Abstract
Aims
To evaluate associations of treatment and an ‘additive genetic efficacy score’ (AGES) based on dopamine functional polymorphisms with time to first smoking lapse and point prevalence abstinence at end of treatment among participants enrolled in two randomized clinical trials of smoking cessation therapies.
Design
Double-blind pharmacogenetic efficacy trials randomizing participants to active or placebo bupropion. Study 1 also randomized participants to cognitive-behavioral smoking cessation treatment (CBT) or this treatment with CBT for depression. Study 2 provided standardized behavioural support.
Setting
Two Hospital-affiliated clinics (Study 1), and two University-affiliated clinics (Study 2).
Participants
N=792 self-identified white treatment-seeking smokers aged ≥18 years smoking ≥10 cigarettes per day over the last year.
Measurements
Age, gender, Fagerström Test for Nicotine Dependence, dopamine pathway genotypes (rs1800497 [ANKK1 E713K], rs4680 [COMT V158M], DRD4 exon 3 Variable Number of Tandem Repeats polymorphism [DRD4 VNTR], SLC6A3 3' VNTR) analyzed both separately and as part of an AGES, time to first lapse, and point prevalence abstinence at end of treatment.
Findings
Significant associations of the AGES (hazard ratio = 1.10, 95% Confidence Interval [CI] = 1.06–1.14], p=0.0099) and of the DRD4 VNTR (HR = 1.29, 95%CI 1.17–1.41, p=0.0073) were observed with time to first lapse. A significant AGES by pharmacotherapy interaction was observed (β [SE]=−0.18 [0.07], p=0.016), such that AGES predicted risk for time to first lapse only for individuals randomized to placebo.
Conclusions
A score based on functional polymorphisms relating to dopamine pathways appears to predict lapse to smoking following a quit attempt, and the association is mitigated in smokers using bupropion.
Keywords: Bupropion, genetic, pharmacogenetic analysis, randomized clinical trial, first lapse
INTRODUCTION
While smoking cessation markedly reduces the risk of morbidity and premature death (1), sustained abstinence rates with the best available treatments (bupropion, nicotine replacement therapy or varenicline and behavioral counseling) do not exceed 50% by the end of treatment (2–5). Twin and family studies indicate that nicotine dependence and ability to quit smoking have genetic determinants (6–11). Candidate gene investigations of smoking cessation pharmacotherapies have reported associations between genetic variants in multiple pharmacodynamic (e.g., catecholamine, cholinergic, opioid receptors) and pharmacokinetic (e.g., cytochrome p450 [CYP] 2A6 & 2B6) pathways implicated in nicotine dependence and treatment response (12–16). Sustained-release bupropion hydrochloride, a first line treatment for smoking cessation, is an atypical antidepressant with dopaminergic (17) and noradrenergic (17) reuptake inhibition and cholinergic (18) properties and it is metabolized to hydroxybupropion and other active metabolites by CYP2B6 (19, 20).
Numerous studies have reported moderation of bupropion efficacy for smoking cessation by single genetic polymorphisms (21). The limitation of this approach include a lack of statistical power to detect effects of single variants, genetic confounders and non-genetic confounders (22). The use of an additive genetic scale has the advantages of considering the collective impact of several variants, relying on prior knowledge of alleles (in contrast to agnostic genome-wide association [GWAS] approaches) and provides greater statistical power than modeling each variant individually if the assumptions of the additive genetic scale accurately model the biological pathway under study. McGeary and colleagues (23) reported the use of a proof-of-concept, bupropion-specific dopamine pathway additive genetic risk score that was associated with smoking cessation in a previous clinical trial of abstinent, alcoholic smokers (23), that assumes an additive genetic model of four dopamine-based risk alleles using the following criteria for marker selection: (i) evidence of moderation of drug response in at least two separate clinical trials; (ii) evidence of functional impact of variant on gene expression and/or endophenotypes of nicotine dependence in humans; and (iii) presence of gene within a relevant pharmacological pathway for the medication (bupropion) and nicotine dependence. This model incorporates four target genes based on evidence from single gene association studies examining variants in two genes associated with expression of dopamine D2 (DRD2) and D4 (DRD4) receptors, one for catechol-O-methyl transferase (COMT), and another for the dopamine transporter (SLC6A3). A systematic review and network analysis by Wang and Li suggested that the dopamine pathway loci with the strongest evidence of association with smoking cessation were DRD2 rs1800497, DRD4 VNTR, COMT rs4680 & SLC6A3 VNTR)(24). Two recent fMRI studies provide biologically plausible evidence of dopamine pathway genetic scores brain reward circuitry activation. Nikolova and colleagues reported that a similar, five locus (DRD2 −141C Del, DRD2 rs1800497, DRD4 VNTR, COMT rs4680 & SLC6A3 VNTR) dopamine pathway genetic risk score predicted 10.9% of the inter-individual variation in ventral striatum reactivity – a probable site of action for bupropion(25), which was greater than the contribution of any single variant alone (26) and Stice and colleagues also reported that a multilocus dopamine score using the same variants but a different scoring and weighting scheme was associated with brain reward circuitry reactivity (27).
Genome-wide investigations suggest that hundreds of polymorphisms moderate the efficacy of bupropion and nicotine replacement therapy (NRT) for smoking cessation, yet the variation in drug response explained by any particular allele is minor (28, 29). We are aware of only one other additive genetic predictive test for smoking cessation (30, 31) that used nominal associations from GWAS investigations of clinical trials (32, 33). The present investigation applies an alternative, pharmacological candidate gene approach by developing a predictive efficacy score for drug response to bupropion based on a priori hypotheses regarding the contributions of specific variants in both the pharmacodynamic pathway of nicotine and the dopamine pathways associated with both smoking cessation and bupropion effects. Supplemental Table S1 provides descriptions and rationale for selection of each polymorphism included in the bupropion AGES for smoking cessation.
