Abstract
Background
Few studies have examined the acute effects of autologous hematopoietic stem cell transplant (Au-HSCT) on the neuropsychological functioning of patients with multiple myeloma (MM). We examined the prevalence of cognitive deficits after induction chemotherapy (pre-AuHSCT) in patients with MM, determined clinically significant changes in cognitive function 1 and 3 months post-AuHSCT, and identified patients who may be vulnerable to cognitive decline during this period.
Methods
53 patients with MM were recruited pre-AuHSCT. Neuropsychological tests measuring multiple cognitive domains (attention, psychomotor speed, learning/memory, language, executive function, motor function) were administered pre-AuHSCT and 1 and 3 months post-AuHSCT. A pretreatment assessment was not available. An Overall Cognitive Function Index (OCFI) was computed to determine cognitive impairment pre-AuHSCT, and a practice-effect-adjusted Reliable Change Index was used to determine cognitive change over time.
Results
Overall, deficits were more frequent in learning/memory, executive function, motor function, and psychomotor speed. Pre-AuHSCT, 47% of patients (25/53) exhibited cognitive impairment based on the OCFI. One month post-AuHSCT, 49% (20/41) demonstrated clinically significant decline on ≥1 measures; 3 months post-AuHSCT, 48% (14/29) showed decline on ≥1 measures. Older patients, minorities, those with advanced disease, more induction cycles, and postinduction deficits showed greater vulnerability to decline.
Conclusions
Nearly half of the patients showed vulnerability to impairment in learning/memory or executive function after receiving induction therapy, and the prevalence of impairment remained high post-AuHSCT. Awareness of cognitive impairment and associated risk factors in actively treated patients is important for considering psychosocial or other support for patients with acute cognitive symptoms.
Keywords: cognitive impairment, MDASI, autologous transplant
INTRODUCTION
The introduction of new treatment agents and autologous hematopoietic stem cell transplantation (AuHSCT) over the past decade has resulted in increased survival for patients with multiple myeloma (MM).1,2 However, patients are faced with significant symptom burden resulting from their disease and from treatment-related toxicities.3,4 Cognitive dysfunction induced by cancer or aggressive cancer therapy can be a persistent survivorship issue for patients because of its potential to affect daily-functioning, occupational, and social activities.
A limited number of studies have investigated neuropsychological functioning in bone marrow transplant recipients. Few have had samples that included patients with MM,5–7 and most were studies of patients who underwent allogeneic transplant.6–9 Additionally, some qualitative studies have reported cognitive impairment associated with either disease or treatment in MM patients and its consequent interference in patients’ personal and professional lives.10,11 Some of these studies were limited by cross-sectional designs8,9 and relatively small sample sizes.5,8–11 To our knowledge, few studies have examined neuropsychological function in samples of patients with a variety of hematological malignancies undergoing either autologous or allogeneic HSCT.6,7,12,13 Although chronic effects of transplantation were assessed in these studies, acute effects in the critical posttransplant period (particularly at 1 and 3 months) have not previously been examined in a homogenous sample of MM patients undergoing AuHSCT.
Results from an objective evaluation of cognitive function at these time points and the identification of factors that may contribute to cognitive decline have important implications for patient and clinician expectations post-AuHSCT. Correlations between patient performance on objective neuropsychological tests and patient self-evaluation of cognitive function have been reported, but largely in breast cancer. Concordance or discordance between these outcomes after induction chemotherapy (ie, pre-AuHSCT) and longitudinally (ie, post-AuHSCT) has not been investigated in patients with MM.
In light of the above, our objectives were to: 1) report the incidence of postinduction cognitive deficits in patients with MM, 2) present clinically significant changes in cognitive function 1 month and 3 months post-AuHSCT; 3) identify subgroups of patients who may be vulnerable to cognitive dysfunction by examining potential predictors of cognitive decline before and after AuHSCT, and 4) present an evaluation of patients’ objective performance on neuropsychological tests compared with their self-appraisal of cognitive function before and after AuHSCT.
METHODS
Study Site and Participants
Patients were recruited from The University of Texas MD Anderson Cancer Center, Houston, Texas between 2008 and 2011. Eligible patients had a confirmed diagnosis of MM, had received induction therapy and were approved to receive AuHSCT, were ≥18 years old, could speak and understand English, and were able to give informed consent. Patients unable to use a telephone-based Interactive Voice Response (IVR) system were excluded. Written informed consent was obtained from all patients and the study was approved by the MD Anderson Institutional Review Board.
