Abstract
The endoplasmic-reticulum (ER) stress response constitutes a cellular process that is triggered by a variety of conditions that disturb folding of proteins in the ER. Eukaryotic cells have developed an evolutionarily conserved adaptive mechanism, the unfolded protein response (UPR), which aims to clear unfolded proteins and restore ER homeostasis. In cases where ER stress cannot be reversed, cellular functions deteriorate, often leading to cell death. Accumulating evidence implicates ER stress-induced cellular dysfunction and cell death as major contributors to many diseases, making modulators of ER stress pathways potentially attractive targets for therapeutics discovery. Here, we summarize recent advances in understanding the diversity of molecular mechanisms that govern ER stress signaling in health and disease.
1. Introduction
The endoplasmic reticulum (ER) is the central intracellular organelle in the secretory pathway. It is responsible for protein translocation, protein folding, and protein post-translational modifications that allow further transport of proteins to the Golgi apparatus and ultimately to vesicles for secretion or display on the plasma surface. Perturbations in ER function, a process named “ER-stress”, trigger the unfolded protein response (UPR), a tightly orchestrated collection of intracellular signal transduction reactions designed to restore protein homeostasis. The UPR is distinguished by the action of three signaling proteins named IRE1α (inositol-requiring protein-1α), PERK (protein kinase RNA (PKR)-like ER kinase), and ATF6 (activating transcription factor 6). Under physiological conditions, the luminal domains of PERK and ATF6 proteins are bound to the ER resident chaperone BiP (Binding immunoglobulin Protein), which keeps them inactive. When unfolded proteins accumulate in the ER, BiP is released from these complexes to assist with the folding of accumulated proteins [1]. By comparison to the UPR modulators PERK and ATF6, which are modulated by association with BiP, IRE1α appears to become activated when unfolded proteins bind directly to it. Upon activation, PERK, IRE1α and ATF6 induce signal transduction events that alleviate the accumulation of misfolded proteins in the ER by increasing expression of ER chaperones, inhibiting protein entry into the ER by arresting mRNA translation, and stimulating retrograde transport of misfolded proteins from the ER into the cytosol for ubiquitination and destruction by a process named ERAD (ER-assisted degradation).
This review focuses on the (a) signal transduction pathways involved in the UPR and their connections to cell death mechanisms; (b) UPR-independent mechanisms of ER stress-induced cell death, (c) suppressors of ER stress-mediated cell death; (d) ER stress and autophagy; and (e) diseases where ER stress plays a pathological role.
2. ER stress, UPR signaling, and cell death regulation
Under conditions when ER stress is chronically prolonged and the protein load on the ER greatly exceeds its fold capacity, cellular dysfunction and cell death often occur. Among the UPR signaling pathways IRE1α is a key molecule that functions as a rheostat capable of regulating cell fate. Various studies support the concept that IRE1α binds directly to unfolded proteins, including structural studies of the conserved core of yeast IRE1α ER luminal domain, analysis of synthetic peptide interactions with IRE1a in vitro, and protein interaction studies performed using intact cells [1]. In this model, BiP is not pivotal to switch the UPR on and off as previously believed. Instead, BiP desensitizes IRE1α to low levels of stress and acts as a timer to modulate the response time to the level of stress by assisting IRE1α deactivation once ER homeostasis is reestablished. Several lines of evidence argue that alternative outputs from IRE1α–mediated downstream signaling dictate opposing cell fates (survival or death) during stress, which are greatly influenced by the intensity and longevity of ER stress [2].
IRE1α is a transmembrane protein that consists of an N-terminal luminal sensor domain, a single transmembrane domain and C-terminal cytosolic effector that is responsible for both protein kinase and endoribonuclease activities. Accumulation of unfolded proteins in the ER stimulates IRE1α oligomerization in ER membranes and autophosphorylation of IRE1α’s cytosolic domain [3]. The RNase domain processes an intron from the X box-binding protein-1 (XBP-1) mRNA to allow production of the XBP-1 protein. Of note, XBP-1 is not the only RNA targeted by the RNase activity of IRE1α. For example, IRE1α also controls its own expression by cleaving its own mRNA [4]. Moreover, IRE1α cleaves microRNAs that control the levels of caspase family cell death proteases. The XBP-1 protein binds to promoters of several genes involved in UPR and ERAD (ER-assisted degradation) to restore protein homeostasis and promote cytoprotection.
