Abstract
N-methyl-d-aspartate (NMDA) receptors are major glutamatergic receptors involved in most excitatory neurotransmission in the brain. The transcriptional regulation of NMDA receptors is not fully understood. Previously, we found that the GluN1 and GluN2B subunits of the NMDA receptor are regulated by nuclear respiratory factors 1 and 2 (NRF-1 and NRF-2). NRF-1 and NRF-2 also regulate all 13 subunits of cytochrome c oxidase (COX), a critical energy-generating enzyme, thereby coupling neuronal activity and energy metabolism at the transcriptional level. Specificity Protein (Sp) is a family of transcription factors that bind to GC-rich regions, with Sp1, Sp3, and Sp4 all binding to the same cis- motifs. Sp1 and Sp3 are ubiquitously expressed, whereas Sp4 expression is restricted to neurons and testicular cells. Recently, we found that the Sp1 factor regulates all subunits of COX. The goal of the present study was to test our hypothesis that the Sp factors also regulate specific subunits of NMDA receptors, and that they function with NRF-1 and NRF-2 via one of three mechanisms: complementary, concurrent and parallel, or a combination of complementary and concurrent/parallel. By means of multiple approaches we found that Sp4 functionally regulated GluN1, GluN2A, and GluN2B, but not GluN2C. On the other hand, Sp1 and Sp3 did not regulate these subunits as previously thought. Our data suggest that Sp4 operates in a complementary and concurrent/parallel manner with NRF-1 and NRF-2 to mediate the tight coupling between energy metabolism and neuronal activity at the molecular level.
Keywords: Sp4, GluN1, GluN2A, GluN2B, NMDA, Gene regulation
1. Introduction
N-methyl-D-aspartate (NMDA) receptors are excitatory glutamatergic receptors ubiquitously expressed in the brain. They play critical roles in processes ranging from basic excitatory neurotransmission to the complex phenomenon of synaptic plasticity, learning, and memory (for review see [1]). NMDA receptors are heterotetrameric, voltage-dependent, ligand-gated ion channels composed of the ubiquitous GluN1 subunit in various combinations with the GluN2A-D, or GluN3A-B subunits [2]. The properties of NMDA receptors are dictated by their subunit composition, with the majority composed of two GluN1 and two GluN2A or GluN2B subunits [3]. The GluN1/GluN2A receptors, expressed predominantly in the adult brain, are faster-acting than the GluN1/GluN2B receptors, which are widely expressed in both the adult and neonatal brains [2]. The GluN2C, GluN2D, GluN3A, and GluN3B receptor subunits are more developmentally and regionally specified [4, 5].
Our laboratory has shown that GluN1 and GluN2B are transcriptionally regulated by nuclear respiratory factors 1 (NRF-1) and 2 (NRF-2) via a concurrent and parallel mechanism [6, 7]. NRF-1 and NRF-2 each also regulates all 13 subunits of cytochrome c oxidase (COX), an enzyme critical for energy generation in neurons [8–10]. Neuronal activity and energy metabolism are tightly coupled. A requirement for NRF-1 and NRF-2 in this coupling is revealed when a reduction in the expression of COX, GluN1, and GluN2B induced by TTX blockade is rescued by an over-expression of NRF-1 and NRF-2, and an up-regulation of these proteins induced by KCl depolarization is suppressed by a knock-down of NRF-1 or NRF-2 [6, 7, 9, 10]. The question arises as to whether other transcription factors are also involved in this co-regulation.
Specificity protein (Sp) is a family of zinc-finger transcription factors that binds to GC-rich regulatory regions of genes [11]. Three members, namely Sp1, Sp3, and Sp4, possess two glutamine-rich domains that bind and compete for the same cis- motifs: the GC box ‘GGGCGG’ with high affinity, or the GT and CT boxes with significantly lower affinities (‘GGGTGG’ and ‘CCCTCC’, respectively) [11, 12]. Sp1 and Sp3 are ubiquitously expressed and are involved in a wide variety of cellular processes [13]. Unlike Sp1 and Sp3, the expression of Sp4 is restricted to neurons and testicular cells [14].
Recently, we found that Sp1 factor, like NRF-1 and NRF-2, transcriptionally regulates all subunit genes of COX [15]. We sought to determine if Sp1, Sp3, and/or Sp4 regulate specific subunits of NMDA receptors. If so, do these transcription factors operate via complementary, concurrent and parallel, or a combined complementary and concurrent/parallel mechanism with NRF-1 and NRF-2? In the complementary mechanism, Sp factors regulate NMDA receptor subunits complementary to those regulated by NRF-1 and NRF-2. In the concurrent and parallel mechanism, Sp factors, NRF-1, and NRF-2 jointly regulate the same NMDA receptor subunit genes in a parallel fashion (both are stimulatory). In a combination of the complementary and concurrent/parallel mechanisms, a subset of subunit genes is controlled by all three factors, whereas a different subset is controlled by Sp factors but not NRF-1 or NRF-2.
The goal of the present study was to test our hypothesis that Sp1, Sp3, and Sp4 also mediate the transcriptional coupling of synaptic transmission and energy metabolism.
2. Material and Methods
All experiments and animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publications No. 80–23, revised 1996), and all protocols were approved by the Medical College of Wisconsin Animal Care and Use Committee (approval can be provided upon request). All efforts were made to minimize the number of animals used and their suffering.
2.1. Cell culture
Murine neuroblastoma (N2a) cells (ATCC, Manassas, VA, USA) were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 50 units/mL penicillin, and 100 μg/ mL streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified atmosphere with 5% CO2.
Mouse primary visual cortex neurons were cultured as described previously for rats [16]. Briefly, 1-day-old neonatal mouse pups were killed by decapitation. Brains were removed from the skull and the meninges were removed. Cortical tissue was dissected, trypsinized, and triturated to release individual neurons. Primary cortical neurons were plated in 35 mm poly-L-lysine-coated dishes at a density of 200,000 cells/dish. Cells were maintained in Neurobasal-A media supplemented with B27 (Invitrogen). Ara-C (Sigma, St Louis, MO, USA) was added to the media to suppress the proliferation of glial cells.
2.2. In silico analysis of promoters of murine NMDA receptor subunit genes
DNA sequences surrounding the transcription start points (TSPs) of N-methyl-D-aspartate (NMDA) receptor subunit genes (Grin1 and Grin2a-c) were derived from the NCBI mouse genome database (Grin1 GenBank ID: NC_000068.7, Grin2a GenBank ID: NC_000082.6, Grin2b GenBank ID: NC_000072.6, Grin2c GenBank ID: NC_000077.6). Sequences encompassing 1 kb upstream and 1 kb downstream of the TSP of each gene were analyzed. Computer-assisted search for Sp1 binding motif ‘GGGCGG’, or the atypical Sp1 binding motif ‘GGGTGG’, or their complements, was conducted on each promoter. These motifs were applicable to Sp1, Sp3, or Sp4.
Alignment of human, mouse, and rat promoter sequences was performed with NCBI’s Ensembl interface. Mouse NMDA receptor promoter sequences were compared with those of rat and human genomic sequences for the conservation of the Sp binding motif.
2.3. Electrophoretic mobility shift and supershift assays
Electrophoretic mobility shift assays (EMSA) for possible Sp1, Sp3, and Sp4 interactions with putative binding elements on all NMDA receptor subunit promoters were carried out with a few modifications from methods previously described [8]. Briefly, based on in silico analysis, oligonucleotide probes with putative Sp binding motifs on each NMDA receptor subunit promoter were synthesized (Table 1A), annealed, and labeled by a Klenow fragment (Invitrogen) fill-in reaction with [α-32P] dATP (50 μCi/200 ng; Perkin-Elmer, Shelton, CT, USA). Mouse primary visual cortical tissue and HeLa nuclear extract was isolated using methods described previously [17]. Each labeled EMSA probe was incubated with 2 μg of calf thymus DNA and 10 μg of mouse cortical and/or 10 μg of HeLa nuclear extract. The probe reaction was processed for EMSA. Supershift assays were performed with 1 μg of Sp1, 3, or 4 specific antibody (Sp1, polyclonal rabbit antibody, H-225, SC14027, Santa Cruz Biotechnology (SCBT), Santa Cruz, CA, USA; Sp3, polyclonal rabbit antibody, H-225, SC13018, SCBT; Sp4, polyclonal rabbit antibody, V-20, SC645, SCBT) added to the probe/nuclear extract mixture and incubated for 20 min at 24°C. When tested with western blots using mouse whole brain extract, the Sp1, Sp3, and Sp4 antibodies gave two adjacent bands at the appropriate molecular weights corresponding to the phosphorylated and non-phosphorylated forms of these transcription factors. For competition, a 100-fold excess of unlabeled oligonucleotides were incubated with the nuclear extract before the addition of labeled oligonucleotides. Shift reactions were loaded onto 4.5% polyacrylamide gel (58:1, Acrylamide:Bisacrylamide) and run at 200 V for 3 h in 0.25X Tris-borate-EDTA buffer. Results were visualized by autoradiography and exposed on film. Mouse GM3 Synthase with known Sp1 binding site was designed as previously described [18] and used as a positive control. Sp1 mutants with mutated sequences, as shown in Table 1B, were used as negative controls.
Table 1A.