The use of a genetic efficacy score has the potential to simplify the incorporation of multiple informative polymorphisms to the process of genetically tailored treatment for smoking cessation. Here we report evidence of bupropion moderation of a smoking cessation outcome by an additive genetic efficacy score (AGES) composed of polymorphisms previously associated with smoking cessation and prior evidence of effects on gene expression and nicotine dependence endophenotypes in neuroimaging studies (See Supplemental Table S1 for details). We were interested in the effect of the AGES on days to first lapse because this phenotype (a) is predictive of longer-term abstinence outcomes (34); (b) spans the full period of exposure to drug during which gene × drug interactions are biologically plausible and more likely to be detected; (c) complies with recommendations from the Society for Research on Nicotine and Tobacco to report survival analyses as well as point-prevalence abstinence (34); (d) would be testing the proof of concept AGES using a phenotype (time to first smoking lapse) (TTFSL) not previously reported in extant publications of these four genetic variants using these two clinical trial samples, which reported point-prevalence and continuous abstinence; and (e) was measured during the interval with the highest risk of relapse. Given observations from previously published studies described above, we hypothesized that: (i) individuals with higher AGES scores on bupropion would be less likely to lapse and relapse during treatment and that (ii) individuals with lower scores would be more at risk of relapse on placebo, compared with individuals with lower scores on bupropion, respectively.
METHODS
Participant characteristics, detailed procedures, recruitment details, and methods are described in detail elsewhere for both Study 1 (35, 36) and Study 2 (37, 38). We have reported previous analyses for some of these genes for both studies using point prevalence outcomes and continuous/prolonged abstinence, but heretofore we have not published results for time to first lapse in association with these variants (36). The following is a brief overview of both studies used in the current analyses.
Study 1
Participants were European-ancestry smokers randomized in double-blind manner to bupropion or placebo, stratified by gender, current depressive symptoms and nicotine dependence severity, using the urn randomization technique. Ancestry was assessed by self-report of “White, non-Hispanic” racial/ethnic heritage. Patients were randomized to either bupropion (150 mg/day for the first 3 days, followed by 300 mg/day), or matching placebo, for 12 weeks and either cognitive behavioral therapy (CBT) or CBT with additional emphasis on treatment of depressive symptoms (CBT-D) – both of which involved 12 two-hour sessions delivered at one of two sites at regional medical centers assigned at random (see Brown et al. (35) for more details about recruitment and behavioral interventions).
Study 2
Participants were European-ancestry smokers randomized to either bupropion or placebo and all participants received behavioral counseling treatment concurrently. Ancestry was assessed by self-report of “White, non-Hispanic” racial/ethnic heritage confirmed by ancestry-informative markers. Treatment consisted of bupropion (150 mg/day for the first 3 days, followed by 300 mg/day), or matching placebo, for 10 weeks and counseling which took place at one of two sites (see Lerman et al. (37) for more details).
Measures
Measured domains available from both studies included (a) descriptive and diagnostic measures, (b) level of nicotine dependence, and (c) smoking outcomes. Participants provided background information including age, gender, years of education, marital status, number of years of regular smoking, and average number of cigarettes per day. Current and past Axis I diagnoses were determined with the Structured Clinical Interview for DSM-IV Non-patient Edition (39). Severity of nicotine dependence was assessed using the Fagerström Test of Nicotine Dependence (FTND) (40), a six-item measure with total scores ranging from 0 to 10, with higher scores indicating higher levels of nicotine dependence. In an effort to characterize two key clinical milestones, the focus of the present investigation is on two separate phenotypes during the period of treatment. The primary outcome of interest was time to first lapse defined as the first time smoking a single puff or more of a cigarette since the quit day. We then examined point-prevalence abstinence at end of treatment (EOT). In an effort to reduce multiple comparisons, we did not evaluate follow-up post EOT in the present investigation, in part because results from single marker variants and bupropion response at these end points have been reported in previous publications (36, 41–44).
Genotyping
Blood samples for both studies were collected and processed after informed consent using methods described in detail in previous publications (36, 37). DNA was genotyped at the Laboratory of Molecular Carcinogenesis Laboratory at the Georgetown University Lombardi Comprehensive Cancer Center (DRD2 rs1800497 & SLC6A3 VNTR – Studies 1 and 2) (Washington, D.C.), the University of Pennsylvania (COMT rs4680 – Study 2), the Primary Care Genetics Laboratory & Translational Research Center (DRD2 rs1800497 from additional Study 1 participants) (Pawtucket, RI), and the National Institute on Drug Abuse Molecular Neurobiology Laboratory (DRD4 VNTR & COMT rs4680 – Study 1) (Baltimore, MD), and the SRI International Center for Health Sciences Molecular Genetics Laboratory (DRD4 VNTR – Study 2) (Menlo Park, CA) using methods previously described (44–50).
Genetic efficacy score
An additive, continuous genetic efficacy (AGES) score was calculated for each locus (range 0–2) based on the number of putative ‘efficacy’ alleles for moderation of bupropion efficacy for smoking cessation (i.e., [COMT: 0 = GG, 1 = GA; 2 = AA]; [DRD2: 0 = AA, 1 = AG, 2 = GG]; [DRD4: 0 = SS; 1 = SL; 2 = LL]; [SLC6A3: 0 = 99; 1 = 9*; 2 = **]) (23). An AGES quotient was calculated for each participant based on the number of efficacy alleles they possessed divided by the total number of possible alleles (range: 0–8). The quotient adjusts for missing genetic data such that the denominator would be 8 if data for all 4 polymorphisms was available but would only be 6 if one of the genotypes were not available. The AGES quotient was calculated only for participants with genotype data available for two or more polymorphisms (see Table 1). The same algorithm for coding AGES was used in both studies.
Table 1.
