- 18.Joseph RW, Couriel DR, Komanduri KV. Chronic graft-versus-host disease after allogeneic stem cell transplantation: challenges in prevention, science, and supportive care. J Support Oncol. 2008;6:361–72. [PubMed] [Google Scholar]
- 19.Zhang Y, Sandy AR, Wang J, Radojcic V, Shan GT, Tran IT, et al. Notch signaling is a critical regulator of allogeneic CD4+ T-cell responses mediating graft-versus-host disease. Blood. 2011;117:299–308. doi: 10.1182/blood-2010-03-271940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran IT, Sandy AR, Carulli AJ, Ebens C, Chung J, Shan GT, et al. Blockade of individual Notch ligands and receptors controls graft-versus-host disease. J Clin Invest. 2013;123:1590–604. doi: 10.1172/JCI65477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mochizuki K, Xie F, He S, Tong Q, Liu Y, Mochizuki I, et al. Delta-like Ligand 4 Identifies a Previously Uncharacterized Population of Inflammatory Dendritic Cells That Plays Important Roles in Eliciting Allogeneic T Cell Responses in Mice. J Immunol. 2013;190:3772–82. doi: 10.4049/jimmunol.1202820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandy A, Chung J, Toubai T, Shan G, Tran I, Friedman A, et al. T cell-specific Notch inhibition blocks graft-versus-host disease by inducing a hyporesponsive program in alloreactive CD4+ and CD8+ T cells. J Immunol. 2013;190:5818–28. doi: 10.4049/jimmunol.1203452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–33. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louvi A, Artavanis-Tsakonas S. Notch and disease: A growing field. Semin Cell Dev Biol. 2012;23:473–80. doi: 10.1016/j.semcdb.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon WR, Vardar-Ulu D, Histen G, Sanchez-Irizarry C, Aster JC, Blacklow SC. Structural basis for autoinhibition of Notch. Nat Struct Mol Biol. 2007;14:295–300. doi: 10.1038/nsmb1227. [DOI] [PubMed] [Google Scholar]
- 26.Tian L, Wu X, Chi C, Han M, Xu T, Zhuang Y. ADAM10 is essential for proteolytic activation of Notch during thymocyte development. Int Immunol. 2008;20:1181–7. doi: 10.1093/intimm/dxn076. [DOI] [PubMed] [Google Scholar]
- 27.Gibb DR, El Shikh M, Kang DJ, Rowe WJ, El Sayed R, Cichy J, et al. ADAM10 is essential for Notch2-dependent marginal zone B cell development and CD23 cleavage in vivo. J Exp Med. 2010;207:623–35. doi: 10.1084/jem.20091990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, et al. A presenilin-1-dependent -secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–22. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 29.Tamura K, Taniguchi Y, Minoguchi S, Sakai T, Tun T, Furukawa T, et al. Physical interaction between a novel domain of the receptor Notch and the transcription factor RBP-J kappa/Su(H) Curr Biol. 1995;5:1416–23. doi: 10.1016/s0960-9822(95)00279-x. [DOI] [PubMed] [Google Scholar]
- 30.Petcherski AG, Kimble J. Mastermind is a putative activator for Notch. Curr Biol. 2000;10:R471–3. doi: 10.1016/s0960-9822(00)00577-7. [DOI] [PubMed] [Google Scholar]
- 31.Wu L, Aster JC, Blacklow SC, Lake R, Artavanis-Tsakonas S, Griffin JD. MAML1, a human homologue of drosophila mastermind, is a transcriptional co-activator for NOTCH receptors. Nat Genet. 2000;26:484–9. doi: 10.1038/82644. [DOI] [PubMed] [Google Scholar]
- 32.Nam Y, Sliz P, Song L, Aster JC, Blacklow SC. Structural basis for cooperativity in recruitment of MAML coactivators to Notch transcription complexes. Cell. 2006;124:973–83. doi: 10.1016/j.cell.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 33.Varnum-Finney B, Wu L, Yu M, Brashem-Stein C, Staats S, Flowers D, et al. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. J Cell Sci. 2000;113(Pt 23):4313–8. doi: 10.1242/jcs.113.23.4313. [DOI] [PubMed] [Google Scholar]