EMSA Probes. Positions of probes are given relative to TSP. Putative Sp1 binding sites are underlined.
| Gene Promoter | Position | EMSA Sequence |
|---|---|---|
| Grin1 | −208/−188 | F: 5′ TTTTGGAAGCGGGGGCGGTGGGAGG 3′ |
| R: 5′ TTTTCCTCCCACCGCCCCCGCTTCC 3′ | ||
| Grin2a | +46/+67 | F: 5′ TTTTGCATCCTGGGCGGGTGTGTGC 3′ |
| R: 5′ TTTTGCACACACCCGCCCAGGATGC 3′ | ||
| Grin2b | +155/+174 | F: 5′ TTTTGATGTCCCCGCCCTCCCCGC 3′ |
| R: 5′ TTTTGCGGGGAGGGCGGGGACATC 3′ | ||
| Grin2c | −56/−32 | F: 5′ TTTTCGGCTGGGGCGGGCCGGGGCGGGGC 3′ |
| R: 5′ TTTTGCCCCGCCCCGGCCCGCCCCAGCCG 3′ | ||
| GM3 Synthase | −58/−38 | F: 5′ TTTTGCGCGACCCCGCCCCCGCCTA 3′ |
| R: 5′ TTTTTAGGCGGGGGCGGGGTCGCGC 3′ |
Table 1B.
Mutant EMSA Probes. Positions of probes are given relative to TSP. Mutated Sp1 binding sites are underlined.
| Gene Promoter | Position | Sequence |
|---|---|---|
| Grin1 | −208/−188 | F: 5′ TTTTGGAAGCGTTTTTTGTGTGAGG 3′ |
| R: 5′ TTTTCCTCACACAAAAAACGCTTCC 3′ | ||
| Grin2a | +46/+67 | F: 5′ TTTTGCATCCTAAACAAATGTGTGC 3′ |
| R: 5′ TTTTGCACACATTTGTTTAGGATGC 3′ | ||
| Grin2b | +155/+174 | F: 5′ TTTTGATGTCTTTTTTCTTTTCGC 3′ |
| R: 5′ TTTTGCGAAAAGAAAAAAGACATC 3′ |
2.4. Chromatin immunoprecipitation (ChIP) assays in murine visual cortical tissue
ChIP assays were performed similar to those described previously [7]. Briefly, 0.1 g of murine visual cortical tissue was used for each immunoprecipitation reaction. From fresh murine brain, the visual cortex was quickly dissected and cut into small pieces. The finely chopped visual cortical tissue was fixed with 2% formaldehyde for 20 min at 24 °C. Cells were then resuspended in swelling buffer (5 mM PIPES, pH 8.0, 85 mM KCl, and 1% Nonidet P-40 (Sigma, St Louis, MO, USA), with protease inhibitors added right before use) and homogenized 10 times in a small pestle Dounce tissue homogenizer (5 mL). Nuclei were then isolated by centrifugation before being subjected to sonication in SDS lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl, pH 8.1 (Sigma)). The sonicated lysate was immunoprecipitated with either 2 μg of Sp1 polyclonal rabbit antibody, 2 μg of Sp3 polyclonal rabbit antibody, 2 μg of Sp4 polyclonal rabbit antibody, or 2 μg of anti-nerve growth factor receptor (NGFR) p75 polyclonal goat antibody (sc-6188, SCBT). Semi-quantitative PCR was performed using 1/20th of precipitated chromatin. Primers encompassing putative Sp1 binding sites near TSPs of NMDA receptor subunit genes (identified in in silico analysis) were designed (Supplementary Table 1) as previously described [10]. GM3 Synthase and neurotrophin 3 promoters with known Sp binding sites were used as positive controls [18, 19], and β-actin promoter was used as a negative control (Supplementary Table 1). PCR reactions were carried out with DreamTaq polymerase (Thermo-Fisher Scientific, Waltham, MA, USA). The use of cycling parameters and PCR additives significantly improved the quality and reproducibility of ChIP and are listed in Supplementary Table 1. PCR products were visualized on 2% agarose gels stained with ethidium bromide.
2.5. Construction and transfection of luciferase reporter vectors for promoter mutagenesis study
Luciferase reporter constructs of Grin1, Grin2a, and Grin2b gene promoters were made by PCR cloning their proximal promoter sequences using genomic DNA prepared from mouse N2a cells as a template. Digestion with restriction enzymes was performed, followed by ligation of the product directionally into pGL3 basic luciferase vector (E1751, Promega, Madison, WI, USA). Sequences of primers used for PCR cloning and restriction enzymes used for digestion are provided in Supplementary Table 2A. Site-directed mutation of putative Sp binding sites on each promoter was generated using QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). Primers for mutagenesis are listed in Supplementary Table 2B. All constructs were verified by sequencing.
Each promoter construct was transfected into N2a cells in a 24-well plate using Lipofectamine 2000 (Invitrogen) and cell lysates harvested after 48 h. Each well received 0.6 μg of reporter construct and 0.06 μg of pRL-TK renilla luciferase vector (E2241, Promega), a vector with thymidine kinase promoter that constitutively expressed renilla luciferase. Transfected neurons were stimulated with KCl at a final concentration of 20 mM in the culture media for 5 h as previously described [20]. After 5 h of treatment, cell lysates were harvested and measured for luciferase activity as described previously [20]. Data from six independent transfections were averaged for each promoter construct.
2.6. Plasmid construction of Sp1, Sp3, or Sp4 shRNA vectors, transfection, and KCl treatment
Sp1 silencing was carried out using a combination of three to five Sp1-specific 19–25 nt hairpin RNA (shRNA) against Sp1 (sc-29488, SCBT). Sp3 and Sp4 silencing was carried out using specific shRNA sequences against murine Sp3 and Sp4 that were cloned into the pLKO.1 TRC cloning vector (Plasmid 10878, Addgene, Cambridge, MA, USA). Target Sp3 and Sp4 shRNA sequences were chosen from the RNAi Consortium’s Public TRC Cloning Database at the Broad Institute and are listed in Supplementary Table 3. The pLKO.1 non-mammalian shRNA control vector, which contains a scrambled shRNA sequence that targets no known mammalian genes, was used as the negative control (SHC002, Sigma).
For transfection, N2a cells were plated at 60% confluency in 6-well dishes. Cells were co-transfected the day after plating with either the Sp1, Sp3, or Sp4 shRNA constructs (3 μg) and turboGFP (1 μg) vectors or the pLKO.1 non-mammalian control (3μg) and the turboGFP (1 μg) vector using 5 μl of JetPrime transfection reagent (PolyPlus Transfection, Illkirch, France) per well. Puromycin at a final concentration of 5μg/mL was added to the culture medium 1.5 days after transfection to select for purely transfected cells. Green fluorescence was observed to monitor transfection efficiency. Transfection efficiency for N2a cells was around 75%; however, puromycin selection effectively yielded 100% transfected cells. N2a cells transfected with shRNA against Sp1, Sp3, and Sp4 were further stimulated with KCl at a final concentration of 20 mM in the culture media for 5 h as previously described [20]. After 5 h of treatment, cells were harvested for RNA and protein isolation.
For transfection of primary neuronal cultures, disassociated neurons were plated at a density of 2 × 105 cells/well and transfected 5 days post-plating with Sp1, Sp3, or Sp4 shRNA constructs (2 μg) or the pLKO.1 non-mammalian control (2 μg) using 10 μl of Neurofect transfection reagent per well. TurboGFP (0.5 μg) vector was added to visualize transfection efficiency in each well. Transfection efficiency was around 40 – 50%; however, puromycin selection effectively yielded 100% transfected cells.
2.7. Sp1, Sp3, and Sp4 over-expression and TTX treatment
The human Sp1, Sp3, and Sp4 cDNA clones were obtained from Open Biosystems (Lafayette, CO, USA) and cloned into pcDNA Dest40 vector using Gateway Multisite Cloning kit (Invitrogen) according to the manufacturer’s instructions and as described previously [15].
Transfection procedure for N2a cells and primary neuronal culture was similar to that described above with the modification that either 1.5 μg of Sp1, Sp3, and Sp4 over-expression vector, or 1 μg of the pcDNA3.1 empty vector and 0.5 μg of turboGFP vector were used for both N2a cells and primary neuronal cultures. Green fluorescence was used to monitor transfection efficiency. Transfected N2a cells were impulse blocked for 3 days with TTX at a final concentration of 0.4 μM and starting on the day after plating as previously described [20]. N2a cells were harvested for RNA and protein isolation four days after transfection, whereas primary neuronal cultures were harvested 2 days after transfection.
2.8. RNA isolation and cDNA synthesis
Total RNA was isolated using TRIZOL (Invitrogen) according to the manufacturer’s instructions. 1 μg of total RNA was treated with Dnase I and the reaction stopped with heating at 65°C in the presence of EDTA. cDNA was synthesized using iScript cDNA synthesis kit (170-8891, BioRad, Hercules, CA, USA) according to the manufacturer’s instructions.
2.9. Real-time quantitative PCR
Real-time quantitative PCR was carried out in a Cepheid Smart Cycler Detection system (Cepheid, Sunnyvale, CA, USA) and/or the iCycler System (BioRad) using the IQ Sybr Green SuperMix (170-8880, BioRad) following the manufacturer’s protocols and as described previously. The primer sequences used are shown in Supplementary Table 4. Primers were optimized to yield 95% – 105% reaction efficiency with PCR products run on agarose gel to verify the correct amplification length. Melt curve analyses verified the formation of a single desired PCR product in each PCR reaction. Mouse β-actin for N2a and Gapdh for primary neurons were used as internal controls, and the 2−ΔΔCT method was applied to quantify the relative amount of transcripts.