Measures
Neuropsychological assessment
A battery of neuropsychological tests designed to measure multiple cognitive domains (attention, psychomotor speed, learning/memory, language, executive function, and motor function) was administered. These tests are widely used, standardized psychometric instruments that have demonstrated sensitivity to the effects of cancer treatment. All participants completed a neurocognitive assessment pre-AuHSCT and 1 and 3 months post-AuHSCT.
Beck Depression Inventory-II
To examine the effect of depressed mood on cognitive function, we assessed patients’ depressive symptoms using the Beck Depression Inventory-II (BDI-II), a widely used 21-item instrument for measuring the intensity of depressive symptoms.14 Each item is rated on a 0–3 numeric scale, resulting in a maximum attainable score of 63. A higher total score indicates more depressive symptoms. Clinically significant depressed mood was operationally defined as a BDI-II score ≥14.14 Depressive symptoms were assessed at the same time as the neurocognitive tests.
M. D. Anderson Symptom Inventory multiple myeloma module
Patients’ self-report of cognitive function was obtained from responses to 2 questions on the M. D. Anderson Symptom Inventory multiple myeloma module (MDASI-MM)15: “Your problem with remembering things at its worst?” and “Your problem with paying attention (concentrating) at its worst?” On a 0–10 scale (0 = not present, 10 = the worst severity imaginable), the MDASI-MM measures the severity of patients’ physical, affective, and cognitive symptoms and the functional interference caused by symptoms.16 MDASI-MM assessments were collected in person by research staff twice a week from pre-AuHSCT until 30 days post-AuHSCT, then weekly up to 12 months using an IVR system.
Statistical Analysis
To facilitate comparisons among measures, we converted raw cognitive test scores to standardized scores (z-scores; mean = 0, SD = 1) using published normative data17–21 that adjusts for age, education, handedness, and sex (where appropriate). An index for each patient’s pre-AuHSCT (postinduction) overall cognitive function (OCFI) was operationally defined as impaired (OCFI-Impaired, patients with z-scores ≤ −1.5 on 2 or more tests, or ≤ −2.0 on a single test) or not impaired (OCFI-Not Impaired, all other patients).22 This dichotomization was designed to minimize the number of potential false-positive errors resulting from multiple tests and to determine frequency of impairment rather than low performance.
Descriptive statistics were generated for predictor variables. Linear regression modeling was used to examine predictors of postinduction cognitive impairment. Candidate variables included age, education, sex, minority status (race), comorbidities, depressed mood, disease stage, and number of induction therapy cycles pre-AuHSCT). For each cognitive outcome, a final model of main effects was obtained using backwards stepwise regression and a significance criterion of P < .05 for variable retention. Correlations between patients’ objective neuropsychological test scores and self-appraisal scores were examined.
To determine clinically and statistically meaningful change in cognitive function at 1 month post-AuHSCT compared with pre-AuHSCT, and at 3 months post-AuHSCT compared with 1 month post-AuHSCT, we performed longitudinal analysis using a practice-effect–adjusted23 Reliable Change Index (RCI-PE).24 The published studies from which the RCI-PE was determined for each test are listed in Table 2. A backwards stepwise linear regression was conducted to determine predictors of cognitive decline at 1 and 3 months post-AuHSCT relative to previous performance. Examined variables were the same as those at pre-AuHSCT, with the addition of pre-AuHSCT deficits. A final model of main effects was obtained for each cognitive outcome using stepwise selection and a significance criterion of P < .05 for variable retention.
Table 2.
Mean Score and Impairment Frequency Pre-AuHSCT on Each Neuropsychological Test Grouped by Principal Cognitive Domain (N = 53)
| Cognitive Domain | Test | Abbreviation | RCI Study | Mean (SDa) | Impairedb (%) |
|---|---|---|---|---|---|
|
| |||||
| Attention | WAIS-III Digit Span | WAIS-III DSpan33 | Wechsler, 198133 | ||
| WAIS-III Digit Span forward | 0.45 (0.98) | 1.9 | |||
| WAIS-III Digit Span backward | 0.35 (0.99) | 0.0 | |||
|
| |||||
| Psychomotor speed | WAIS-III Digit Symbol | WAIS-III Symbol33 | Wechsler, 198133 | 0.34 (0.92) | 1.9 |
| Trail Making Test Part A | TMT-A34 | Levine, 200435 | 0.38 (1.29) | 11.3 | |
|
| |||||
| Learning/ memory | HVLT-R Total Recall | HVLT-R36 | Benedict, 199817 | −0.45 (1.12) | 18.9 |
| HVLT-R Delayed Recall | −0.36 (1.43) | 16.9 | |||
| HVLT-R Delayed Recognition | −0.29 (1.16) | 11.3 | |||
|
| |||||
| Executive function | Trail Making Test Part B | TMT-B34 | Levine, 200435 | −0.71 (3.39) | 28.3 |
| MAE Controlled Oral Word Association | COWA37 | Ruff, 199619 | −0.51 (1.15) | 20.8 | |
|
| |||||
| Motor | GPB-D (dominant hand) | GPB-D38 | Dikmen, 1999 | −0.61 (0.80) | 1.9 |
| GPB-ND (nondominant hand) | GPB-ND38 | −0.75 (0.65) | 0.0 | ||
Abbreviations: AuHSCT, autologous hematopoietic stem cell transplant; RCI, Reliable Change Index; SD, standard deviation from the mean; WAIS-III, Wechsler Adult Intelligence Scale-Third Edition; HVLT-R, Hopkins Verbal Learning Test-Revised; MAE, Multilingual Aphasia Examination; COWA: GPB, Grooved Pegboard
z-scores (mean of 0, SD of 1).