- 34.Ohishi K, Varnum-Finney B, Bernstein ID. Delta-1 enhances marrow and thymus repopulating ability of human CD34(+)CD38(-) cord blood cells. J Clin Invest. 2002;110:1165–74. doi: 10.1172/JCI16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–7. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, et al. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464:1052–7. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- 37.Wolfe MS. gamma-Secretase in biology and medicine. Semin Cell Dev Biol. 2009;20:219–24. doi: 10.1016/j.semcdb.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, et al. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol. 2002;14:637–45. doi: 10.1093/intimm/dxf030. [DOI] [PubMed] [Google Scholar]
- 39.Weng AP, Nam Y, Wolfe MS, Pear WS, Griffin JD, Blacklow SC, et al. Growth suppression of pre-T acute lymphoblastic leukemia cells by inhibition of notch signaling. Mol Cell Biol. 2003;23:655–64. doi: 10.1128/MCB.23.2.655-664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maillard I, Weng AP, Carpenter AC, Rodriguez CG, Sai H, Xu L, et al. Mastermind critically regulates Notch-mediated lymphoid cell fate decisions. Blood. 2004;104:1696–702. doi: 10.1182/blood-2004-02-0514. [DOI] [PubMed] [Google Scholar]
- 41.Tu L, Fang TC, Artis D, Shestova O, Pross SE, Maillard I, et al. Notch signaling is an important regulator of type 2 immunity. J Exp Med. 2005;202:1037–42. doi: 10.1084/jem.20050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maillard I, Tu L, Sambandam A, Yashiro-Ohtani Y, Millholland J, Keeshan K, et al. The requirement for Notch signaling at the beta-selection checkpoint in vivo is absolute and independent of the pre-T cell receptor. J Exp Med. 2006;203:2239–45. doi: 10.1084/jem.20061020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koch U, Radtke F. Notch and cancer: a double-edged sword. Cell Mol Life Sci. 2007;64:2746–62. doi: 10.1007/s00018-007-7164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aster JC, Pear WS, Blacklow SC. Notch signaling in leukemia. Annu Rev Pathol. 2008;3:587–613. doi: 10.1146/annurev.pathmechdis.3.121806.154300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Vlierberghe P, Ferrando A. The molecular basis of T cell acute lymphoblastic leukemia. J Clin Invest. 2012;122:3398–406. doi: 10.1172/JCI61269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–58. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 47.Koch U, Fiorini E, Benedito R, Besseyrias V, Schuster-Gossler K, Pierres M, et al. Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J Exp Med. 2008;205:2515–23. doi: 10.1084/jem.20080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hozumi K, Mailhos C, Negishi N, Hirano K, Yahata T, Ando K, et al. Delta-like 4 is indispensable in thymic environment specific for T cell development. J Exp Med. 2008;205:2507–13. doi: 10.1084/jem.20080134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sambandam A, Maillard I, Zediak VP, Xu L, Gerstein R, Aster J, et al. Notch signaling controls the generation and differentiation of early T lineage progenitors. Nat Immunol. 2005;6:663–70. doi: 10.1038/ni1216. [DOI] [PubMed] [Google Scholar]
- 50.Tan JB, Visan I, Yuan JS, Guidos CJ. Requirement for Notch1 signals at sequential early stages of intrathymic T cell development. Nat Immunol. 2005;6:671–9. doi: 10.1038/ni1217. [DOI] [PubMed] [Google Scholar]