Characteristics of Randomized Clinical Trials of Bupropion Combined in Pooled Analyses
| Study 1 (Brown Trial) (N = 356) |
Study 2 (Penn/PNAT Trial) (N = 436) |
||||
|---|---|---|---|---|---|
| Baseline measures | Bupropion (n = 175) |
Placebo (n = 181) |
Bupropion (n = 235) |
Placebo (n = 201) |
|
| Female | 88 (50.3%) | 89 (49.2%) | 126 (54%) | 110 (55%) | |
| Age | 45.8 (10.9%) | 46.0 (10.8%) | 44.6 (11.8%) | 44.7 (11.2%) | |
| FTND | 6.2 (1.7%) | 6.2 (1.8%) | 5.1 (2.1%) | 5.2 (2.2%) | |
| CPD | 24.2 (9.3%) | 25.2 (10.3%) | 21.4 (9.0%) | 21.9 (9.7%) | |
| Genotype | |||||
| AGES quotient | 4.1 (1.4) | 4.4 (1.3) | 4.3 (1.4) | 4.4 (1.4) | |
| DRD2 rs1800497 | AA | 10 (6%) | 13 (7%) | 11 (5%) | 10 (5%) |
| AG | 79 (45%) | 65 (36%) | 78 (33%) | 54 (27%) | |
| GG | 86 (49%) | 103 (57%) | 129 (55%) | 126 (63%) | |
| Missing | 0 | 0 | 17 (7%) | 11 (5%) | |
| DRD4 VNTR | SS | 116 (66%) | 113 (62%) | 164 (70%) | 124 (62%) |
| SL | 44 (25%) | 48 (27%) | 51 (22%) | 60 (30%) | |
| LL | 2 (1%) | 8 (4%) | 8 (3%) | 9 (4%) | |
| Missing | 13 (8%) | 12 (7%) | 12 (5%) | 8 (4%) | |
| COMT rs4680 | GG | 50 (29%) | 38 (21%) | 58 (25%) | 68 (34%) |
| GA | 72 (41%) | 90 (50%) | 93 (40%) | 59 (29%) | |
| AA | 43 (24%) | 48 (26%) | 69 (29%) | 48 (24%) | |
| Missing | 10 (6%) | 5 (3%) | 15 (6%) | 26 (13%) | |
| SLC6A3 VNTR | ** | 73 (42%) | 93 (51%) | 114 (49%) | 105 (52%) |
| 9* | 53 (30%) | 51 (28%) | 95 (40%) | 71 (35%) | |
| 99 | 14 (8%) | 11 (6%) | 18 (8%) | 16 (8%) | |
| Missing | 35 (20%) | 26 (15%) | 8 (3%) | 9 (5%) | |
Note. Values represent means (standard deviation) or frequencies (%).All genotypes were in approximate Hardy-Weinberg Equilibrium. AGES = Additive Genetic Efficacy Scale. FTND = Fagerström Test for Nicotine Dependence (FTND) (45). VNTR = variable number of tandem repeats.
Statistical analyses
We used maximum likelihood estimation of mixed-effects Cox proportional hazards regression and mixed effects logistic regression models of the two trials combined with a random effect representing variability across sites (n=4) when testing whether AGES quotient moderated survival to day of first lapse after quit day and biochemically verified seven-day point prevalence abstinence at the end of treatment (PPA-EOT). Self-reports of no smoking in the past seven days was confirmed with saliva cotinine values >15ng/ml and expired CO <10ppm. Missed self-reports of smoking status were presumed to be smoking in this intention-to-treat analysis. Age, gender and level of nicotine dependence were included as covariates in all analyses.
Models were first fit to estimate the main effects of medication and counseling conditions and the relationship of AGES with risk for smoking lapse, followed by an evaluation of whether this relationship differed for those allocated to bupropion or placebo (AGES × drug interaction). This primary moderation (AGES × drug) analysis was followed by the removal of AGES index for a post-hoc estimate of the set of four individual genetic risk indices representing a count of respective hypothesized risk alleles (range 0–2). All statistical analyses, tables, and figures were generated using R statistical software (http://www.r-project.org/) packages coxme (51), rms (52) and LME4 (53).
RESULTS
Participants
Baseline characteristics (sex, age, race, treatment allocation, cigarettes per day, FTND score, loss to follow-up) were similar across studies (Table 1), although participants in Study 1 reported somewhat higher FTND scores (6 vs. 5, p < 0.05) and smoked more cigarettes per day (25 vs. 22, p < 0.05). The mean age of all participants was 45 years (Standard Deviation [SD] = 12), and mean cigarette consumption was 23 cigarettes/day (SD = 10). There were no significant differences in other baseline measures between active and placebo groups in either study. There were 356 participants in Study 1 and 436 participants in Study 2 with sufficient genotype and smoking outcome data for analyses, constituting the final study population of N=792 for the present investigation.
Genotype and AGES distribution
The mean AGES for active and placebo groups combined was similar in Studies 1 and 2. However, AGES was lower in the active treatment group (4.1; SD = 1.4) compared to the placebo group (4.4; SD = 1.3) (p = 0.02) in Study 1, while there were no significant differences in mean AGES between active (4.3; SD = 1.4) and placebo (4.4; SD = 1.4) groups in Study 2. Missing genotype data ranged from 0–20% for Study 1 and 3–13% for Study 2. None of the genotypes deviated from Hardy-Weinberg equilibrium except for COMT rs4680 in Study 2 (48).
Abstinence outcomes
Results of Cox proportional hazards models evaluating time to first lapse using maximum likelihood estimation and logistic regression models evaluating PPA are summarized in Table 2. Proportional hazards assumptions for the model were supported by evaluation of Schoenfeld residuals (X2 = 13.86, p<0.13). We first confirmed the pharmacological and behavioral treatment effects of the trials, independent of participants’ genetic status. In both samples we confirmed a similarly strong effect of bupropion in reducing the risk for smoking lapse and PPA at the end of treatment (p < 0.001). The main effect of AGES on risk for smoking lapse during treatment was statistically significant (hazard ratios (22) of 1.10, 95% CI = 1.06 – 1.14) but, on PPA-EOT, it was not statistically significant (odds ratio = 1.00, 95% CI = 0.94 – 1.05) (Table 2). We also report the performance of individual genotypes on risk of lapse or PPA-EOT using the additive modeling – counting risk alleles (0,1,2). The only main effect of any single marker genotype was DRD4 in increasing risk for smoking lapse (HR = 1.29; 95% CI = 1.17 – 1.41; p<0.008).