Percentage of patients with a z-score ≤ −2.0 for this test. Overall, 25/53 (47.2%) of patients were cognitively impaired per the OCFI.
We sought to determine if objective decline in patients’ cognitive performance (assessed by the RCI-PE) was related to subjective changes in cognitive function (assessed by the minimally important difference [MID] criterion) between pre-AuHSCT and 1 month post-AuHSCT and between 1 and 3 months post-AuHSCT. MDASI-MM responses were taken from the responses provided on the same day as cognitive testing at the pre-AuHSCT, 1 month post-AuHSCT, and 3 months post-AuHSCT time points. Subjective cognitive decline on the MDASI-MM items “difficulty remembering” and “difficulty paying attention” was categorized based on the MID25 requiring a 2-point or greater increase in symptom severity. Chi-square analysis was conducted between the objective tests and the subjective cognitive function items.
All analyses were conducted using SPSS (version 19 for Windows, Chicago, IL).
RESULTS
Fifty-three patients were enrolled and underwent neuropsychological evaluation pre-AuHSCT. Demographic and clinical characteristics are shown in Table 1. Patients’ mean age was 58 years; most were white non-Hispanic, had at least a high-school education, and were not working. More than half had received 3–4 cycles of induction therapy prior to AuHSCT.
Table 1.
Demographic and Clinical Characteristics of Patients (N = 53)
| Mean age at time of assessment, years (SD) | 57.8 (8.2) |
|---|---|
| n (%) | |
| Male | 33 (62.3) |
| Married | 39 (73.6) |
| Race | |
| White non-Hispanic | 42 (79.3) |
| Other | 11 (20.7) |
| Employment status | |
| Working (full or part time) | 17 (32.1) |
| Retired | 12 (22.6) |
| Other | 24 (45.3) |
| Years of education | |
| ≤12 | 9 (16.9) |
| 13–16 | 31 (58.5) |
| ≥17 | 13 (24.5) |
| Charlson comorbidity score | |
| 0 | 37 (69.8) |
| 1 | 10(18.9) |
| 2 | 4(7.6) |
| 3 | 2(3.8) |
| Most-prevalent comorbid conditions | |
| Diabetes | 7 (13.2) |
| Renal disease | 4 (7.6) |
| Connective-tissue disease | 4 (7.6) |
| Myocardial infarction | 4 (7.6) |
| Disease stage | |
| 1 | 30 (56.6) |
| 2 | 13 (24.5) |
| 3 | 10 (18.9) |
| Number of induction cycles | |
| 1–2 | 11 (22.9) |
| 3–4 | 28 (58.3) |
| ≥5 | 9 (18.7) |
| Induction regimen | |
| Bortezomib-based | 46 (86.8) |
| Other | 7 (13.2) |
Pre-AuHSCT Impairment and Correlations between Objective and Subjective Measures
Pre-AuHSCT, overall cognitive functioning for 47.2% (25/53) of patients was impaired (OCFI-Impaired). Patients’ mean scores and the impairment frequency for each test are shown in Table 2. Patients exhibited deficits more frequently in learning/memory and executive function relative to normal expectations.
Results from stepwise regression indicated that older patients were more likely to exhibit impairment in psychomotor speed (TMT-A) (P = .006; Table 3). Minority patients exhibited higher impairment in attention (WAIS-III DSpan) and learning/memory (HVLT-R Total Recall, HVLT-R Delayed Recall, and HVLT-R Delayed Recognition) (all P < .05). Sex, years of education, depressed mood, comorbidities, disease stage, and number of induction therapy cycles did not emerge as significant predictors of cognitive performance.
TABLE 3.