- 51.Bell JJ, Bhandoola A. The earliest thymic progenitors for T cells possess myeloid lineage potential. Nature. 2008;452:764–7. doi: 10.1038/nature06840. [DOI] [PubMed] [Google Scholar]
- 52.Wolfer A, Wilson A, Nemir M, MacDonald HR, Radtke F. Inactivation of Notch1 impairs VDJbeta rearrangement and allows pre-TCR-independent survival of early alpha beta Lineage Thymocytes. Immunity. 2002;16:869–79. doi: 10.1016/s1074-7613(02)00330-8. [DOI] [PubMed] [Google Scholar]
- 53.Ciofani M, Schmitt TM, Ciofani A, Michie AM, Cuburu N, Aublin A, et al. Obligatory role for cooperative signaling by pre-TCR and Notch during thymocyte differentiation. J Immunol. 2004;172:5230–9. doi: 10.4049/jimmunol.172.9.5230. [DOI] [PubMed] [Google Scholar]
- 54.Ciofani M, Zuniga-Pflucker JC. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat Immunol. 2005;6:881–8. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- 55.Wolfer A, Bakker T, Wilson A, Nicolas M, Ioannidis V, Littman DR, et al. Inactivation of Notch 1 in immature thymocytes does not perturb CD4 or CD8T cell development. Nat Immunol. 2001;2:235–41. doi: 10.1038/85294. [DOI] [PubMed] [Google Scholar]
- 56.Tanigaki K, Tsuji M, Yamamoto N, Han H, Tsukada J, Inoue H, et al. Regulation of alphabeta/gammadelta T cell lineage commitment and peripheral T cell responses by Notch/RBP-J signaling. Immunity. 2004;20:611–22. doi: 10.1016/s1074-7613(04)00109-8. [DOI] [PubMed] [Google Scholar]
- 57.Osborne BA, Minter LM. Notch signalling during peripheral T-cell activation and differentiation. Nat Rev Immunol. 2007;7:64–75. doi: 10.1038/nri1998. [DOI] [PubMed] [Google Scholar]
- 58.Amsen D, Antov A, Flavell RA. The different faces of Notch in T-helper-cell differentiation. Nat Rev Immunol. 2009;9:116–24. doi: 10.1038/nri2488. [DOI] [PubMed] [Google Scholar]
- 59.Radtke F, Macdonald HR, Tacchini-Cottier F. Regulation of innate and adaptive immunity by Notch. Nat Rev Immunol. 2013;13:427–437. doi: 10.1038/nri3445. [DOI] [PubMed] [Google Scholar]
- 60.Saito T, Chiba S, Ichikawa M, Kunisato A, Asai T, Shimizu K, et al. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity. 2003;18:675–85. doi: 10.1016/s1074-7613(03)00111-0. [DOI] [PubMed] [Google Scholar]
- 61.Lewis KL, Caton ML, Bogunovic M, Greter M, Grajkowska LT, Ng D, et al. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity. 2011;35:780–91. doi: 10.1016/j.immuni.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Varnum-Finney B, Purton LE, Yu M, Brashem-Stein C, Flowers D, Staats S, et al. The Notch ligand, Jagged-1, influences the development of primitive hematopoietic precursor cells. Blood. 1998;91:4084–91. [PubMed] [Google Scholar]
- 63.Karanu FN, Murdoch B, Gallacher L, Wu DM, Koremoto M, Sakano S, et al. The notch ligand jagged-1 represents a novel growth factor of human hematopoietic stem cells. J Exp Med. 2000;192:1365–72. doi: 10.1084/jem.192.9.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karanu FN, Murdoch B, Miyabayashi T, Ohno M, Koremoto M, Gallacher L, et al. Human homologues of Delta-1 and Delta-4 function as mitogenic regulators of primitive human hematopoietic cells. Blood. 2001;97:1960–7. doi: 10.1182/blood.v97.7.1960. [DOI] [PubMed] [Google Scholar]
- 65.Varnum-Finney B, Brashem-Stein C, Bernstein ID. Combined effects of Notch signaling and cytokines induce a multiple log increase in precursors with lymphoid and myeloid reconstituting ability. Blood. 2003;101:1784–9. doi: 10.1182/blood-2002-06-1862. [DOI] [PubMed] [Google Scholar]