Table 2.
Cox Proportional Hazards of Time to First Lapse and Logistic Regression Models for Biochemically Verified Point Prevalence Abstinence, Genotype and AGES in Combined Analyses (N=792)
| Time to First Smoking Lapse | Abstinence at End of Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio |
Beta (SE) | Z | P | Odds Ratio | Beta (SE) | Z | P | |
| Baseline covariates | ||||||||
| Age | 1.01 | 0.01 (0.14) | 0.80 | 0.4300 | 1.01 | 0.01 (0.01) | 1.22 | 0.222 |
| Sex | 1.52 | 0.42 (0.10) | 4.08 | <0.001 | 0.76 | −0.28 (0.16) | −1.74 | 0.081 |
| FTND | 1.48 | 0.14 (0.03) | 5.12 | <0.001 | 0.89 | −0.12 (0.04) | −2.85 | 0.004 |
| *BT | 1.31 | 0.27 (0.14) | 1.91 | 0.0560 | 1.27 | 0.24 (0.25) | 0.96 | 0.339 |
| Drug | 0.57 | −0.56 (0.10) | −5.54 | <0.001 | 2.06 | 0.72 (0.16) | 4.50 | <0.001 |
| Site (SD, n=4) | -- | 0.13 | -- | -- | -- | 0.32 | -- | -- |
| Genotype | ||||||||
| DRD2 rs1800497 B | 1.00 | 0.00 (0.09) | 0.02 | 0.990 | 0.98 | −0.02 (0.15) | −0.17 | 0.867 |
| DRD4 VNTR B | 1.29 | 0.25 (0.09) | 2.68 | 0.007 | 0.85 | −0.16 (0.17) | −0.96 | 0.334 |
| COMT rs4680 B | 1.02 | 0.02 (0.07) | 0.24 | 0.810 | 1.09 | 0.09 (0.12) | 0.75 | 0.450 |
| SLC6A3 VNTR B | 0.89 | −0.11 (0.08) | −1.36 | 0.170 | 1.21 | 0.19 (0.14) | 1.40 | 0.162 |
| AGES | 1.10 | 0.09 (0.04) | 2.58 | 0.009 | 1.00 | 0.00 (0.06) | −0.07 | 0.941 |
| AGES × DrugA | -- | −0.18 (0.07) | −2.40 | 0.016 | 1.16 | 0.15 (0.12) | 1.25 | 0.213 |
Note. AGES = Additive Genetic Efficacy Scale. BT = behavioral treatment. FTND = Fagerström Test for Nicotine Dependence (FTND) (45). VNTR = variable number of tandem repeats. Drug = bupropion vs. placebo. SD = standard deviation of effect of study recruitment site. SE = standard error.
AGES × Drug=effects evaluated in models including planned covariates, counseling condition and lower order terms
Individual genes were entered as a block in models without AGES scores.
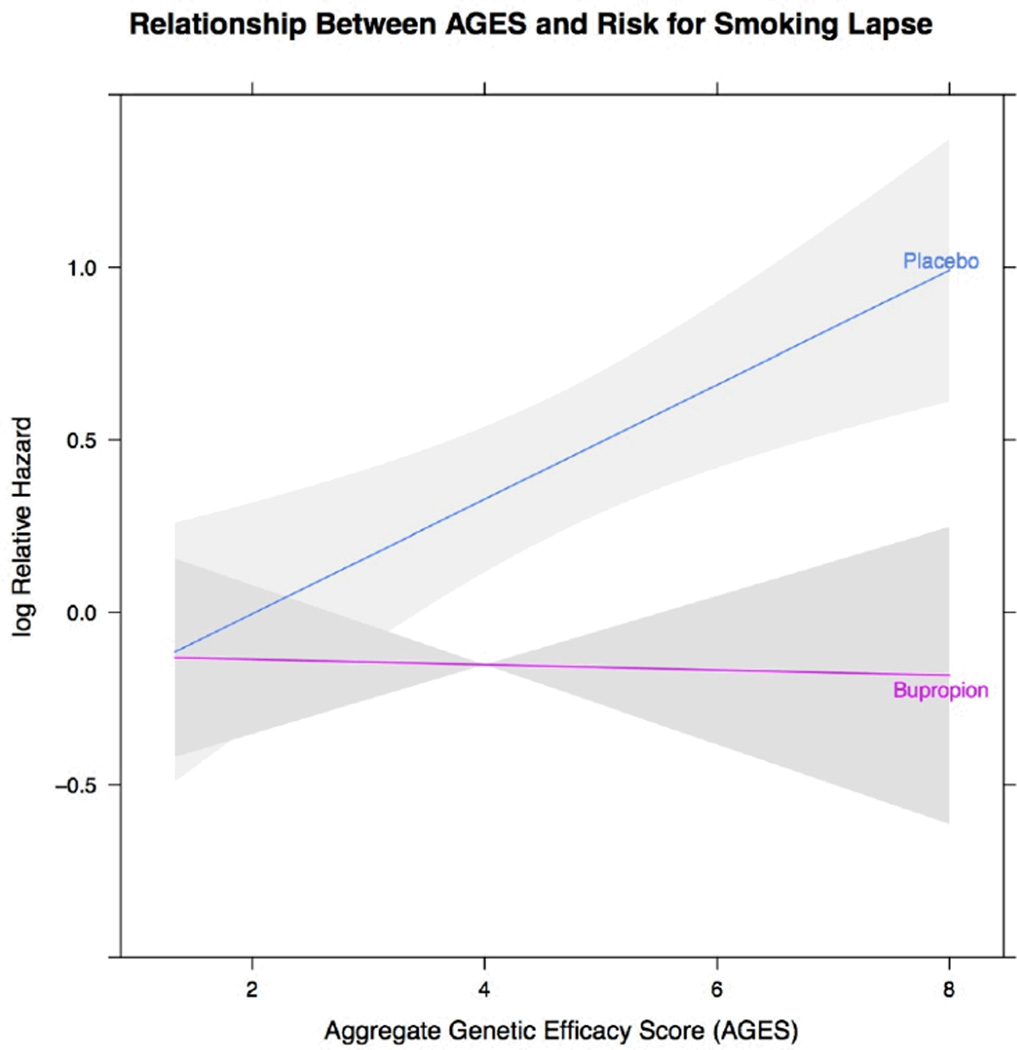
We then evaluated the primary moderation hypothesis, by combining genetic status (AGES) and treatment in an interaction term and examined the relationship with smoking lapse risk and PPA-EOT. The relationship between the interaction term (AGES × drug) and risk for smoking lapse is depicted in Figure 1 along with shaded regions indicating 95% confidence limits. Participants with lower AGES had similar risk of lapse whether they received placebo or active drug treatment. However, as AGES increased, the risk of lapse increased in a linear fashion for those receiving placebo. Those participants with the highest AGES have the highest risk of smoking lapse on placebo when compared to active drug. We did not observe a significant moderating effect of AGES with PPA-EOT (p<0.22). When evaluating the odds of being abstinent at the end of treatment those participants with varying levels of AGES scores had similar odds of abstinence in line with the effects of active or placebo treatment received. We explored potential interaction of AGES with gender and FTND. When added as a set, neither of these interaction terms of AGES with FTND (β=0.001; se=0.02, p<0.97) or gender (b=−0.06; se=0.07; p<0.40) were statistically significant in models evaluating lapse risk. Interaction terms evaluating AGES with FTND (β =−0.02; se=0.03, p<0.50) or gender (β =0.04; se= 0.12; p<0.73) also were not significant in the PPA-EOT model.
Figure 1.
Displays the risk for lapse among smokers receiving bupropion or placebo as AGES scores increase. Grey regions represent 95% confidence limits.
DISCUSSION