2.10. Western blot analysis
KCl and TTX treatments, Sp1, Sp3, and Sp4 shRNA and over-expression samples, along with appropriate controls, were harvested in sample buffer (50 mM Tris-HCl pH 6.8, 1% SDS, 10% glycerol, 12.5 mM EDTA). Nuclear extract from mouse cortical tissue, cultured primary cortical neurons, N2a cells, and HeLa cells were extracted as described for EMSA above. To load nuclear extract proportional to the cytoplasmic volume, an equal amount of proteins from cytoplasmic extracts was loaded for control and for KCl samples. Based on the ratio between nuclear and cytoplasmic volumes for each of the samples, the proportional amount of nuclear extract was loaded. Protein samples were loaded onto 10% SDS-PAGE gel and electrophoretically transferred onto polyvinylidene difluoride membranes (Bio-Rad). Subsequent to blocking, blots were incubated in primary antibodies against Sp1 (1:1000; Santa Cruz), Sp3 (1:1000; Santa Cruz), Sp4 (1:1000; Santa Cruz), GluN1 (1:1000; Millipore Chemicon, Billerica, MA, USA), GluN2A (1:600; 19953-1-AP, ProteinTech, Chicago, IL, USA), and GluN2B (1:1000; 1498-NR2B, PhosphoSolutions, Aurora, CO, USA). β-actin (1:5000; Sigma) served as a loading control for cytoplasmic extracts and for Sp1, Sp3, and Sp4 shRNA and over-expression samples. TATA binding protein (1:1000; 1TBP18; Abcam; San Franscisco, CA, USA) was the loading control to determine the relative levels of Sp1, Sp3, and Sp4 in different cell types. NeuN (1:250; Millipore) was used to determine nuclear contamination of cytoplasmic extracts. Secondary antibodies used were goat-anti-rabbit and goat-anti-mouse antibodies (Vector Laboratories, Burlingame, CA, USA). Blots were then reacted with the ECL reagent (Pierce, Rockford, IL, USA) and exposed to autoradiographic film (RPI, Mount Prospect, IL, USA). Quantitative analyses of relative changes were done with Gel Doc (BioRad, Hercules, CA, USA).
2.11. Statistical analysis
Significance among group means was determined by analysis of variance (ANOVA). Significance between two groups was analyzed by Student’s t-test. P-values of 0.05 or less were considered significant.
3. Results
3.1. In silico analysis of the Grin1 and Grin2a-c proximal promoter regions
In silico analysis of the proximal promoters of murine NMDA receptor subunit genes in the DNA sequence 1 kb upstream and 1 kb downstream of TSP revealed Sp binding motifs. The GC box motif (GGGCGG) was found on the Grin1, Grin2a, and Grin2c promoters, and the GC box with a tandem CT box (CCCTCC) was found on the Grin2b promoter (see Table 1A for binding motifs).
3.2. Sp4 is prevalent in mouse visual cortical nuclear extract
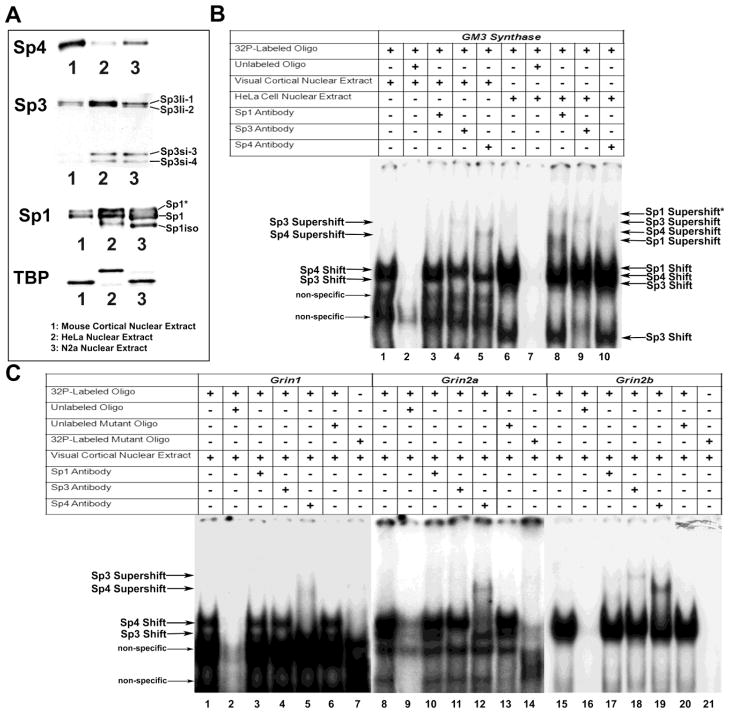
Relative levels of Sp1, Sp3, and Sp4 in mouse visual cortical tissue, N2a, and HeLa cells were probed with antibodies against each Sp factor. Equal amounts of nuclear extracts were loaded and probed with Tata box binding protein (TBP) as the loading control (Figure 1A). The difference in the molecular weights between the samples corresponds to the difference in the molecular weights of the protein in mice and humans. As shown in Figure 1A, Sp4 was present in greater amount in mouse visual cortical tissue nuclear extract than in N2a cells and least in HeLa cell nuclear extract. Sp3 was most prevalent in HeLa cell nuclear extract, less so in N2a cells and least in mouse visual cortical tissue nuclear extract (Figure 1A). The same was true for Sp1 (Figure 1A). Furthermore, we found that N2a and HeLa cells contained smaller isoforms of Sp1 (Figure 1A, Sp1iso) [21] and Sp3 (Sp3si-3 and Sp3si-4) [22] that were not present in mouse visual cortical tissue (Figure 1A). Sp1 was also found in its phosphorylated form in all nuclear lysates (Figure 1A, Sp1*) [23]. The slight downward shift of Sp1iso in N2a cells may be due to post-translational modification differences in mouse N2a and human HeLa cancer cell lines (Figure 1A).
Figure 1.
Relative levels of the Sp factors and In vitro binding of Sp factors to the GM3 Synthase, Grin1, 2a, and 2b promoters. (A) To determine relative levels of Sp factors, equal amounts of nuclear extract from mouse visual cortical tissue, HeLa cell, and N2a cells were probed with Sp4, Sp3, Sp1, or Tata box binding protein (TBP) antibodies. Sp4 levels were highest in mouse cortical tissue, and conversely, Sp3 and Sp1 levels were lowest in mouse cortical tissue as compared to HeLa or N2a cells. In addition, HeLa and N2a cell nuclear extracts contained smaller isoforms of Sp1 (Sp1iso) and Sp3 (Sp3si-3 and Sp3si-4) not present in mouse cortical extract. The phosphorylated form of Sp1 was also found in all nuclear extracts (Sp1*). TBP was used as the loading control and showed similar levels in all nuclear extracts. B and C show In vitro binding of Sp factors to putative binding sites on the GM3 Synthase, Grin1, 2a, 2b, and 2c promoters with EMSA and supershift assays. 32P-labeled oligonucleotides, excess unlabeled oligos as competitors, excess unlabeled mutant Sp oligos as competitors, mouse visual cortical or HeLa nuclear extract, and Sp1, Sp3, or Sp4 antibodies are indicated by a “+” or a “−“ sign. Arrowheads indicate specific Sp1, Sp3, or Sp4 shift, supershift, and non-specific complexes. (B) GM3 Synthase was used as a positive control with mouse cortical nuclear extract (B, lanes 1 – 5) or HeLa cell extract (B, lanes 6 – 10). Incubation with cortical or HeLa nuclear extract revealed specific Sp1, 3, or 4 shift bands (B, lanes 1 and 6, respectively). The location of each band was clearly identified on shorter gel exposure. When excess unlabeled competitor was added, it did not yield a band (B, lanes 2 and 7). The addition of Sp1 antibody yielded two specific supershift bands for HeLa nuclear extract (B, lane 8) corresponding to the presence of tandem Sp binding. The addition of Sp1 antibody did not yield specific supershift bands with cortical nuclear extract (B, lane 3). The addition of Sp3 antibody yielded specific supershift bands with both cortical and HeLa cell extract (B, lanes 4 and 9, respectively), as did the addition of Sp4 antibody (B, lanes 5 and 10, respectively). (C) Incubation of cortical nuclear extract with Grin1, 2a, or 2b probes yielded specific Sp1 and 3 shift bands (C, lanes 1, 8, and 15, respectively). This shift band was competed out with an excess addition of cold probes (C, lanes 2, 9, and 16, respectively). Labeled Grin1, 2a, or 2b probes with mutant Sp sites did not yield specific Sp shift bands (C, lanes 7, 14, and 21). The addition of unlabeled mutant competitor probes to the respective Grin1, 2a, or 2b probes did not compete out the specific shift bands (C, lanes 6, 13, and 20). The addition of Sp1 antibody did not yield supershift bands for Grin1, 2a, or 2b (C, Lanes 3, 10, and 17). The addition of Sp3 antibody did not yield supershift bands for Grin1 or 2a (C, lanes 4 and 11, respectively) but did yield a band for Grin2b (C, lane 18). The addition of Sp4 antibody resulted in supershift bands for Grin1, 2a, and 2b (C, lanes 5, 12, and 19, respectively).
3.3. In vitro binding of Sp4 to putative sites on the Grin1, Grin2a, and Grin2b promoters
The electrophoretic mobility shift assays (EMSA) and supershift assays were performed to determine Sp1, Sp3, and Sp4’s ability to bind its candidate site in vitro. GM3 synthase promoter with known Sp1 binding sites (2 tandem GC boxes) served as the positive control. When incubated with mouse visual cortical tissue nuclear extract, GM3 synthase formed specific DNA/Sp3 and DNA/Sp4 shift and supershift complexes (Figure 1B, lanes 1 and 4 for Sp3, and 1 and 5 for Sp4, respectively) but did not form a DNA/Sp1 shift or supershift complex (Figure 1B, lanes 1 and 3 respectively). We reasoned that the lack of Sp1 shift and supershift was due to insufficient Sp1 in mouse cortical nuclear extract. We, therefore, incubated GM3 synthase with HeLa nuclear extract, which is known to contain Sp1 [18]. In the presence of HeLa nuclear extract, GM3 synthase formed specific DNA/Sp1, DNA/Sp3, and DNA/Sp4 shift and super shift complexes (Figure 1B, lane 6 for Sp1/Sp3/Sp4 shift, lanes 8, 9, and 10 for Sp1, Sp3, and Sp4 supershifts, respectively). As Sp1 is capable of homotypic interactions that lead to multimeric complexes [11], the higher supershift may be two Sp1 proteins bound to tandem Sp sites (Figure 1B, lane 10, Sp1 Supershift*). The supershift for Sp4 was faint in HeLa cells (Figure 1B, lane 10), consistent with reported neuronal distribution of Sp4 [14]. The shift bands for visual cortical and HeLa nuclear extract were competed out with the addition of cold competitor (Figure 1B, lanes 2 and 7, respectively).
