Abstract
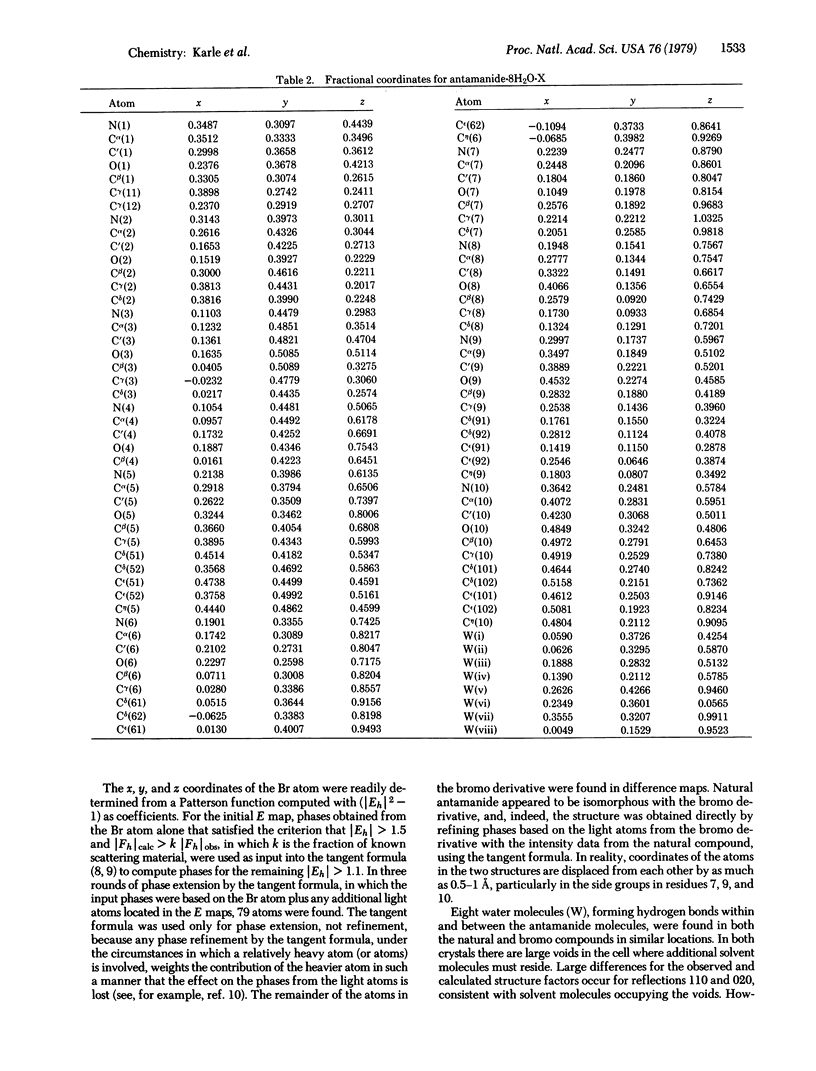
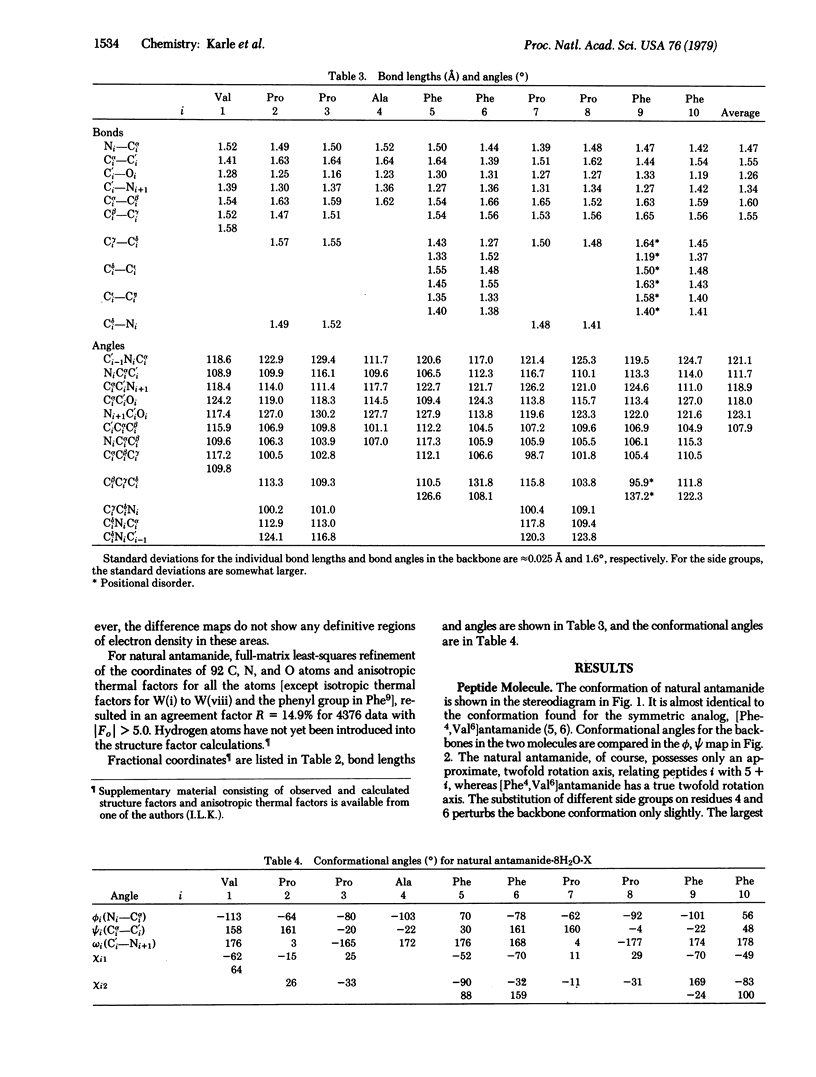
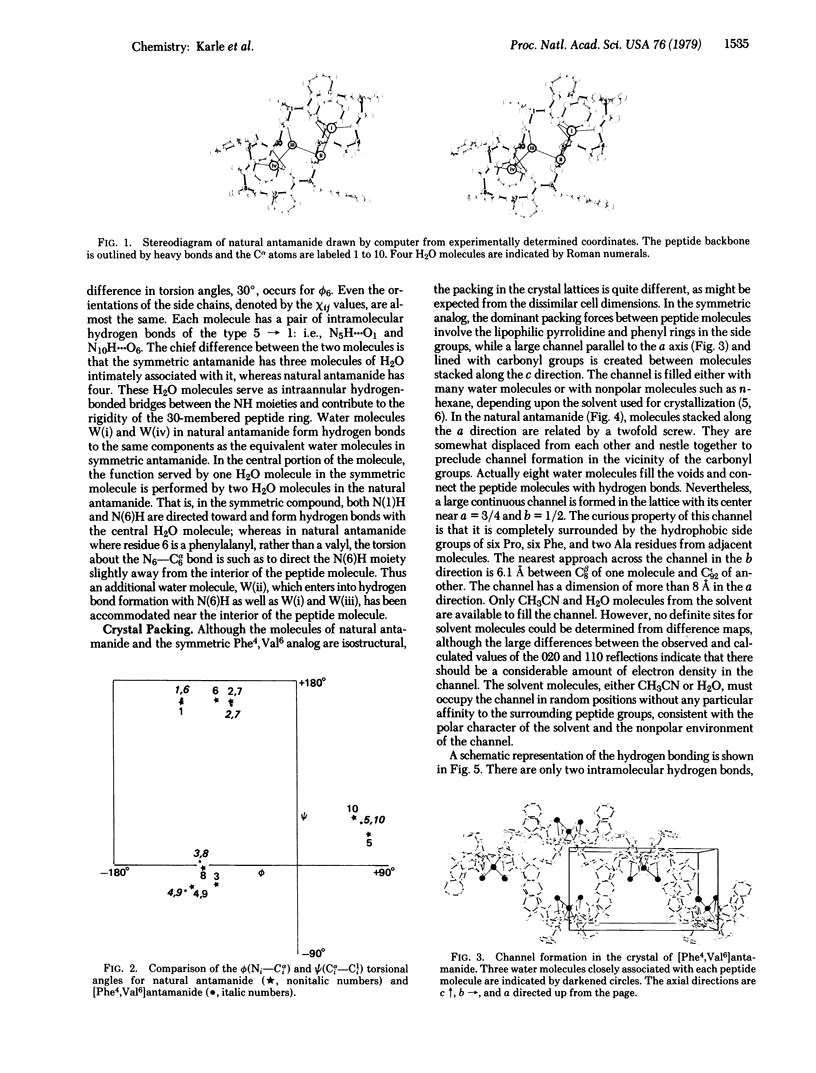
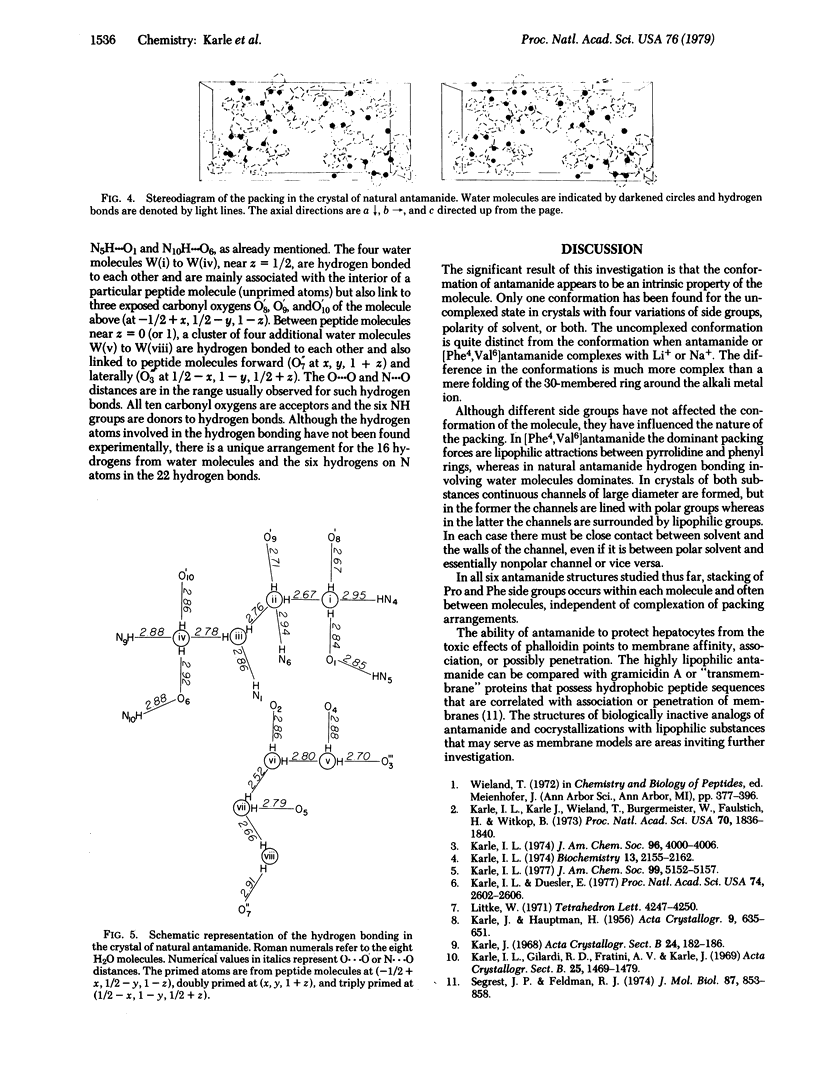
Natural antamanide, a cyclic decapeptide from Amanita phalloides, has been shown to have the same conformation for the backbone and comparable side groups as the biologically active synthetic [Phe4, Val6]antamanide. Packing in the crystal is quite different for the two compounds. Large channels with a polar lining are formed in the crystals of [Phe4, Val6]antamanide, whereas in natural antamanide large channels in the crystal are completely surrounded by the lipophilic side groups of the Pro, Phe, and Ala residues. Antamanide, C64H78N10O10·8H2O·X, crystallizes in space group P212121 with a = 15.88 Å, b = 34.88 Å, and c = 13.61 Å.