Significant Predictors of Cognitive Deficit Pre-AuHSCT for Neuropsychological Tests (N= 53)
| Testa | Adjusted R2 | Race (White Non- Hispanic vs. Other) | Age | ||
|---|---|---|---|---|---|
| Beta | P | Beta | P | ||
| WAIS-III Digit Span | 0.083 | 0.806 | 0.029 | ||
| TMT-A | 0.141 | −0.069 | 0.006 | ||
| HVLT-R Total Recall | 0.162 | 1.144 | 0.003 | ||
| HVLT-R Delayed Recall | 0.076 | 1.072 | 0.035 | ||
| HVLT-R Delayed Recognition | 0.089 | 0.831 | 0.025 | ||
Abbreviations: AuHSCT, autologous hematopoietic stem cell transplant; WAIS-III, Wechsler Adult Intelligence Scale-Third Edition; TMT, Trail Making Test; HVLT-R, Hopkins Verbal Learning Test-Revised; COWA, Controlled Oral Word Association; GPB, Grooved Pegboard
No model emerged for the following tests: WAIS-III Digit Symbol, TMT-B, COWA, GPB-D (dominant hand), and GPB-ND (nondominant hand).
Pearson correlations were significant between patients’ self-appraisal on the MDASI-MM “difficulty remembering” item and objective tests in the learning/memory domain (r = −0.32 for HVLT-R Total Recall, P = .018, and r = −0.44 for HVLT-R Delayed Recognition, P = .001) and in the attention and psychomotor speed domains (r = −0.33 for WAIS-III DSpan, P = .014; r = −0.34 for WAIS-III DSymbol, P = .013). Correlations were also significant between the MDASI-MM “difficulty paying attention” item and objective tests in the attention and learning/memory domains (r = −0.35 for WAIS-III DSpan, P = .009; r = −0.32 for HVLT-R Total Recall, P = .018; r = −0.30 for HVLT-R Delayed Recognition, P = .027) and for 1 test in the motor domain (r = −0.26 for GPB-ND, P = .057).
Post-AuHSCT Longitudinal Analysis of Cognitive Deficits
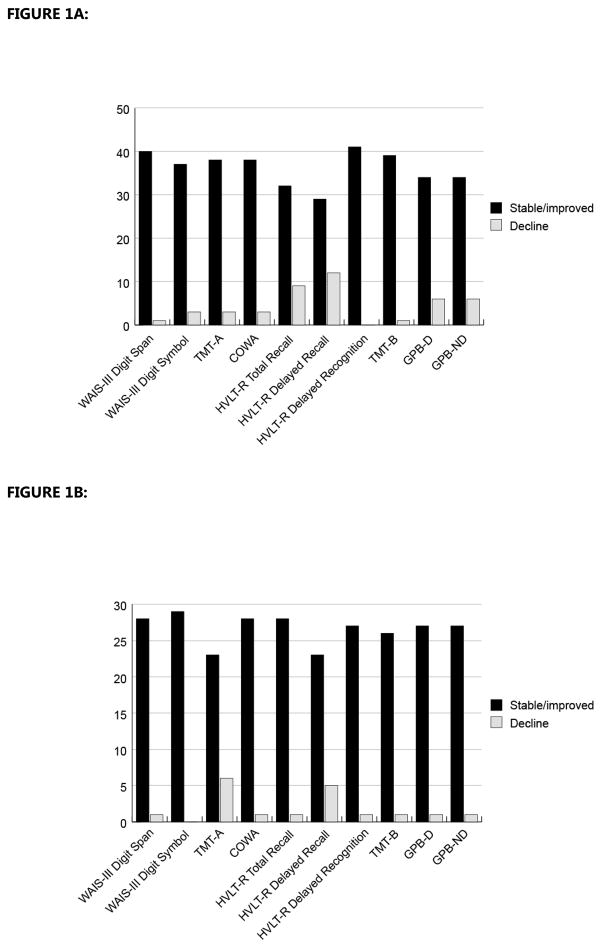
Data was available for 41 patients 1 month post-AuHSCT and for 29 patients 3 months post-AuHSCT. Attrition of the remaining patients was not due to death or known disability, but due to these patients being lost to follow-up (did not return calls), their request not to participate, or their not completing the cognitive assessments at these time points. Patients did not provide specific reasons for non-compliance. At 1 month post-AuHSCT, 48.8% of patients (20/41) demonstrated clinically significant decline based on the RCI-PE from their postinduction performance on 1 or more measure: 19.5% (8/41) showed decline on 1 measure, 9.8% (4/41) on 2 measures, and 19.5% (8/41) on 3 or more measures. These declines were primarily observed in the learning/memory (22.0% for HVLT-R Total Recall; 29.3% for HVLT-R Delayed Recall) and motor-function domains (15.0% for GPB-D; 15.0% for GPB-ND) (Figure 1A).