Mouse visual cortical tissue nuclear extract incubated with putative Sp sites on the Grin1, Grin2a, Grin2b, and Grin2c promoters gave positive shift and supershift bands for Grin1, 2a, and 2b probes (Figure 1C) but not for the Grin2c probe (data not shown). Specifically, Sp3 and Sp4 shift bands for Grin1, Grin2a, and Grin2b were observed (Figure 1C, lanes 1, 8, and 15, respectively) that were competed out by cold competitors (Figure 1C, lanes 2, 9, and 16). Supershift bands with Sp4 antibody were present for Grin1, Grin2a, and Grin2b (Figure 1C, lanes 5, 12, and 19, respectively), whereas Sp1 and Sp3 bands were not present for Grin1 and Grin2a, and were very faint for Grin2b (Figure 1C, lanes 3, 4, 10, 11, 17, and 18). An excess of unlabeled Grin1, Grin2b, and Grin3a probes with mutated Sp binding sites were added to respective radiolabeled probes, and they did not compete out the shift reactions (Figure 1C, lanes 6, 13, and 20, respectively). Shift reactions with mutated Sp sites on Grin1, Grin2a, and Grin2b did not reveal Sp binding (Figure 1C, lanes 7, 14, and 21, respectively).
3.4. In vivo interactions of Sp4 with Grin1, Grin2a, and Grin2b promoters in mouse visual cortex
The chromatin immunoprecipitation (ChIP) assay was performed to verify Sp1, Sp3, and Sp4 protein interaction with the Grin1 and Grin2a-c gene promoters in mouse visual cortical nuclear extract. Briefly, sonicated nuclear lysates from visual cortical tissue were immunoprecipitated with Sp1, Sp3, or Sp4 antibody and the resulting DNA was subjected to PCR analysis using primers surrounding the putative Sp binding sites identified by in silico analysis. As a control for the immunoprecipitation reaction, nerve growth factor receptor (NGFR) antibody was used. To eliminate the possibility of a bead-to-DNA interaction, an additional “no antibody” control was used. As Sp factors regulate GM3 Synthase [18], and Sp4 specifically regulates Neurotrophin 3 [19], primers against these gene promoters were used as positive controls for the immunoprecipitation. Primers in the coding region of β-actin that did not contain Sp binding sites were used as a negative control. As a positive control for the PCR reaction, 0.5% and 0.1% input DNA were used. Parallel PCR amplification of all controls and the immunoprecipitated samples was done to determine Sp factor binding.
As seen in Figure 2, agarose gel analysis of PCR products revealed specific bands for all input DNA controls. Furthermore, Sp4 immunoprecipitated sample revealed an enriched band for GM3 Synthase and Neurotrophin 3 positive controls, as well as for Grin1, Grin2a, and Grin2b. An enriched band was not present for β-actin negative control nor for Grin2c. Immunoprecipitated NGFR and “no antibody” negative controls did not reveal enrichment in any gene regions tested. There was also no enrichment of DNA above negative controls in the Sp1 or Sp3 immunoprecipitated samples for any of the tested regions (data not shown).
Figure 2.
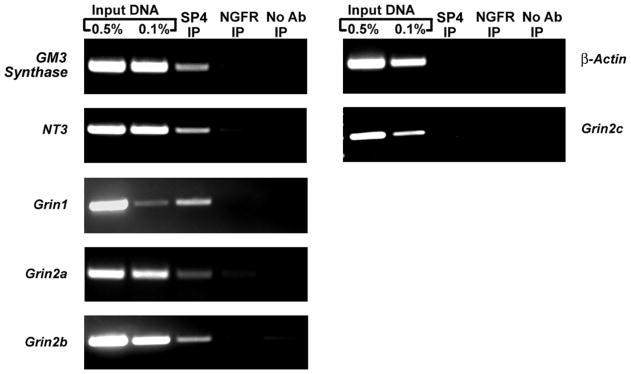
In vivo ChIP assays for Sp4 interaction with NMDA receptor subunits in mouse visual cortical tissue. Chromatin was precipitated with anti Sp4 antibodies (Sp4 IP lane), anti-nerve growth factor receptor p75 antibody (negative control, NGFR IP lane) or no antibody (negative control, no Ab lane). Control reactions for PCR were performed with 0.5% (input 0.5% IP lane) and 0.1% (input 0.1% IP lane) of input chromatin. GM3 synthase and Neurotrophin 3 were used as positive controls, and β-actin was used as a negative control. Results indicate interactions of Sp4 with Grin1, Grin2a, and Grin2b but not with Grin2c.
3.5. Effect of mutated Sp4 binding sites on the Grin1, Grin2a, and Grin2b promoters in N2a cells
Grin1, Grin2a, and Grin2b promoters that bound to Sp4 in vitro and in vivo were each cloned into pGL3 basic luciferase vectors. Site-directed mutations of the putative Sp4 binding sites identified by specific EMSA shift and supershift reactions were constructed. Transfection of control or mutated Sp promoter regions into N2a cells revealed a significant 34%, 38%, and 31% decrease in promoter activity of Grin1, Grin2a, and Grin2b promoters containing the mutated Sp4 motif, respectively (P < 0.001 for all, Figure 3).
Figure 3.
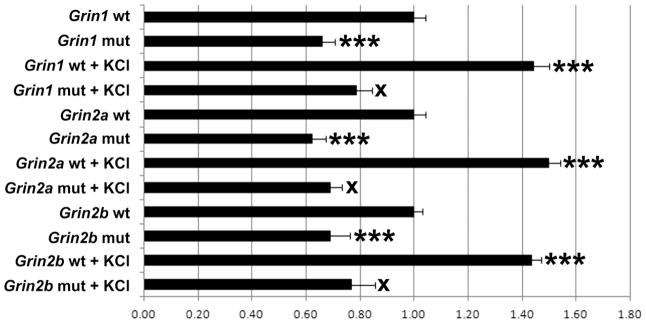
Site-directed mutational analysis of promoters of wild type (wt) and those with mutated Sp4 binding site (mut) for Grin1, Grin2a, and Grin2b genes in N2a cells. Mutating the Sp4 binding sites on Grin1, Grin2a, and Grin2b genes resulted in significant decreases in luciferase activity. KCl depolarization significantly increased promoter activity in all wild types, but not in the Grin1, Grin2a, and Grin2b promoters with mutated Sp sites. N = 6 for each construct. ***= P < 0.001; X = NS. All mutants and wild type + KCl are compared to the wild type. All mutant + KCl are compared to mutants.
3.6. Effect of mutated Sp4 binding sites on the response of Grin1, Grin2a, and Grin2b promoters to KCl depolarizing treatment in N2a cells
To verify that Sp4 binding is necessary for the up-regulation of Grin1, Grin2a, and Grin2b transcripts by KCl stimulation, N2a cells with wild type or Sp4 mutated Grin promoters were subjected to KCl depolarizing stimulation. As shown in Figure 3, KCl induced significant increases of 44%, 50%, and 43% in the expression of Grin1, Grin2a, and Grin2b promoters, respectively (P < 0.001 for all). The activity-driven increase was abolished by mutating the Sp4 binding site (Figure 3), confirming a requirement for Sp4 binding in the KCl depolarization-induced up-regulation of the Grin1, Grin2a, and Grin2b transcripts.
3.7. Effect of silencing Sp1, Sp3, and Sp4 on Grin1 and Grin2a-c in N2a cells
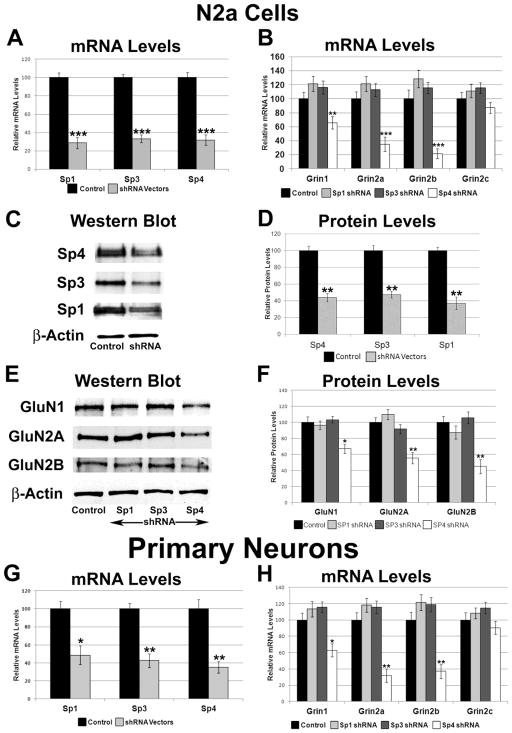
To determine the effect of silencing Sp1, Sp3, or Sp4 transcript on the expression of GluN1, GluN2A, GluN2B, and GluN2C, a combination of 2 – 5 plasmid vectors expressing small hairpin RNA (shRNA) against Sp1, Sp3, or Sp4 mRNA were used. Analysis of cDNAs of N2a cells transfected with shRNA vectors was done using quantitative real-time PCR and the 2−ΔΔCT method. β-actin was used as the internal control. Silencing of Sp4 resulted in significant decreases of 68% in Sp4 mRNA (P < 0.001, Figure 4A), and significant decreases of 34%, 65%, and 78% in Grin1, Grin2a and Grin2b mRNA levels, respectively (P < 0.01, P < 0.001, and P < 0.001, respectively; Figure 4B). With silencing, protein levels of Sp4 decreased significantly to 56% (P < 0.01, Figure 4C–D), and protein levels of GluN1, GluN2A, and GluN2B significantly decreased by 33%, 45%, and 55%, respectively (P < 0.05, P < 0.01, and P < 0.01, respectively; Figure 4E–F). mRNA levels of Grin2c did not change significantly with Sp4 silencing (Figure 4B).