Keywords: bound water, solvent channels, hydrogen bonds, x-ray analysis
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Karle I. L. Conformation of the lithium ion complex of antamanide, a cyclic decapeptide and ion carrier, in the crystalline state. J Am Chem Soc. 1974 Jun 12;96(12):4000–4006. doi: 10.1021/ja00819a044. [DOI] [PubMed] [Google Scholar]
- Karle I. L., Duesler E. Arrangement of water molecules in cavities and channels of the lattice of [Phe4Val6]antamanide dodecahydrate. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2602–2606. doi: 10.1073/pnas.74.7.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karle I. L., Karle J., Wieland T., Burgermeister W., Faulstich H., Witkop B. Conformations of the li-antamanide complex and na-[phe, val]antamanide complex in the crystalline state. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1836–1840. doi: 10.1073/pnas.70.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karle I. L. The conformation of the sodium complex of a biologically active analog of antamanide in the crystalline state. Biochemistry. 1974 May 7;13(10):2155–2162. doi: 10.1021/bi00707a025. [DOI] [PubMed] [Google Scholar]
- Karle I. L. [Phe4, Val6]antamanide crystallized from methyl acetate/n-hexane. Conformation and packing. J Am Chem Soc. 1977 Jul 20;99(15):5152–5157. doi: 10.1021/ja00457a041. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Feldmann R. J. Membrane proteins: amino acid sequence and membrane penetration. J Mol Biol. 1974 Aug 25;87(4):853–858. doi: 10.1016/0022-2836(74)90090-4. [DOI] [PubMed] [Google Scholar]