FIGURE 1.
FIGURE 1A. Clinically Significant Changes in Cognitive Function from pre-AuHSCT (N = 53) to 1 Month Post-AuHSCT (N = 41), based on the RCI-PE
FIGURE 1B. Clinically Significant Changes in Cognitive Function from 1 Month (N = 41) to 3 Months Post-AuHSCT (N = 29), based on the RCI-PE
Abbreviations: AuHSCT, autologous hematopoietic stem cell transplant; RCI-PE, practice-effect–adjusted Reliable Change Index; WAIS-III, Wechsler Adult Intelligence Scale-Third Edition; TMT, Trail Making Test; HVLT-R, Hopkins Verbal Learning Test-Revised; COWA, Controlled Oral Word Association; GPB-D, Grooved Pegboard, dominant hand; GPB-ND, Grooved Pegboard, nondominant hand.
Results from stepwise regression 1 month post-AuHSCT indicated that patients’ pre-AuHSCT deficits were the strongest predictor of cognitive decline at this time point, particularly in the learning/memory, psychomotor speed, and executive-function domains (HVLT-R Delayed Recall, P = .005; WAIS-III DSymbol, P = .003; COWA, P <.001; TMT-B, P < .001). Additionally, minority status was a predictor of decline for 1 test in the learning/memory domain (HVLT-R Total Recall, P = .021) as well as in the psychomotor speed domain (WAIS-III DSymbol, P = .015; TMT-A, P = .028). Education also was a predictor of decline in the learning/memory and psychomotor-speed domains (WAIS-III DSymbol, P = .001; HVLT-R Total Recall, P = .030). Among the clinical variables examined, number of cycles of induction therapy was associated with COWA score (P = .020), advanced disease stage with GPB-D score (P = .011), and Charlson comorbidity score with TMT-B score (P = .014).
At the 3-month follow-up post-AuHSCT, approximately 48% of patients (14/29) showed clinically significant decline on 1 or more measures relative to their performance 1 month post-AuHSCT: 31.0% (9/29) showed decline on 1 measure, 13.8% (4/29) on 2 measures, and 3.5% (1/29) on 3 measures. Of those who demonstrated decline 1 month post-AuHSCT, 50% (8/16) demonstrated decline again 3 months post-AuHSCT. Of those who did not demonstrate decline 1 month post-AuHSCT, 46% (6/13) demonstrated decline between 1 and 3 months post-AuHSCT. This decline was primarily observed in learning/memory (17.9% for HVLT-R Delayed Recall) and psychomotor speed (20.7% for TMT-A) (Figure 1B).
Variables similar to those at 1 month were associated with cognitive decline 3 months post-AuHSCT. Pre-AuHSCT cognitive deficits continued to be associated with decline in COWA (P < .001) and TMT-B (P < .001); education continued to be associated with WAIS-III DSymbol (P = .008), and number of induction cycles continued to be associated with COWA (P = .004). Age emerged as a significant predictor of declining performance in psychomotor-speed and motor domains (TMT-A, P = .042; GPB-D, P = .043). Additionally, disease stage emerged as a predictor of COWA score (P = .001) and HVLT-R Total Recall score (P =.027).
The presence of pre-AuHSCT cognitive deficits did not significantly influence patients’ likelihood to return for their 1-month post-AuHSCT follow-up; of the 12 patients who did not return at 1 month, 4 had exhibited cognitive impairment pre-AuHSCT and 8 had not (Χ2=1.192, P = .275). Similarly, of the 12 patients who did not return for their 3 month post-AuHSCT follow-up, 4 had demonstrated clinically significant decline 1 month post-AuHSCT and 8 had not (Χ2=1.620, P = .203).
Association between Change in Cognitive Performance and Patients’ Self-Appraisal of Change in Cognitive Function
Patients who showed an objective decline in the learning/memory domain between pre-AuHSCT and 1 month post-AuHSCT were more likely to report increased difficulty with memory (HVLT-R Total Recall, Χ2 = 7.281, P = .007; HVLT-R Delayed Recall, Χ2 = 10.712, P = .001) and attention (HVLT-R Delayed Recall, Χ2 = 7.791, P = .005) on the MDASI-MM compared with patients who did not show decline on these measures. Additionally, deficits in motor function 1 month post-AuHSCT were associated with reports of increased difficulty with memory (GPB-D, Χ2 = 4.270, P = .039; GPB-ND, Χ2 = 4.270, P = .039). No additional statistically significant associations were found between objective cognitive function and subjective cognitive ratings 1 or 3 months post-AuHSCT.