Pooled analyses that combined samples from two separate pharmacogenetic trials found that the AGES formula had both a main effect and a moderating effect in altering the efficacy of bupropion (vs. placebo) on time to first lapse. The main effect finding suggests that AGES captures genetic variation that might alter several endophenotypes underpinned by dopaminergic circuitry that promote lapse behavior. Candidate endophenotypes include inhibitory control (54), attentional bias toward smoking stimuli (54), urge to smoke (55), anhedonia (54), diminished positive affect (55), and negative affect (55).
The AGES × drug interaction was significant illustrating that smokers with different AGES scores responded differently to bupropion. As illustrated in Figure 1, AGES increased lapse risk among smokers randomized to placebo relative to those who received bupropion, suggesting that bupropion offsets increased propensity to lapse with higher AGES scores. Other pharmacogenetic investigations from these trials and others (including additional genetic markers combinations) have shown effects of genotype on smoking cessation in placebo groups (43, 56, 57). Bupropion is known to alter reuptake of synaptic dopamine (18). Hence, the significant AGES × drug effect enhances the biological plausibility that AGES might be a genetic marker of lapse risk in untreated smokers attempting to maintain abstinence rather than a proxy for some other non-biological environmentally-mediated process that may impact lapse (e.g., low socioeconomic status) (58).
AGES did not have a significant main effect or interactive effect with drug assignment on PPA measured at EOT. This may indicate that TTFSL reflects a more refined phenotype better suited for identifying genetic effects. Successful cessation depends upon (i) attaining initial abstinence, (ii) maintaining abstinence without a lapse, and (iii) if a lapse occurs, avoiding full blown relapse to pre-cessation levels of smoking (59). Cessation failure can represent a breakdown at any one of these stages, each of which could reflect different underlying causes. Patient characteristics such as psychopathology, gender, and nicotine dependence are differentially predictive with regards to explaining variance in risk of lapse in comparison to other milestones and PPA (60, 61). If genetic factors operate similarly, it is possible that the genetic variation in dopamine as indicated by the AGES might have a notably stronger effect on increasing risk of lapse in comparison to some of the other cessation milestones. A recent study showed that bupropion was specifically effective at offsetting risk of initial lapse (62). At the same time, the fact that AGES did not predict PPA at EOT suggests that other factors are important for explaining the resumption of regular smoking behavior, which is a paramount clinical outcome in smoking cessation.
The strengths of the present investigation include its relevance to clinical translation towards personalized medicine for smoking cessation, the use of an a priori formula based on biological knowledge, rigorous placebo-controlled design and application of a refined cessation phenotype (i.e., days to lapse) that may be more sensitive for detecting treatment effects than composite phenotypes (e.g., point-prevalence abstinence) (59, 62). Moreover, these results add to an emerging literature utilizing formulas designed to aggregate variation in multiple dopamine polymorphisms on various phenotypes (23, 26, 27). Notably, McGeary et al. investigated an AGES that included several of the variants studied here in a placebo-controlled bupropion smoking cessation trial in 90 alcoholics and found no main effects or moderation of treatment efficacy on relapse risk (23). The current sample was larger and afforded more statistical power to highlight the potential utility of AGES in the general population of treatment-seeking smokers.