Figure 4.
Effect of RNA interference-mediated silencing of Sp1, Sp3, or Sp4 on the expression of the NMDA receptor subunit genes. (A) Real-time PCR revealed a down-regulation of Sp1, Sp3, and Sp4 transcripts in N2a cells transfected with Sp1, Sp3, and Sp4 shRNA, respectively. N = 6. (B) mRNA levels of Grin1, Grin2a, and Grin2b were decreased with Sp4 shRNA but not with Sp3 or Sp1 shRNA. N = 6. (C–D) Western blots revealed a down-regulation of Sp4, Sp3, and Sp1 proteins in Sp4, Sp3, and Sp1 shRNA-transfected N2a cells, respectively. β-actin served as loading control and a representative blot is shown. N = 3. (E–F) Silencing of Sp4 reduced the protein levels of GluN1, GluN2A, and GluN2B, whereas silencing of Sp1 and Sp3 did not significantly change these subunit levels. β-actin served as a loading control. N = 3. (G) Primary neurons transfected with Sp1, Sp3, or Sp4 shRNA showed decreases in Sp1, Sp3, and Sp4 transcript, respectively. N = 3. (H) mRNA levels of Grin1, Grin2a, and Grin2b were decreased with Sp4 shRNA but not with Sp3 or Sp1 shRNA in primary neurons. Grin2c levels did not decrease with Sp1, Sp3, or Sp4 shRNA in primary neurons. N = 3. *= P < 0.01, **= P < 0.01, and ***= P < 0.001.
Silencing of Sp1 and Sp3 resulted in a 71% and 66% decrease in Sp1 and Sp3 mRNA levels, respectively (P < 0.001 for both, Figure 4A), and a 63% and 52% in Sp1 and Sp3 protein levels, respectively (P < 0.01 for both, Figure 4C–D). However, silencing neither Sp1 nor Sp3 significantly changed mRNA levels of Grin1 and Grin2a-c (Figure 4B), or protein levels of GluN1, GluN2A, or GluN2B (Figure 4E–F).
3.8. Effect of silencing Sp1, Sp3, and Sp4 on Grin1 and Grin2a-c in primary neurons
To determine if the effect of silencing Sp factors, especially the lack of an effect seen with silencing Sp1 and Sp3, was specific to N2a cells, we transfected cultured primary mouse cortical neurons with the same shRNAs as we used for N2a cells. Gapdh was used as the internal control. In these neurons, the silencing of Sp4 resulted in a 64% decrease in mRNA levels of Sp4 (P < 0.01, Figure 4G), and a significant 37%, 68%, and 63% decrease in mRNA levels of Grin1, Grin2a, and Grin2b, respectively (P < 0.05, P < 0.01, and P < 0.01, respectively, Figure 4H). No significant change was observed for Grin2c (Figure 4H).
Silencing of Sp1 or Sp3 resulted in significant decreases in Sp1 or Sp3 mRNA levels (51% and 57%, respectively, P < 0.01 for both, Figure 4G), but did not significantly change mRNA levels of Grin1, Grin2a, Grin2b, and Grin2c (Figure 4H).
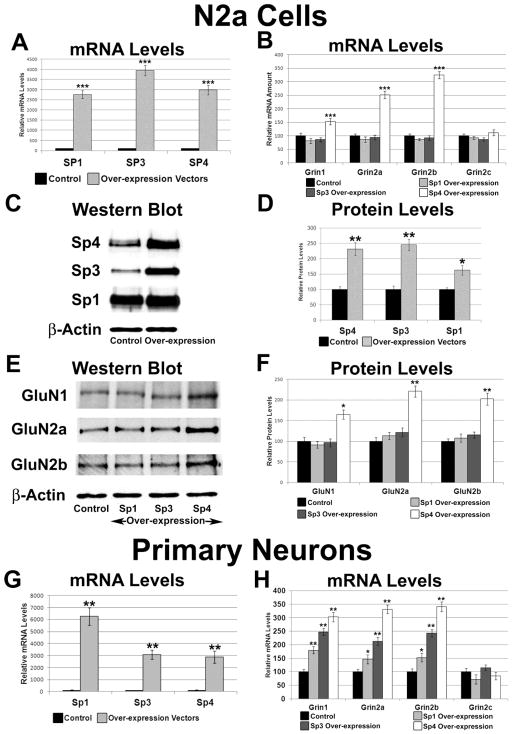
3.9. Effect of over-expressing Sp1, Sp3, and Sp4 on Grin1 and Grin2a-c in N2a Cells
To determine the effect of over-expressing Sp1, Sp3, or Sp4 on transcript levels of Grin1 and Grin2a-c, plasmids over-expressing Sp1, Sp3, or Sp4 were transfected into N2a cells. β-actin was used as the internal control. Sp4 over-expression resulted in an approximately 30-fold increase in Sp4 mRNA levels (P < 0.001, Figure 5A), and significant increases by 53%, 152%, and 224% in mRNA levels of Grin1, Grin2a, and Grin2b, respectively (P < 0.001 for all; Figure 5B). mRNA levels of Grin2c did not change significantly (Figure 5B). Sp4 over-expression led to a significant 132% increase in Sp4 protein levels (P < 0.01, Figure 5C–D), and significant increases by 65%, 121%, and 102% in protein levels of GluN1, GluN2A, and GluN2B, respectively (P < 0.05, P < 0.01, and P < 0.01, respectively, Figure 5E–F).
Figure 5.
Effect of Sp1, Sp3, and Sp4 over-expression on the transcript and protein levels of NMDA receptor subunit genes. (A) Real-time PCR revealed an up-regulation of Sp1, Sp3, and Sp4 transcripts in N2a cells transfected with Sp1, Sp3, and Sp4 over-expression vectors, respectively. N = 6. (B) mRNA levels of Grin1, Grin2a, and Grin2b were increased with Sp4 over-expression but not with Sp3 or Sp1 over-expression. N = 6. (C-D) Western blots revealed an up-regulation of Sp4, Sp3, and Sp1 protein with Sp4, Sp3, and Sp1 over-expression, respectively, in N2a cells. β-actin served as a loading control and a representative blot is shown. N = 3. (E–F) Over-expression of Sp4 increased protein levels of GluN1, GluN2A, and GluN2B, whereas over-expression of Sp1 and Sp3 did not significantly change these subunit levels. β-actin served as a loading control. N = 3. (G) Primary neurons transfected with Sp1, Sp3, or Sp4 over-expression showed increases in Sp1, Sp3, and Sp4 transcripts, respectively. N = 3. (H) mRNA levels of Grin1, Grin2a, and Grin2b were increased with Sp4 over-expression but not with Sp3 or Sp1 over-expression in primary neurons. Grin2c levels did not increase with Sp1, Sp3, or Sp4 overexpression in primary neurons. N = 3. *= P < 0.01, **= P < 0.01, and ***= P < 0.001.
Sp1 or Sp3 over-expression led to a significant 27 and 39-fold increase in their respective mRNA levels (P < 0.001 for both; Figure 5A), and a significant 63% and 146% increase in their respective protein levels (P < 0.01 and P < 0.05, respectively, Figure 5C–D). However, over-expression of Sp1 or Sp3 did not significantly change mRNA levels of Grin1 and Grin2a-c (Figure 5B) or protein levels of GluN1, GluN2A, and GluN2B (Figure 5E–F).
3.10. Effect of over-expressing Sp1, Sp3, and Sp4 on Grin1 and Grin2a-c in primary neurons
To determine whether the lack of an effect seen with over-expression of Sp1 and Sp3 was restricted to N2a cells, cultured primary mouse cortical neurons were transfected with Sp1, Sp3, or Sp4 over-expression vectors. Gapdh was used as the internal control. Over-expression of Sp4 in cultured mouse cortical neurons resulted in a 29-fold increase in mRNA levels of Sp4 (P < 0.01, Figure 5G), with a 203%, 231%, and 241% increase in mRNA levels of Grin1, Grin2a, and Grin2b, respectively (P < 0.01 for all; Figure 5H). Grin2c levels were not changed by Sp4 over-expression (Figure 5H).
Over-expression of Sp1 or Sp3 resulted in a significant 63 and 31-fold increase in respective mRNA levels (P < 0.01 for both; Figure 5G), and significant changes in mRNA levels of Grin1, Grin2a, and Grin2b (Figure 5H), but not Grin2c (Figure 5H). The increase in mRNA levels of Grin1, Grin2a, and Grin2b with Sp1 and Sp3 over-expression was significantly less than that seen with Sp4 over-expression.