DISCUSSION
This study evaluated the acute effects of induction chemotherapy and AuHSCT on the cognition of patients with MM. According to the OCFI classification criteria (OCFI-Impaired, patients with z-scores ≤ −1.5 on 2 or more tests, or ≤−2.0 on a single test; OCFI-Not Impaired, all other patients22), 47.2% of our MM patient sample exhibited cognitive impairment postinduction (pre-AuHSCT). Impairment continued to be high over time, as nearly 49% of patients at 1 month and 48% at 3 months post-AuHSCT exhibited deficits on 1 or more measures. Learning/memory showed the greatest vulnerability to impairment at all 3 time points; executive function showed greater vulnerability at the pre-AuHSCT time point, motor function at 1 month post-AuHSCT, and psychomotor speed at 3 months post-AuHSCT. The presence of pre-AuHSCT deficits after induction chemotherapy, especially in learning/memory and executive function, are consistent with results from previous studies.12,13
Chi-square analyses conducted 1 and 3 months post-AuHSCT indicated that attrition of approximately 23% of patients at 1 month and 29% at 3 months was not significantly influenced by prior cognitive impairment on any test. Of patients who showed reliable decline in cognitive performance 1 month post-AuHSCT (relative to their pre-AuHSCT performance), 50% showed decline again at 3 months; of those who did not, 46% showed decline at 3 months. Importantly, patients who showed decline at 3 months post-AuHSCT did so primarily on HVLT-R Delayed Recall (learning/memory domain) and TMT-A (psychomotor speed domain). Only 25% of patients displayed stable or improving performance on all cognitive measures throughout the study. Our results concur with those from other studies showing that neurocognitive functioning is negatively affected in the immediate posttransplant period.7,12
Previous studies12,26 have identified increasing age and less education as predictive of cognitive decline in patients undergoing treatment for cancer; our results concur with these conclusions. Additionally, several studies8,12,27,28 in mixed samples of patients undergoing hematopoietic stem cell transplant support that pretransplant chemotherapy may be implicated in posttransplant deficits, a fact that may account for our observation that postinduction deficits and increasing number of chemotherapy cycles were strong predictors of subsequent decline. Regarding affective status, most previous studies,12,26,29 like the current study, have not found a significant association between patients’ mood and changes in cognition. Overall, our results indicate that, although the elderly and minority patients are most likely to exhibit impairment on specific measures both before and after AuHSCT, those with more induction therapy cycles, more postinduction deficits, and more-advanced disease may be most vulnerable to overall cognitive decline post-AuHSCT.
Most previous studies suggest that self-perceived cognitive functioning does not correlate highly with objective measures of cognitive function, although low to moderate (but statistically significant) correlations have been reported.30–32 Our study analogously found low to moderate but significant correlations between responses to the MDASI-MM items “difficulty remembering” and “difficulty paying attention” and objective tests in learning/memory, attention, motor function, and psychomotor speed. One month post-AuHSCT, associations were observed between objective cognitive decline (in learning/memory and motor function) and patients’ self-report of decline in memory and attention as assessed by the MDASI-MM. None of these associations persisted 3 months post-AuHSCT.
Our study had limitations. It lacked a preinduction assessment, as the patient cohort was enrolled after induction therapy in readiness for AuHSCT. Cognitive impairment has been observed in patients with hematological and other malignancies prior to administration of systemic chemotherapy, and it is likely that deficits may be associated with effects of both disease and induction regimens. Future studies should incorporate a preinduction therapy baseline assessment to enable identification of impaired cognitive function attributable to induction chemotherapy and to control for various pretransplant treatments. Additionally, whereas we accounted for the impact of 23% attrition 1 month post-AuHSCT and 29% 3 months post-AuHSCT, we lacked long-term follow up data (example, 1 year plus) that would reveal the trajectory of patients’ cognitive functioning over an extended period of time post-AuHSCT.
To our knowledge, our study is the first to report the incidence of acute cognitive deficits specifically in patients with MM undergoing AuHSCT and to describe clinically significant changes in cognitive function 1 and 3 months thereafter. Our results suggest that postinduction cognitive impairment in patients with MM is high, and that reliable decline 1 month post-AuHSCT relative to pre-AuHSCT, and 3 months relative to 1 month post-AuHSCT, occurs in a substantial number of patients on 1 or more measures of cognitive function. Older patients, minorities, and those with advanced disease, more induction cycles, or postinduction deficits may be more vulnerable to cognitive decline, particularly in learning/memory, psychomotor speed, and motor function. Survivors expecting to quickly resume work that involves high cognitive demand immediately posttransplant may benefit from awareness of these potential challenges. Clinician awareness of cognitive impairment and associated risk factors postinduction and in the period three months after transplant in this population is equally crucial, so that vocational counseling or other psychosocial support can be provided to the patients who may need it.