Limitations of these trials have been described previously (38), and include lack of generalizability to individuals of non-European descent, probable unmeasured genetic heterogeneity not captured by these four variants (63), modest sample size, and minor methodological differences across the two studies such as shorter duration of pharmacological and behavioral treatment, less intensive counseling and lower EOT abstinence rates in Study 2 (10 weeks) vs. Study 1 (12 weeks), as discussed in other publications (38, 64). Fewer people were genotyped for SLC6A3 in Study 1 and participants were not included in AGES unless they had at least 2/4 markers. Hence, there are fewer participants with SCL6A3 represented in AGES in comparison to those with DRD4, which could contribute to differences in EOT-PPA outcomes between the previously published papers involving the two RCTs in this report (38). In addition, because this initial investigation aimed solely to test whether or not the AGES predicts treatment response, we did not analyze the analytic and clinical utility of particular AGES cutoffs that could be applied in clinical settings such as receiver-operator curves and/or comparisons of the predictive value of AGES to validated non-genetic predictors of smoking cessation (e.g., FTND). Another limitation is that the multilocus dopamine genetic score explains a fraction of the phenotypic variances in reward pathway responsivity and smoking cessation and there are many other genetic variants that were not included in the AGES. Several investigations have reported associations with smoking cessation with polymorphisms in cholinergic receptor (e.g., CHRNA5-A3-B4 (14, 56, 57, 65); CHRNB2 (14, 41, 57)) and other genes indirectly related to dopamine neurotransmission (e.g., GALR1 or FREQ) (66, 67) or the metabolism of bupropion (CYP2B6) (36, 37), but these polymorphisms have not – to our knowledge – been replicated in clinical trials of bupropion demonstrating gene × drug interactions. However, dopamine pathway polymorphisms have been the most widely reported pharmacological pathway influencing bupropion efficacy for smoking cessation in retrospective analyses of clinical trials (12, 13, 21). In addition, the AGES formula applied equal weights to each polymorphism as a default, counted efficacy alleles for each polymorphism and assumed linear influence for each additional allele based on an additive model assumption, tallied the additive effects across the variants which does not allow for possible interactive effects across different variants (i.e., epistasis), and was limited in candidate gene selection.
Based on the results of this study and others (23, 26, 27), we anticipate future refinement of AGES for bupropion and other smoking cessation medications to come with advancement of basic knowledge regarding the functional effects of particular genetic variants on biological pathways implicated in smoking cessation and bupropion pharmacology, incorporation of additional genes and more complete variant coverage in relevant biological pathways (12, 67), weighting of each efficacy allele, excluding genes and variants that play little role, and determining whether additive versus dominant models are most appropriate for incorporated loci. We anticipate that this initial AGES study will help lay the groundwork for future efforts aimed to develop and refine AGES formulas for smoking cessation treatment response.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Christopher Jepson (University of Pennsylvania), Faith Allen (UCSF), Ruth Krasnow (SRI International), Denise Nishita (SRI International), Garrett Sullivan (Brown University), Li Su (formerly SRI International) and Yungang He (formerly SRI International), for curation and management of clinical data, for management of biospecimens and for generation and management of genotyping data.
This work was supported by National Institutes of Health grants DA-027331 (SPD), DA-017441 (SPD), DA-014276 (RN), GM-061374 (SPD) and a research support is gratefully acknowledged from the Institute of Medicine and the Bristol University Institute for Advanced Studies Benjamin Meaker Visiting Professorship for SPD; DA-025041 (AML), HL-032318 and CA-084719 (RN); DA-014276 (RAB); CA-063532 and CA-084718 (CL), DA-020830 (AWB, CL, GES, NLB & RFT); MRM is a member of the UK Centre for Tobacco Control Studies, a UKCRC Public Health Research: Centre of Excellence and funding for from the British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, and the National Institute for Health Research, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged.
SPD is a scientific advisor with Genophen and participated in a one-day workshop with Pfizer. MRM has received research support from Pfizer and GlaxoSmithKline. CL has served as a consultant and has received research funding from Astra Zeneca, Glaxo Smith Kline and Pfizer. RFT has participated in one-day workshops with Novartis and McNeil.
Footnotes
Declarations of interest:
The other authors have nothing to disclose.
REFERENCES
- 1.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 2.Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007:CD000031. doi: 10.1002/14651858.CD000031.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;4:CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD000146. doi: 10.1002/14651858.CD000146.pub4. [DOI] [PubMed] [Google Scholar]
- 5.Stead LF, Lancaster T. Behavioural interventions as adjuncts to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev. 2012;12:CD009670. doi: 10.1002/14651858.CD009670.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Carmelli D, Swan GE, Robinette D, Fabsitz R. Genetic influence on smoking--a study of male twins. N Engl J Med. 1992;327:829–833. doi: 10.1056/NEJM199209173271201. [DOI] [PubMed] [Google Scholar]
- 7.Heath AC, Martin NG, Lynskey MT, Todorov AA, Madden PA. Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Res. 2002;5:113–124. doi: 10.1375/1369052022983. [DOI] [PubMed] [Google Scholar]
- 8.Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 9.Broms U, Silventoinen K, Madden PA, Heath AC, Kaprio J. Genetic architecture of smoking behavior: a study of Finnish adult twins. Twin Res Hum Genet. 2006;9:64–72. doi: 10.1375/183242706776403046. [DOI] [PubMed] [Google Scholar]
- 10.True WR, Heath AC, Scherrer JF, et al. Genetic and environmental contributions to smoking. Addiction. 1997;92:1277–1287. [PubMed] [Google Scholar]