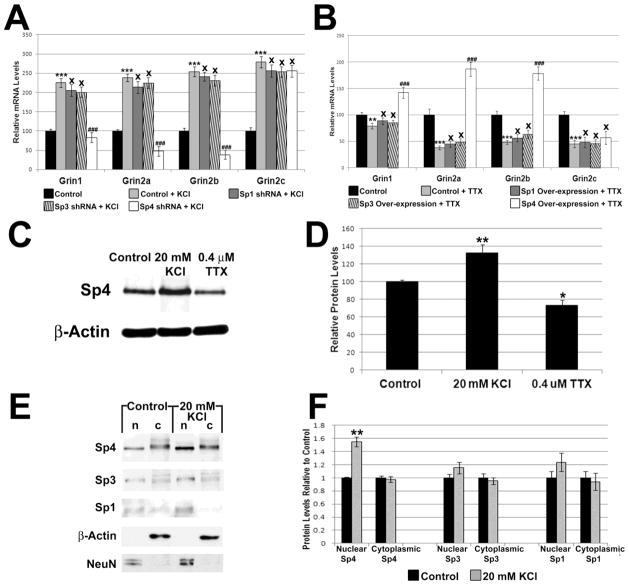
3.11. Silencing of Sp4 abolished the KCl-induced up-regulation of Grin1, Grin2a, and Grin2b transcripts in N2a Cells
We have previously shown that NMDA subunits are up-regulated in response to physiological concentrations of KCl, a depolarizing agent that increases neuronal activity [6]. To determine if the Sp factors are necessary for this up-regulation, control N2a cells and cells transfected with Sp1, Sp3, or Sp4 shRNA were subjected to 20 mM KCl for 5 h. As seen in Figure 6A, KCl depolarization significantly increased Grin1, Grin2a, and Grin2b transcript levels by 125%, 138%, and 154%, respectively (P < 0.001 for all). In the presence of Sp4 shRNA, KCl failed to increase transcript levels of Grin1, Grin2a, and Grin2b (Figure 6A). On the other hand, Sp1 or Sp3 shRNA did not prevent activity-mediated increases in Grin1, Grin2a, and Grin2b transcript levels (Figure 6A). Transcript levels of Grin2c increased significantly with KCl treatment (P < 0.001; Figure 6A) but was not significantly changed in the presence of Sp1, Sp3, or Sp4 shRNA (Figure 6A).
Figure 6.
Effect of increased or decreased neuronal activity on Sp factors and on target genes in the presence of Sp1, Sp3, or Sp4 silencing or over-expression. (A) N2a cells treated for 5 h with 20 mM KCl revealed an up-regulation of all transcripts as compared to controls. In the presence of Sp4 silencing, 5 h treatment with 20 mM KCl failed to up-regulate the transcripts of Grin1, Grin2a, and Grin2b, but had no effect on Grin2c. Sp3 and Sp1 silencing did not prevent KCl-induced up-regulation of Grin1 and Grin2a-c subunits. N = 6. (B) N2a cells treated for 3 days with 0.4 μM TTX revealed a down-regulation of Grin1 and Grin2a-c subunits as compared to controls. Over-expression of Sp4 rescued the down-regulation of the Grin1, Grin2a, and Grin2b transcripts, but not that of Grin2c. Over-expression of Sp1 or Sp3 did not rescue the down-regulation of Grin1 and Grin2a-c transcripts seen with KCl treatment. N = 6. (C–D) KCl-induced activity increased protein levels of Sp4, whereas TTXinduced impulse blockade decreased Sp4 protein levels in primary neurons. N = 3. (E–F) Increased neuronal activity led to an increase in the nuclear Sp4 but not cytoplasmic Sp4. Nuclear and cytoplasmic levels of Sp1 and Sp3 did not change significantly. β-actin served as a loading control and indicated no cytoplasmic contamination of the nuclear fraction. NeuN was present only in the nucleus and indicated no nuclear contamination of the cytoplasmic fraction. N = 3. *= P < 0.01, **= P < 0.01, and ***= P < 0.001; (A–B) ### = P < 0.001 and X = non-significant when compared to KCl- or TTX-treated samples
3.12. Over-expression of Sp4 rescued the TTX-mediated down-regulation of Grin1, Grin2a, and Grin2b in N2a cells
Our laboratory has shown previously that NMDA subunit transcript levels are down-regulated in response to 0.4 μM TTX [6]. To determine if over-expression of Sp factors can rescue NMDA transcript levels suppressed by TTX-induced impulse blockade, control N2a cells and cells transfected with Sp1, Sp3, or Sp4 over-expression vectors were subjected to 0.4 μM TTX treatment for 3 days. Control cells exposed to TTX showed a significant decrease of 21%, 63%, and 52% in transcript levels of Grin1, Grin2a, and Grin2b, respectively (P < 0.01, P < 0.001, and P < 0.001, respectively; Figure 6B). Cells that were transfected with Sp4 over-expression vectors rescued the down-regulation seen with TTX treatment, with an increase in Grin1, Grin2a, and Grin2b transcript levels of 42%, 86%, and 78%, respectively, as compared to controls (P < 0.001 for all as compared to TTX alone; Figure 6B). Grin2c transcript level decreased significantly with TTX treatment (P < 0.001, Figure 6B) but was not rescued with Sp4 over-expression (Figure 6B). Over-expression of Sp1 or Sp3 did not rescue the TTX-mediated decrease in transcript levels of Grin1, Grin2a, Grin2b, and Grin2c (Figure 6B).
3.13. Effect of KCl and TTX on Sp4 levels in primary neurons
To determine if Sp4 itself is regulated by neuronal activity, we analyzed its protein levels in the presence of KCl or TTX. As shown in Figure 6C–D, Sp4 protein levels increased significantly by 33% with 5 h of KCl depolarizing treatment (P < 0.01) and decreased significantly by 26% (P < 0.05) with 0.4 uM TTX treatment in primary neurons.
To determine if increased Sp4 expression seen with KCl treatment represents new synthesis and nuclear translocation of Sp4, we harvested the nuclear and cytoplasmic fractions from cultured mouse cortical neurons treated with and without KCl. We loaded an equal amount of cytoplasmic extract for both control and treated cells. We also loaded an equal amount of nuclear extracts proportional to the cytoplasmic volume for both control and treated cells. β-actin was used as the cytoplasmic loading control. Its absence in the nuclear fraction indicated that the fraction was free of cytoplasmic contamination (Figure 6E). NeuN, a neuronal DNA binding protein and nuclear marker, was present in the nuclear fraction only (Figure 6E), indicating that the cytoplasmic fraction was free of nuclear contamination. The intensity of NeuN did not change significantly with KCl treatment (data not shown).
Depolarizing treatment led to a significant 54% increase in Sp4 protein in the nuclear fraction as compared to controls (P < 0.01, Figure 6E–F), whereas the cytoplasmic fraction of Sp4 was not significantly changed (Figure 6E–F). This indicates that there was new synthesis and nuclear translocation of Sp4 in primary neurons. On the other hand, depolarizing treatment did not lead to a significant increase in the cytoplasmic or nuclear fractions of Sp1 or Sp3.
3.14. Homology
The functional Sp binding sites are conserved among mice, rats, and humans for Grin1, Grin2a, and Grin2b (Figure 7).
Figure 7.
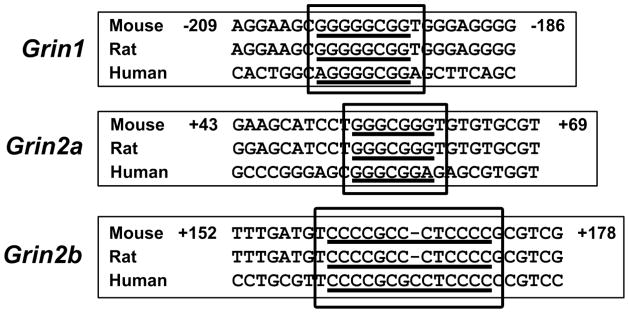
Aligned partial sequences of Grin1, Grin2a, and Grin2b promoters from mouse, rat, and human showed conserved Sp binding sites.
4. Discussion
Using multiple approaches, including EMSA and supershift assays, ChIP in primary visual cortical tissue, promoter mutational analysis, over-expression and knock-down experiments, the present study documents for the first time that specificity protein 4 (Sp4) functionally regulates the expression of NMDA receptor subunits GluN1, GluN2A, and GluN2B (Grin1, Grin2a, and Grin2b, respectively). Moreover, silencing Sp4 prevented the up-regulation of Grin1, Grin2a, and Grin2b mRNA levels induced by depolarizing stimulation, whereas over-expressing Sp4 rescued mRNA levels suppressed by TTX-induced impulse blockade. The Sp regulatory sites tested in this study are conserved among mice, rats, and/or humans.
The NMDA receptor is a family of glutamatergic receptors that mediate the majority of excitatory neurotransmission in the brain. They are voltage-dependent, ligand-gated, heterotetrameric ion channels composed of GluN1 in various combinations with GluN2A-D and/or GluN3A-B subunits [1]. When activated, NMDA receptors exhibit a relatively higher permeability to Ca2+ ion than the AMPA or kainite excitatory receptors [24]. NMDA receptors are functionally linked to a variety of normal neuronal processes, including neural development, plasticity, learning, and memory [25–27]. However, with excessive Ca2+ influx, NMDA receptors are implicated in neuropathological conditions, such as excitotoxicity and many neurodegenerative diseases [28]. Thus, the proper regulation of NMDA receptors is crucial for the health and survival of neurons in the CNS.