Acknowledgments
Funding: This work was supported in part by the Hawn Foundation and the National Cancer Institute (P01 CA124787 to C.S.C and MD Anderson Cancer Center Support Grant P30 CA016672). The sponsors played no role in the study design, data collection, analysis, interpretation, or preparation of the report.
The authors thank Jeanie Woodruff, BS, ELS, Department of Symptom Research, MD Anderson, for editorial assistance, and Melissa S.Y. Thong, PhD, Department of Medical Psychology and Neuropsychology, Tilburg University, The Netherlands, for assistance with data preparation for statistical analysis.
Footnotes
Disclosures: The authors made no disclosures.
Previous presentation: Invited oral presentation at the American Society of Hematology 54th Annual Meeting and Exposition, Atlanta GA, Dec 8–11, 2012
References
- 1.Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111(5):2521–6. doi: 10.1182/blood-2007-08-104984. [DOI] [PubMed] [Google Scholar]
- 2.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–20. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catley L, Anderson K. Strategies to improve the outcome of stem cell transplantation in multiple myeloma. Hematol J. 2004;5(1):9–23. doi: 10.1038/sj.thj.6200322. [DOI] [PubMed] [Google Scholar]
- 4.Chapko MK, Syrjala KL, Schilter L, Cummings C, Sullivan KM. Chemoradiotherapy toxicity during bone marrow transplantation: time course and variation in pain and nausea. Bone Marrow Transplant. 1989;4(2):181–6. [PubMed] [Google Scholar]
- 5.Ahles TA, Tope DM, Furstenberg C, Hann D, Mills L. Psychologic and neuropsychologic impact of autologous bone marrow transplantation. J Clin Oncol. 1996;14(5):1457–62. doi: 10.1200/JCO.1996.14.5.1457. [DOI] [PubMed] [Google Scholar]
- 6.Syrjala KL, Artherholt SB, Kurland BF, et al. Prospective neurocognitive function over 5 years after allogeneic hematopoietic cell transplantation for cancer survivors compared with matched controls at 5 years. J Clin Oncol. 2011;29(17):2397–404. doi: 10.1200/JCO.2010.33.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syrjala KL, Dikmen S, Langer SL, Roth-Roemer S, Abrams JR. Neuropsychologic changes from before transplantation to 1 year in patients receiving myeloablative allogeneic hematopoietic cell transplant. Blood. 2004;104(10):3386–92. doi: 10.1182/blood-2004-03-1155. [DOI] [PubMed] [Google Scholar]
- 8.Andrykowski MA, Schmitt FA, Gregg ME, Brady MJ, Lamb DG, Henslee-Downey PJ. Neuropsychologic impairment in adult bone marrow transplant candidates. Cancer. 1992;70(9):2288–97. doi: 10.1002/1097-0142(19921101)70:9<2288::aid-cncr2820700913>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 9.Harder H, Cornelissen JJ, Van Gool AR, Duivenvoorden HJ, Eijkenboom WM, van den Bent MJ. Cognitive functioning and quality of life in long-term adult survivors of bone marrow transplantation. Cancer. 2002;95(1):183–92. doi: 10.1002/cncr.10627. [DOI] [PubMed] [Google Scholar]
- 10.Molassiotis A, Wilson B, Blair S, Howe T, Cavet J. Unmet supportive care needs, psychological well-being and quality of life in patients living with multiple myeloma and their partners. Psychooncology. 2011;20(1):88–97. doi: 10.1002/pon.1710. [DOI] [PubMed] [Google Scholar]
- 11.Potrata B, Cavet J, Blair S, Howe T, Molassiotis A. ‘Like a sieve’: an exploratory study on cognitive impairments in patients with multiple myeloma. Eur J Cancer Care (Engl) 2010;19(6):721–8. doi: 10.1111/j.1365-2354.2009.01145.x. [DOI] [PubMed] [Google Scholar]
- 12.Friedman MA, Fernandez M, Wefel JS, Myszka KA, Champlin RE, Meyers CA. Course of cognitive decline in hematopoietic stem cell transplantation: a within-subjects design. Arch Clin Neuropsychol. 2009;24(7):689–98. doi: 10.1093/arclin/acp060. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs SR, Small BJ, Booth-Jones M, Jacobsen PB, Fields KK. Changes in cognitive functioning in the year after hematopoietic stem cell transplantation. Cancer. 2007;110(7):1560–7. doi: 10.1002/cncr.22962. [DOI] [PubMed] [Google Scholar]
- 14.Beck AT, Brown G, Steer RA. Beck Depression Inventory II Manual. San Antonio, TX: 1996. [Google Scholar]