- 11.McCaffery JM, Papandonatos GD, Lyons MJ, et al. Educational attainment, smoking initiation and lifetime nicotine dependence among male Vietnam-era twins. Psychol Med. 2008;38:1287–1297. doi: 10.1017/S0033291707001882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold AB, Lerman C. Pharmacogenetics of smoking cessation: role of nicotine target and metabolism genes. Hum Genet. 2012 doi: 10.1007/s00439-012-1143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.David SP, Munafò MR. Genetic variation in the dopamine pathway and smoking cessation. Pharmacogenomics. 2008;9:1307–1321. doi: 10.2217/14622416.9.9.1307. [DOI] [PubMed] [Google Scholar]
- 14.King DP, Paciga S, Pickering E, et al. Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo-controlled clinical trials. Neuropsychopharmacology. 2011;37:641–650. doi: 10.1038/npp.2011.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kortmann GL, Dobler CJ, Bizarro L, Bau CH. Pharmacogenetics of smoking cessation therapy. Am J Med Genet B Neuropsychiatr Genet. 2009;153B:17–28. doi: 10.1002/ajmg.b.30978. [DOI] [PubMed] [Google Scholar]
- 16.Ray R, Tyndale RF, Lerman C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J Neurogenet. 2009;23:252–261. doi: 10.1080/01677060802572887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fryer JD, Lukas RJ. Noncompetitive functional inhibition at diverse, human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine, and ibogaine. J Pharmacol Exp Ther. 1999;288:88–92. [PubMed] [Google Scholar]
- 18.Ascher JA, Cole JO, Colin JN, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995;56:395–401. [PubMed] [Google Scholar]
- 19.Faucette SR, Hawke RL, Lecluyse EL, et al. Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab Dispos. 2000;28:1222–1230. [PubMed] [Google Scholar]
- 20.Zhu AZ, Cox LS, Nollen N, et al. CYP2B6 and Bupropion's Smoking-Cessation Pharmacology: The Role of Hydroxybupropion. Clin Pharmacol Ther. 2012;92:771–777. doi: 10.1038/clpt.2012.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kortmann GL, Dobler CJ, Bizarro L, Bau CH. Pharmacogenetics of smoking cessation therapy. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:17–28. doi: 10.1002/ajmg.b.30978. [DOI] [PubMed] [Google Scholar]
- 22.Lotsch J, Fluhr K, Neddermayer T, Doehring A, Geisslinger G. The consequence of concomitantly present functional genetic variants for the identification of functional genotype-phenotype associations in pain. Clin Pharmacol Ther. 2009;85:25–30. doi: 10.1038/clpt.2008.103. [DOI] [PubMed] [Google Scholar]
- 23.McGeary JE, Knopik VS, Hayes JE, et al. Predictors of relapse in a bupropion trial for smoking cessation in recently-abstinent alcoholics: preliminary results using an aggregate genetic risk score. Subst Abuse. 2012;6:107–114. doi: 10.4137/SART.S8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Li MD. Common and unique biological pathways associated with smoking initiation/progression, nicotine dependence, and smoking cessation. Neuropsychopharmacology. 2010;35:702–719. doi: 10.1038/npp.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Culbertson CS, Bramen J, Cohen MS, et al. Effect of bupropion treatment on brain activation induced by cigarette-related cues in smokers. Arch Gen Psychiatry. 2011;68:505–515. doi: 10.1001/archgenpsychiatry.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology. 2011;36:1940–1947. doi: 10.1038/npp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stice E, Yokum S, Burger K, Epstein L, Smolen A. Multilocus genetic composite reflecting dopamine signaling capacity predicts reward circuitry responsivity. J Neurosci. 2012;32:10093–10100. doi: 10.1523/JNEUROSCI.1506-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uhl GR, Drgon T, Johnson C, et al. Genome-wide association for smoking cessation success: participants in the Patch in Practice trial of nicotine replacement. Pharmacogenomics. 2010;11:357–367. doi: 10.2217/pgs.09.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uhl GR, Liu QR, Drgon T, et al. Molecular genetics of successful smoking cessation: convergent genome-wide association study results. Arch Gen Psychiatry. 2008;65:683–693. doi: 10.1001/archpsyc.65.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uhl GR, Drgon T, Johnson C, et al. Genome wide association for smoking cessation success in a trial of precessation nicotine replacement. Mol Med. 2010 doi: 10.2119/molmed.2010.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uhl GR, Walther D, Musci R, et al. Smoking quit success genotype score predicts quit success and distinct patterns of developmental involvement with common addictive substances. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.155. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drgon T, Johnson C, Walther D, et al. Genome-wide association for smoking cessation success: participants in a trial with adjunctive denicotinized cigarettes. Mol Med. 2009;15:268–274. doi: 10.2119/molmed.2009.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uhl GR, Drgon T, Johnson C, et al. Genome-wide association for smoking cessation success: participants in the Patch in Practice trial of nicotine replacement. Pharmacogenomics. 2010;11:357–367. doi: 10.2217/pgs.09.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes JR, Keely JP, Niaura RS, et al. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 35.Brown RA, Niaura R, Lloyd-Richardson EE, et al. Bupropion and cognitive-behavioral treatment for depression in smoking cessation. Nicotine Tob Res. 2007;9:721–730. doi: 10.1080/14622200701416955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.David SP, Brown RA, Papandonatos GD, et al. Pharmacogenetic clinical trial of sustained-release bupropion for smoking cessation. Nicotine Tob Res. 2007;9:821–833. doi: 10.1080/14622200701382033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lerman C, Shields PG, Wileyto EP, et al. Pharmacogenetic investigation of smoking cessation treatment. Pharmacogenetics. 2002;12:627–634. doi: 10.1097/00008571-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 38.David SP, Strong DR, Munafò MR, et al. Bupropion efficacy for smoking cessation is influenced by the DRD2 Taq1A polymorphism: analysis of pooled data from two clinical trials. Nicotine Tob Res. 2007;9:1251–1257. doi: 10.1080/14622200701705027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 40.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 41.Conti DV, Lee W, Li D, et al. Nicotinic acetylcholine receptor beta2 subunit gene implicated in a systems-based candidate gene study of smoking cessation. Hum Mol Genet. 2008;17:2834–2848. doi: 10.1093/hmg/ddn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lerman C, Shields PG, Wileyto EP, et al. Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial. Health Psychol. 2003;22:541–548. doi: 10.1037/0278-6133.22.5.541. [DOI] [PubMed] [Google Scholar]