Sp4 is in the specificity protein/Krüppel-like (SP/KLF) family of zinc finger transcription factors that bind to GC-rich sequences of DNA (for review see [11]). The SP/KLF family currently has 25 members and, of these, Sp4 is structurally most similar to specificity proteins 1 (Sp1) and 3 (Sp3) [11]. Sp4, Sp3, and Sp1 share critical amino acid homology in their DNA binding domains and compete for the same cis- elements [11]. Unlike Sp1 and Sp3, whose expression is ubiquitous, Sp4 is found primarily in the brain and testes, and its neuronal expression increases with development [14]. Hypomorphic Sp4 mice have vacuolization in the hippocampal gray matter and deficits in both sensorimotor gating and contextual memory. Restoration of Sp4 rescues all observable molecular, histological, and behavioral abnormalities in these mice [29]. Sp4-null male mice do not breed but have a normal reproductive system, suggesting a possible deficit in the nervous system [14].
The present study has found that Sp4 exists in greater abundance in the murine brain than in N2a or HeLa cells, whereas the opposite is true for Sp1 and Sp3. Furthermore, mRNA and protein levels of Sp4 are higher than that of Sp1 in primary neurons [30]. On the other hand, Sp1 and Sp3, but not Sp4, are highly expressed in glial cells [30]. Consistent with these findings is our discovery that it is Sp4, not Sp1 or Sp3, that regulates the expression of Grin1, Grin2a, and Grin2b in neurons. N2a cells possess smaller isoforms of Sp1 and Sp3 that are not present in primary neurons (See Figure 1A). The smaller isoforms of Sp3 are known to act as transcriptional repressors and the same may be true for Sp1 [22]. In the absence of the smaller isoforms, primary neurons over-expressing the exogenous full length Sp3 or Sp1 may be activated to express their target genes, whereas such activation was not seen in N2a cells (Figure 5H and 5B).
Of the NMDA receptor subunits, GluN1 is obligatory to the formation of a functional receptor. This subunit binds to the co-agonist glycine, forms the pore of the NMDA channel, and is expressed ubiquitously in both pre- and postnatal brains [5]. Although a binding site for Sp1 was previously reported but not characterized on the Grin1 promoter [1, 31], our present study documents that it is Sp4, and not Sp1, that exerts such functional regulation in neurons.
The GluN2A-D subunits each binds to the agonist glutamate [5]. Most NMDA receptors in the CNS are composed of two GluN1 and two GluN2A or two GluN2B subunits [3]. The expression of GluN1/GluN2A receptors is limited in the neonate but becomes prominent in the adult, whereas the expression GluN1/GluN2B receptors is prominent in both the neonate and adult [32]. The GluN1/GluN2A receptors confer a lower affinity for glutamate and faster channel kinetics than the GluN1/GluN2B receptors [2]. In the adult, the GluN1/GluN2A receptors are prevalent at synaptic sites, whereas the GluN1/GluN2B receptors dominate extra-synaptically, with each receptor subtype playing distinct roles in the coupling to intracellular signaling cascades, synaptic plasticity, and cell death [33–35]. The role of GluN1/GluN2A receptors in synaptic plasticity, whether long term potentiation or long term depression, or the location and conditions under which GluN2A plays a role, is still debated [35–38].
The GluN1/GluN2B receptor has higher affinity for the signaling protein CaMKII than GluN1/GluN2A, interacting directly with CaMKII to modulate synaptic plasticity, learning, and memory [39]. Upon activation, GluN1/GluN2B receptors allow for the entry of large amounts of cations, particularly Ca2+, which is responsible for its functional role in neuronal plasticity, especially long term potentiation, as well as its role in learning and memory [25, 27]. When activated in excess, however, the GluN1/GluN2B receptors are implicated in excitotoxicity and cell death [40]. The regulation of GluN2B is critical for neuronal health, with GluN2B knockout mice dying at birth. On the other hand, GluN2A knockout mice are viable, though possessing neurological deficits [24]. The present study shows for the first time that it is Sp4, and not Sp1 as previously postulated [41, 42], that regulates both GluN2A and GluN2B in neurons.
mRNA levels of GluN1, GluN2A, and GluN2B change in response to perturbations in neuronal activity. Specifically, increasing neuronal activity by KCl-induced depolarization or decreasing neuronal activity by the action potential blocker TTX cause parallel changes in Grin1, Grin2a, and Grin2b [[6] and the present study]. Sp4 itself responds to depolarizing stimulation with its own new synthesis and translocation to the nucleus [the present study], consistent with our hypothesis that Sp4 activates its target genes, Grin1, Grin2a, and Grin2b, in an activity-dependent manner. However, under extreme conditions, such as an hour of excitotoxic insult, Sp4 degrades via a calpain-mediated pathway [30]. With excitotoxicity, synaptic GluN1/GluN2A receptors confer neuroprotection through the activation of an Akt- or ERK-dependent anti-apoptotic signaling pathway [43]. Extra-synaptic GluN1/GluN2B receptors, however, are pro-apopotoic [43]. We propose that Sp4 closely regulates the expression of GluN1, GluN2A, and GluN2B receptor subunits under physiological conditions of neuronal activity, but that under excitotoxic conditions, Sp4 itself is degraded, thereby down-regulating all of its target genes, especially the neuroprotective GluN2A subunit.
The GluN2C and 2D subunits are developmentally and regionally expressed, and relatively little is known about their functions [5]. The GluN3A and 3B subunits bind glycine, but like GluN2C and 2D, are thought to be regionally and developmentally expressed [44]. The present study indicates that GluN2C is not regulated by Sp4, Sp3, nor Sp1. The other subunits are beyond the scope of this study.
Neuronal activity is a highly energy demanding process, and it is coupled to energy metabolism at the cellular level [45]. Recently, we found that this coupling extends to the molecular level in that the same transcription factors, nuclear respiratory factor 1 (NRF-1) and 2 (NRF-2), each co-regulates mediators of energy metabolism and neuronal activity. Both NRF-1 and NRF-2 co-regulate the GluN1 and GluN2B subunits of the NDMA receptor [6, 7] as well as all 13 subunits of cytochrome c oxidase (COX) [9, 10], the terminal enzyme of the mitochondrial electron transport chain critical for energy generation in neurons. The present study documents that Sp4 regulates the GluN1, GluN2A, and GluN2B subunits of the NDMA receptor. Sp1 factor has also been found recently to regulate all 13 subunits of COX [15]. Thus, Sp factors can also mediate the coupling of neuronal activity with energy metabolism. Of the three mechanisms possible for Sp4’s regulation of the NMDA receptor subunit genes with respect to NRF-1 and NRF-2: complementary, concurrent and parallel, and a combination of complementary and concurrent/parallel, the present results are consistent with a combination of the complementary and concurrent/parallel mechanism: complementary for Grin2a, and concurrent/parallel for Grin1 and Grin2b (Figure 8).
Figure 8.
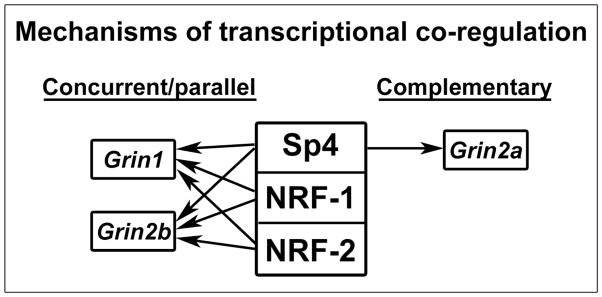
A mechanistic scheme of transcriptional co-regulation by Sp4, NRF-1, and NRF-2. The three factors co-regulate Grin1 and Grin2b in a concurrent and parallel (same direction) manner, but only Sp4 regulates Grin2a, hence via a complementary mechanism.
A possible benefit of a combination of complementary and concurrent/parallel mechanism is that the critical GluN1 and GluN2B subunits are regulated by multiple transcription factors, but that there is a differential regulation for GluN2A. It is likely that Sp4, NRF-2, and NRF-1 transcription factors play different and non-redundant roles in mediating the tight coupling of neuronal activity and energy metabolism under different conditions. Other transcription factors or transcription co-activators may also be involved. Future studies will be directed at these issues.
Supplementary Material
Highlights.
Sp4 regulates the expressions of NMDA receptor subunits GluN1, GluN2A, and GluN2B
Sp4 silencing prevented the KCl-induced up-regulation of Grin1/2a/2b transcripts
Sp4 over-expression rescued TTX-mediated down-regulation of Grin1/2a/2b transcripts
Sp4 regulates Grin1/2b concurrently and Grin2a complementarily with NRF-1 and NRF-2
Sp1 and Sp3 do not regulate the three NMDA receptor subunits regulated by Sp4
Acknowledgments
This work is supported by NIH Grant R01 EY018441 and NIH/NEI Training Grant 1-T32-EY14537. Anusha Priya is a member of the MCW-MSTP which is partially supported by a T32 grant from NIGMS, GM080202.