- 15.Jones D, Vichaya EG, Wang XS, et al. Validation of the M. D. Anderson Symptom Inventory Multiple Myeloma Module. 2012: Abstract presented at the 48th Annual Meeting of the American Society of Clinical Oncology; Chicago, IL. June 1–5, 2012. [Google Scholar]
- 16.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–46. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Benedict RHB, Schretlen D, Lea Groninger. Hopkins verbal learning test revised: normative data and analysis of inter-form and test-restest reliability. The Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 18.Heaton RK, Miller SW, Taylor MJ, et al. Revised comprehensive norms for an expanded Halstead–Reitan battery. Odessa, FL: PAR; 2004. [Google Scholar]
- 19.Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329–38. [PubMed] [Google Scholar]
- 20.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–14. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 21.Wechsler D. Wechsler adult intelligence scale III. Psychological Corporation; San Antonio: 1997. [Google Scholar]
- 22.Wefel JS, Lenzi R, Theriault R, Buzdar AU, Cruickshank S, Meyers CA. ‘Chemobrain’ in breast carcinoma?: a prologue. Cancer. 2004;101(3):466–75. doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- 23.Chelune GJ, Naugle RI, Hea Luders. Individual change after epilepsy surgery: practice effects and base-rate information. Neuropsychology. 1993;7:41–52. [Google Scholar]
- 24.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12–9. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 25.Cleeland CS. The M.D. Anderson Symptom Inventory User Guide. Houston, TX: 2010. [Google Scholar]
- 26.Wefel JS, Vidrine DJ, Veramonti TL, et al. Cognitive impairment in men with testicular cancer prior to adjuvant therapy. Cancer. 2011;117(1):190–6. doi: 10.1002/cncr.25298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyers CA, Byrne KS, Komaki R. Cognitive deficits in patients with small cell lung cancer before and after chemotherapy. Lung Cancer. 1995;12(3):231–5. doi: 10.1016/0169-5002(95)00446-8. [DOI] [PubMed] [Google Scholar]
- 28.Meyers CA. Neuropsychological aspects of cancer and cancer treatment. Physical Medicine and Rehabilitation: State of the Art Reviews. 1994;8:229–241. [Google Scholar]
- 29.Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010 Jul 15;116(14):3348–56. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 30.Weis J, Poppelreuter M, Bartsch HH. Cognitive deficits as long-term side-effects of adjuvant therapy in breast cancer patients: ‘subjective’ complaints and ‘objective’ neuropsychological test results. Psychooncology. 2009;18(7):775–82. doi: 10.1002/pon.1472. [DOI] [PubMed] [Google Scholar]
- 31.Johnson I, Tabbane K, Dellagi L, Kebir O. Self-perceived cognitive functioning does not correlate with objective measures of cognition in schizophrenia. Comprehensive psychiatry. 2011 Nov-Dec;52(6):688–92. doi: 10.1016/j.comppsych.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Ganz PA, Kwan L, Castellon SA, et al. Cognitive Complaints After Breast Cancer Treatments: Examining the Relationship With Neuropsychological Test Performance. J Natl Cancer Inst. 2013;105(11):791–801. doi: 10.1093/jnci/djt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wechsler D. Wechsler Adult Intelligence Scale - Revised. San Antonio, TX: The Psychological Corporation; 1981. [Google Scholar]
- 34.Reitan RM. Trail Making Test Manual for Administration and Scoring. Tucson, AZ: Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- 35.Levine AJ, Miller EN, Becker JT, Selnes OA, Cohen BA. Normative data for determining significance of test-retest differences on eight common neuropsychological instruments. Clin Neuropsychol. 2004;18(3):373–84. doi: 10.1080/1385404049052420. [DOI] [PubMed] [Google Scholar]
- 36.Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with 6 equivalent forms. Clin Neuro-psychol. 1991;5:125–42. [Google Scholar]
- 37.Benton AL, Hamsher K. Multilingual Aphasia Examination. Iowa City, IA: AJA Associates; 1983. [Google Scholar]
- 38.Trites R. Neuropsychological Test Manual. Ottawa, ON: Royal Ottawa Hospital; 1977. [Google Scholar]