- 66.Delaney C, Varnum-Finney B, Aoyama K, Brashem-Stein C, Bernstein ID. Dose-dependent effects of the Notch ligand Delta1 on ex vivo differentiation and in vivo marrow repopulating ability of cord blood cells. Blood. 2005;106:2693–9. doi: 10.1182/blood-2005-03-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dallas MH, Varnum-Finney B, Delaney C, Kato K, Bernstein ID. Density of the Notch ligand Delta1 determines generation of B and T cell precursors from hematopoietic stem cells. J Exp Med. 2005;201:1361–6. doi: 10.1084/jem.20042450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dallas MH, Varnum-Finney B, Martin PJ, Bernstein ID. Enhanced T-cell reconstitution by hematopoietic progenitors expanded ex vivo using the Notch ligand Delta1. Blood. 2007;109:3579–87. doi: 10.1182/blood-2006-08-039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–64. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Varnum-Finney B, Halasz LM, Sun M, Gridley T, Radtke F, Bernstein ID. Notch2 governs the rate of generation of mouse long- and short-term repopulating stem cells. J Clin Invest. 2011;121:1207–16. doi: 10.1172/JCI43868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumano K, Chiba S, Kunisato A, Sata M, Saito T, Nakagami-Yamaguchi E, et al. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. 2003;18:699–711. doi: 10.1016/s1074-7613(03)00117-1. [DOI] [PubMed] [Google Scholar]
- 72.Mancini SJ, Mantei N, Dumortier A, Suter U, Macdonald HR, Radtke F. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood. 2005;105:2340–2. doi: 10.1182/blood-2004-08-3207. [DOI] [PubMed] [Google Scholar]
- 73.Maillard I, Koch U, Dumortier A, Shestova O, Xu L, Sai H, et al. Canonical Notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2008;2:356–66. doi: 10.1016/j.stem.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao J, Graves S, Koch U, Liu S, Jankovic V, Buonamici S, et al. Hedgehog signaling is dispensable for adult hematopoietic stem cell function. Cell Stem Cell. 2009;4:548–58. doi: 10.1016/j.stem.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klinakis A, Lobry C, Abdel-Wahab O, Oh P, Haeno H, Buonamici S, et al. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature. 2011;473:230–3. doi: 10.1038/nature09999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maeda T, Merghoub T, Hobbs RM, Dong L, Maeda M, Zakrzewski J, et al. Regulation of B versus T lymphoid lineage fate decision by the proto-oncogene LRF. Science. 2007;316:860–6. doi: 10.1126/science.1140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee SU, Maeda M, Ishikawa Y, Li SM, Wilson A, Jubb AM, et al. LRF-mediated Dll4 repression in erythroblasts is necessary for hematopoietic stem cell maintenance. Blood. 2013;121:918–29. doi: 10.1182/blood-2012-03-418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Milner LA, Kopan R, Martin DI, Bernstein ID. A human homologue of the Drosophila developmental gene, Notch, is expressed in CD34+ hematopoietic precursors. Blood. 1994;83:2057–62. [PubMed] [Google Scholar]
- 79.Milano F, Heimfeld S, Gooley T, Jinneman J, Nicoud I, Delaney C. Correlation of infused CD3+CD8+ cells with single-donor dominance after double-unit cord blood transplantation. Biol Blood Marrow Transplant. 2013;19:156–60. doi: 10.1016/j.bbmt.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bernstein ID, Boyd RL, van den Brink MR. Clinical strategies to enhance posttransplant immune reconstitution. Biol Blood Marrow Transplant. 2008;14:94–9. doi: 10.1016/j.bbmt.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zakrzewski JL, Kochman AA, Lu SX, Terwey TH, Kim TD, Hubbard VM, et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med. 2006;12:1039–47. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 82.Zakrzewski JL, Suh D, Markley JC, Smith OM, King C, Goldberg GL, et al. Tumor immunotherapy across MHC barriers using allogeneic T-cell precursors. Nat Biotechnol. 2008;26:453–61. doi: 10.1038/nbt1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Awong G, Herer E, Surh CD, Dick JE, La Motte-Mohs RN, Zuniga-Pflucker JC. Characterization in vitro and engraftment potential in vivo of human progenitor T cells generated from hematopoietic stem cells. Blood. 2009;114:972–82. doi: 10.1182/blood-2008-10-187013. [DOI] [PubMed] [Google Scholar]
- 84.Holland AM, Zakrzewski JL, Tsai JJ, Hanash AM, Dudakov JA, Smith OM, et al. Extrathymic development of murine T cells after bone marrow transplantation. J Clin Invest. 2012;122:4716–26. doi: 10.1172/JCI60630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lancrin C, Schneider E, Lambolez F, Arcangeli ML, Garcia-Cordier C, Rocha B, et al. Major T cell progenitor activity in bone marrow-derived spleen colonies. J Exp Med. 2002;195:919–29. doi: 10.1084/jem.20011475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arcangeli ML, Lancrin C, Lambolez F, Cordier C, Schneider E, Rocha B, et al. Extrathymic hemopoietic progenitors committed to T cell differentiation in the adult mouse. J Immunol. 2005;174:1980–8. doi: 10.4049/jimmunol.174.4.1980. [DOI] [PubMed] [Google Scholar]
- 87.Maillard I, Schwarz BA, Sambandam A, Fang T, Shestova O, Xu L, et al. Notch-dependent T-lineage commitment occurs at extrathymic sites following bone marrow transplantation. Blood. 2006;107:3511–9. doi: 10.1182/blood-2005-08-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Radtke F, Fasnacht N, Macdonald HR. Notch Signaling in the Immune System. Immunity. 2010;32:14–27. doi: 10.1016/j.immuni.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 89.Toubai T, Sun Y, Tawara I, Friedman A, Liu C, Evers R, et al. Ikaros-Notch axis in host hematopoietic cells regulates experimental graft-versus-host disease. Blood. 2011;118:192–204. doi: 10.1182/blood-2010-12-324616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roderick JE, Gonzalez-Perez G, Kuksin CA, Dongre A, Roberts ER, Srinivasan J, et al. Therapeutic targeting of NOTCH signaling ameliorates immune-mediated bone marrow failure of aplastic anemia. J Exp Med. 2013;210:1311–1329. doi: 10.1084/jem.20112615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–63. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 92.Riccio O, van Gijn ME, Bezdek AC, Pellegrinet L, van Es JH, Zimber-Strobl U, et al. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9:377–83. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yan M, Callahan CA, Beyer JC, Allamneni KP, Zhang G, Ridgway JB, et al. Chronic DLL4 blockade induces vascular neoplasms. Nature. 2010;463:E6–7. doi: 10.1038/nature08751. [DOI] [PubMed] [Google Scholar]
- 94.Morgan TH. The Theory of the Gene. The American Naturalist. 1917;51:513–44. [Google Scholar]
- 95.Suzuki T, Yokoyama Y, Kumano K, Takanashi M, Kozuma S, Takato T, et al. Highly efficient ex vivo expansion of human hematopoietic stem cells using Delta1-Fc chimeric protein. Stem Cells. 2006;24:2456–65. doi: 10.1634/stemcells.2006-0258. [DOI] [PubMed] [Google Scholar]
- 96.Watts KL, Delaney C, Humphries RK, Bernstein ID, Kiem HP. Combination of HOXB4 and Delta-1 ligand improves expansion of cord blood cells. Blood. 2010;116:5859–66. doi: 10.1182/blood-2010-05-286062. [DOI] [PMC free article] [PubMed] [Google Scholar]