Abbreviations
- Sp
Specificity protein transcription factor
- Grin
Gene name for NMDA receptor
Footnotes
Conflict of Interest: The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McBain CJ, Mayer ML. N-methyl-D-aspartic acid receptor structure and function. Physiol Rev. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- 2.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 3.Behe P, Stern P, Wyllie DJ, Nassar M, Schoepfer R, Colquhoun D. Determination of NMDA NR1 subunit copy number in recombinant NMDA receptors. Proc Biol Sci. 1995;262:205–213. doi: 10.1098/rspb.1995.0197. [DOI] [PubMed] [Google Scholar]
- 4.Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- 5.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 6.Dhar SS, Wong-Riley MT. Coupling of energy metabolism and synaptic transmission at the transcriptional level: role of nuclear respiratory factor 1 in regulating both cytochrome c oxidase and NMDA glutamate receptor subunit genes. J Neurosci. 2009;29:483–492. doi: 10.1523/JNEUROSCI.3704-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Priya A, Johar K, Wong-Riley MT. Nuclear respiratory factor 2 regulates the expression of the same NMDA receptor subunit genes as NRF-1: both factors act by a concurrent and parallel mechanism to couple energy metabolism and synaptic transmission. Biochim Biophys Acta. 2013;1833:48–58. doi: 10.1016/j.bbamcr.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ongwijitwat S, Wong-Riley MT. Is nuclear respiratory factor 2 a master transcriptional coordinator for all ten nuclear-encoded cytochrome c oxidase subunits in neurons? Gene. 2005;360:65–77. doi: 10.1016/j.gene.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Ongwijitwat S, Liang HL, Graboyes EM, Wong-Riley MT. Nuclear respiratory factor 2 senses changing cellular energy demands and its silencing down-regulates cytochrome oxidase and other target gene mRNAs. Gene. 2006;374:39–49. doi: 10.1016/j.gene.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Dhar SS, Ongwijitwat S, Wong-Riley MT. Nuclear respiratory factor 1 regulates all ten nuclear-encoded subunits of cytochrome c oxidase in neurons. J Biol Chem. 2008;283:3120–3129. doi: 10.1074/jbc.M707587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suske G. The Sp-family of transcription factors. Gene. 1999;238:291–300. doi: 10.1016/s0378-1119(99)00357-1. [DOI] [PubMed] [Google Scholar]
- 12.Letovsky J, Dynan WS. Measurement of the binding of transcription factor Sp1 to a single GC box recognition sequence. Nucleic Acids Res. 1989;17:2639–2653. doi: 10.1093/nar/17.7.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang SJ, Liang HL, Ning G, Wong-Riley MT. Ultrastructural study of depolarization-induced translocation of NRF-2 transcription factor in cultured rat visual cortical neurons. Eur J Neurosci. 2004;19:1153–1162. doi: 10.1111/j.1460-9568.2004.03250.x. [DOI] [PubMed] [Google Scholar]
- 14.Supp DM, Witte DP, Branford WW, Smith EP, Potter SS. Sp4, a member of the Sp1-family of zinc finger transcription factors, is required for normal murine growth, viability, and male fertility. Dev Biol. 1996;176:284–299. doi: 10.1006/dbio.1996.0134. [DOI] [PubMed] [Google Scholar]
- 15.Dhar SS, Johar K, Wong-Riley MT. Bigenomic transcriptional regulation of all thirteen cytochrome c oxidase subunit genes by specificity protein 1. Open Biol. 2013;3:120176. doi: 10.1098/rsob.120176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ongwijitwat S, Wong-Riley MT. Functional analysis of the rat cytochrome c oxidase subunit 6A1 promoter in primary neurons. Gene. 2004;337:163–171. doi: 10.1016/j.gene.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 17.Abmayr SM, Yao T, Parmely T, Workman JL. Preparation of nuclear and cytoplasmic extracts from mammalian cells. Curr Protoc Mol Biol. 2006;Chapter 12(Unit 12):11. doi: 10.1002/0471142727.mb1201s75. [DOI] [PubMed] [Google Scholar]
- 18.Xia T, Zeng G, Gao L, Yu RK. Sp1 and AP2 enhance promoter activity of the mouse GM3-synthase gene. Gene. 2005;351:109–118. doi: 10.1016/j.gene.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Ishimaru N, Tabuchi A, Hara D, Hayashi H, Sugimoto T, Yasuhara M, Shiota J, Tsuda M. Regulation of neurotrophin-3 gene transcription by Sp3 and Sp4 in neurons. J Neurochem. 2007;100:520–531. doi: 10.1111/j.1471-4159.2006.04216.x. [DOI] [PubMed] [Google Scholar]
- 20.Dhar SS, Liang HL, Wong-Riley MT. Transcriptional coupling of synaptic transmission and energy metabolism: role of nuclear respiratory factor 1 in co-regulating neuronal nitric oxide synthase and cytochrome c oxidase genes in neurons. Biochim Biophys Acta. 2009;1793:1604–1613. doi: 10.1016/j.bbamcr.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Infantino V, Convertini P, Iacobazzi F, Pisano I, Scarcia P, Iacobazzi V. Identification of a novel Sp1 splice variant as a strong transcriptional activator. Biochem Biophys Res Commun. 2011;412:86–91. doi: 10.1016/j.bbrc.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 22.Kennett SB, Udvadia AJ, Horowitz JM. Sp3 encodes multiple proteins that differ in their capacity to stimulate or repress transcription. Nucleic Acids Res. 1997;25:3110–3117. doi: 10.1093/nar/25.15.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan NY, Khachigian LM. Sp1 phosphorylation and its regulation of gene transcription. Mol Cell Biol. 2009;29:2483–2488. doi: 10.1128/MCB.01828-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sprengel R, Suchanek B, Amico C, Brusa R, Burnashev N, Rozov A, Hvalby O, Jensen V, Paulsen O, Andersen P, Kim JJ, Thompson RF, Sun W, Webster LC, Grant SG, Eilers J, Konnerth A, Li J, McNamara JO, Seeburg PH. Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell. 1998;92:279–289. doi: 10.1016/s0092-8674(00)80921-6. [DOI] [PubMed] [Google Scholar]
- 25.Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 26.Wang CC, Held RG, Chang SC, Yang L, Delpire E, Ghosh A, Hall BJ. A critical role for GluN2B-containing NMDA receptors in cortical development and function. Neuron. 2011;72:789–805. doi: 10.1016/j.neuron.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 27.El Gaamouch F, Buisson A, Moustie O, Lemieux M, Labrecque S, Bontempi B, De Koninck P, Nicole O. Interaction Between alphaCaMKII and GluN2B Controls ERK-Dependent Plasticity. J Neurosci. 2012;32:10767–10779. doi: 10.1523/JNEUROSCI.5622-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pina-Crespo JC, Talantova M, Micu I, States B, Chen HS, Tu S, Nakanishi N, Tong G, Zhang D, Heinemann SF, Zamponi GW, Stys PK, Lipton SA. Excitatory glycine responses of CNS myelin mediated by NR1/NR3 “NMDA” receptor subunits. J Neurosci. 2010;30:11501–11505. doi: 10.1523/JNEUROSCI.1593-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou X, Long JM, Geyer MA, Masliah E, Kelsoe JR, Wynshaw-Boris A, Chien KR. Reduced expression of the Sp4 gene in mice causes deficits in sensorimotor gating and memory associated with hippocampal vacuolization. Mol Psychiatry. 2005;10:393–406. doi: 10.1038/sj.mp.4001621. [DOI] [PubMed] [Google Scholar]
- 30.Mao X, Yang SH, Simpkins JW, Barger SW. Glutamate receptor activation evokes calpain-mediated degradation of Sp3 and Sp4, the prominent Sp-family transcription factors in neurons. J Neurochem. 2007;100:1300–1314. doi: 10.1111/j.1471-4159.2006.04297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamoto S, Sherman K, Bai G, Lipton SA. Effect of the ubiquitous transcription factors, SP1 and MAZ, on NMDA receptor subunit type 1 (NR1) expression during neuronal differentiation. Brain Res Mol Brain Res. 2002;107:89–96. doi: 10.1016/s0169-328x(02)00440-0. [DOI] [PubMed] [Google Scholar]
- 32.Sowa Y, Shiio Y, Fujita T, Matsumoto T, Okuyama Y, Kato D, Inoue J, Sawada J, Goto M, Watanabe H, Handa H, Sakai T. Retinoblastoma binding factor 1 site in the core promoter region of the human RB gene is activated by hGABP/E4TF1. Cancer Res. 1997;57:3145–3148. [PubMed] [Google Scholar]
- 33.Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choo AM, Geddes-Klein DM, Hockenberry A, Scarsella D, Mesfin MN, Singh P, Patel TP, Meaney DF. NR2A and NR2B subunits differentially mediate MAP kinase signaling and mitochondrial morphology following excitotoxic insult. Neurochem Int. 2012;60:506–516. doi: 10.1016/j.neuint.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology. 2007;52:60–70. doi: 10.1016/j.neuropharm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Foster KA, McLaughlin N, Edbauer D, Phillips M, Bolton A, Constantine-Paton M, Sheng M. Distinct roles of NR2A and NR2B cytoplasmic tails in long-term potentiation. J Neurosci. 2010;30:2676–2685. doi: 10.1523/JNEUROSCI.4022-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui Z, Feng R, Jacobs S, Duan Y, Wang H, Cao X, Tsien JZ. Increased NR2A:NR2B ratio compresses long-term depression range and constrains long-term memory. Sci Rep. 2013;3:1036. doi: 10.1038/srep01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, Takahashi E, Li W, Halt A, Wiltgen B, Ehninger D, Li GD, Hell JW, Kennedy MB, Silva AJ. Interactions between the NR2B receptor and CaMKII modulate synaptic plasticity and spatial learning. J Neurosci. 2007;27:13843–13853. doi: 10.1523/JNEUROSCI.4486-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu A, Zhuang Z, Hoffman PW, Bai G. Functional analysis of the rat N-methyl-D-aspartate receptor 2A promoter: multiple transcription starts points, positive regulation by Sp factors, and translational regulation. J Biol Chem. 2003;278:26423–26434. doi: 10.1074/jbc.M211165200. [DOI] [PubMed] [Google Scholar]
- 42.Klein M, Pieri I, Uhlmann F, Pfizenmaier K, Eisel U. Cloning and characterization of promoter and 5′-UTR of the NMDA receptor subunit epsilon 2: evidence for alternative splicing of 5′-non-coding exon. Gene. 1998;208:259–269. doi: 10.1016/s0378-1119(98)00005-5. [DOI] [PubMed] [Google Scholar]
- 43.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 45.Wong-Riley MT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989;12:94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.