- 43.Leventhal AM, David SP, Brightman M, et al. Dopamine D4 receptor gene variation moderates the efficacy of bupropion for smoking cessation. Pharmacogenomics J. 2012;12:86–92. doi: 10.1038/tpj.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bergen AW, Javitz HS, Su L, et al. The DRD4 Exon III VNTR, bupropion, and associations with prospective abstinence. Nicotine Tob Res. 2012 doi: 10.1093/ntr/nts245. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.David SP, Johnstone E, Griffiths SE, et al. No association between functional catechol O-methyl transferase 1947A>G polymorphism and smoking initiation, persistent smoking or smoking cessation. Pharmacogenetics. 2002;12:265–268. doi: 10.1097/00008571-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Leventhal AM, David SP, Brightman M, et al. Dopamine D4 receptor gene variation moderates the efficacy of bupropion for smoking cessation. Pharmacogenomics J. 2010 doi: 10.1038/tpj.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.David SP, Brown RA, Papandonatos GD, et al. Pharmacogenetic clinical trial of sustained-release bupropion for smoking cessation. Nicotine Tob Res. 2007;9:821–833. doi: 10.1080/14622200701382033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berrettini WH, Wileyto EP, Epstein L, et al. Catechol-O-methyltransferase (COMT) gene variants predict response to bupropion therapy for tobacco dependence. Biological Psychiatry. 2007;61:111–118. doi: 10.1016/j.biopsych.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 49.Vandenbergh DJ, Rodriguez LA, Hivert E, et al. Long forms of the dopamine receptor (DRD4) gene VNTR are more prevalent in substance abusers: no interaction with functional alleles of the catechol-o-methyltransferase (COMT) gene. Am J Med Genet. 2000;96:678–683. doi: 10.1002/1096-8628(20001009)96:5<678::aid-ajmg15>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 50.George SR, Cheng R, Nguyen T, Israel Y, O'Dowd BF. Polymorphisms of the D4 dopamine receptor alleles in chronic alcoholism. Biochem Biophys Res Commun. 1993;196:107–114. doi: 10.1006/bbrc.1993.2222. [DOI] [PubMed] [Google Scholar]
- 51.Strong DR, Therneau T. coxme: Mixed Effects Cox Models. R package version 2.2-3. 2012 [Google Scholar]
- 52.Strong DR, Harrell FE. Regression Modeling Strategies. R package version 3.6-2. 2012 [Google Scholar]
- 53.Strong DR, Bates D, Maechler M, Bolker B. lme4: Linear mixed-effects models using S4 classes. R package. 2012 [Google Scholar]
- 54.Powell J, Dawkins L, West R, Pickering A. Relapse to smoking during unaided cessation: clinical, cognitive and motivational predictors. Psychopharmacology (Berl) 2010;212:537–549. doi: 10.1007/s00213-010-1975-8. [DOI] [PubMed] [Google Scholar]
- 55.Strong DR, Kahler CW, Leventhal AM, et al. Impact of bupropion and cognitive-behavioral treatment for depression on positive affect, negative affect, and urges to smoke during cessation treatment. Nicotine Tob Res. 2009;11:1142–1153. doi: 10.1093/ntr/ntp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen LS, Baker TB, Piper ME, et al. Interplay of Genetic Risk Factors (CHRNA5-CHRNA3-CHRNB4) and Cessation Treatments in Smoking Cessation Success. Am J Psychiatry. 2012 doi: 10.1176/appi.ajp.2012.11101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bergen AW, Javitz HS, Krasnow R, et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenet Genomics. 2013;23:94–103. doi: 10.1097/FPC.0b013e32835cdabd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Businelle MS, Kendzor DE, Reitzel LR, et al. Mechanisms linking socioeconomic status to smoking cessation: a structural equation modeling approach. Health Psychol. 2010;29:262–273. doi: 10.1037/a0019285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shiffman S, Scharf DM, Shadel WG, et al. Analyzing milestones in smoking cessation: illustration in a nicotine patch trial in adult smokers. J Consult Clin Psychol. 2006;74:276–285. doi: 10.1037/0022-006X.74.2.276. [DOI] [PubMed] [Google Scholar]
- 60.Leventhal AM, Japuntich SJ, Piper ME, et al. Isolating the role of psychological dysfunction in smoking cessation: relations of personality and psychopathology to attaining cessation milestones. Psychol Addict Behav. 2012;26:838–849. doi: 10.1037/a0028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Japuntich SJ, Leventhal AM, Piper ME, et al. Smoker characteristics and smoking-cessation milestones. Am J Prev Med. 2011;40:286–294. doi: 10.1016/j.amepre.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Japuntich SJ, Piper ME, Leventhal AM, Bolt DM, BAKER TB. The effect of five smoking cessation pharmacotherapies on smoking cessation milestones. J Consult Clin Psychol. 2011;79:34–42. doi: 10.1037/a0022154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bergen AW, Javitz HS, Su L, et al. The DRD4 Exon III VNTR, Bupropion, and Associations With Prospective Abstinence. Nicotine Tob Res. 2012;15:1190–1200. doi: 10.1093/ntr/nts245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sarginson JE, Killen JD, Lazzeroni LC, et al. Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5-A3-B4) predict severity of nicotine addiction and response to smoking cessation therapy. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:275–284. doi: 10.1002/ajmg.b.31155. [DOI] [PubMed] [Google Scholar]
- 66.Dahl JP, Jepson C, Levenson R, et al. Interaction between variation in the D2 dopamine receptor (DRD2) and the neuronal calcium sensor-1 (FREQ) genes in predicting response to nicotine replacement therapy for tobacco dependence. Pharmacogenomics J. 2006;6:194–199. doi: 10.1038/sj.tpj.6500358. [DOI] [PubMed] [Google Scholar]
- 67.Gold AB, Wileyto EP, Lori A, et al. Pharmacogenetic association of the galanin receptor (GALR1) SNP rs2717162 with smoking cessation. Neuropsychopharmacology. 2012;37:1683–1688. doi: 10.1038/npp.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.