In addition to its cytoprotective function, IRE1α also stimulates activation of the Apoptotic-Signaling Kinase-1 (ASK1), which causes activation downstream of stress kinases Jun-N-terminal kinase (JNK) and p38 MAPK that promote apoptosis [5]. Among the apoptosis-inducing substrates of JNK are Bcl-2 and Bim, which are inhibited and activated, respectively, by JNK phosphorylation [6, 7]. In addition, p38 MAPK phosphorylates and activates the transcription factor CHOP, which causes changes in gene expression that favor apoptosis, including increasing expression of Bim and DR5, while decreasing expression of Bcl-2 [8, 9]. Recently, regulated IRE1-dependent decay of mRNA (RIDD) was shown to reduce ER localized mRNAs in both insect (Drosophila) and mammalian (mouse) cells [10] [11]. The RIDD process selectively targets and degrades mRNAs encoding proteins involved in protein folding. Prolonged activation of RIDD signaling can promote cell death through a process shown to be dependent upon the conformational state of IRE1α. Mechanisms that mediate IRE1α-activated RIDD are still unknown and require further investigation.
ATF6 is a transcriptional factor that upon ER stress translocates to the Golgi compartment where it is cleaved by the action of two proteases. The serine protease site-1 (S1P) cleaves ATF6 in the luminal domain, while the N-terminal portion is subsequently cleaved by the metalloprotease site-2 protease (S2P). The cleaved N-terminal cytosolic domain of ATF6 then translocates into the nucleus where it binds to ATF/cAMP response elements (CRE) and ER stress-response elements (ERSE-1) to activate target genes such as BiP, Grp94 and CHOP. In some cells and tissues, ATF6-like bZIP type transcription factors such as OASIS, CREB-H, Tis40 and Luman also participate in UPR signal transduction [12]. Interestingly, functional experiments in a model of ischemia/reperfusion showed that ATF6 protected cardiomyocytes by inducing expression of the protein disulfide isomerase associated 6 (PDIA6) gene, which encodes an ER enzyme that catalyzes protein disulfide bond formation and thus assists with protein folding in the lumen of the ER [13, 14]. Additionally, ER stress can also regulate gene expression via TF6-mediated changes in micro-RNA levels, where it has been hypothesized that ATF6-mediated down-regulation of miR-455 augments expression of calreticulin which has been show to attenuate ER stress after ischemia. Thus, this regulation may contribute to the protective effects of ATF6 in the heart [15].
PERK is the major protein responsible for attenuation of mRNA translation under ER stress, preventing influx of newly synthesized proteins into the already stressed ER compartment. This translational attenuation is mediated by phosphorylation of eukaryotic translation initiation factor 2 (eIF2α). The phosphorylation of eIF2α inhibits the recycling of eIF2α to its active GTP-bound form, which is required for the initiation phase of polypeptide chain synthesis. Phosphorylation of eIF2α also allows for preferential translation of UPR-dependent genes such as the activating transcriptional factor 4 (ATF4). Important targets driven by ATF4 are CHOP (transcriptional factor C/EBP homologous protein), GADD34 (growth arrest and DNA damage-inducible 34), and ATF3. Besides elF2α, PERK can also phosphorylate nuclear erythroid 2 p45-related factor 2 (NRF2), which contributes to dissociation of the NRF2-Keap1 complex and promotes expression of genes containing antioxidant response elements (ARE), preventing oxidative stress by induction of antioxidant genes such as heme oxygenase 1 (HO-1) [16].
If the various UPR-induced mechanisms fail to alleviate ER stress, both the intrinsic and extrinsic pathways for apoptosis can become activated (Figure 1). Players involved in the cell death response include (i) PERK/eIF2α-dependent induction of the pro-apoptotic transcriptional factor CHOP; (ii) IRE1-mediated activation of TRAF2 (tumor necrosis factor receptor associated factor 2), which stimulates the ASK1 (apoptosis signal-regulating kinase 1)/JNK (c-Jun amino terminal kinase) kinase cascade, and (iii) Bax/Bcl2-regulated Ca2+ release from the ER. CHOP/GADD153 (growth arrest/DNA damage) plays a convergent role in the UPR and it has been identified as one of the most important mediators of ER stress-induced apoptosis protein (reviewed by [17]). Apoptosis-relevant targets of the CHOP transcription factor include (i) GADD34; (ii) DR5 (TRAIL Receptor-2), a caspase-activating cell-surface death receptor of the Tumor Necrosis Factor Receptor family; and (iii) Ero1α (endoplasmic reticulum oxidoreductase-1), which hyperoxidizes the ER and promotes cell death. Ero1α can also activate the inositol triphosphate receptor (IP3R) stimulating excessive Ca2+ transport from the ER to the mitochondria, and thereby triggering cell death [18]. CHOP-mediated activation of GADD34 promotes protein dephosphorylation of elF2α reversing translational inhibition [19]. Release of the translational inhibition contributes to accumulation of unfolded proteins in the ER compartment and, at the same time, permits translation of mRNAs encoding pro-apoptotic proteins. Another possible mechanism by which CHOP induces apoptosis is via direct inhibition of Bcl-2 transcription [20] and induction of Bim expression [8].
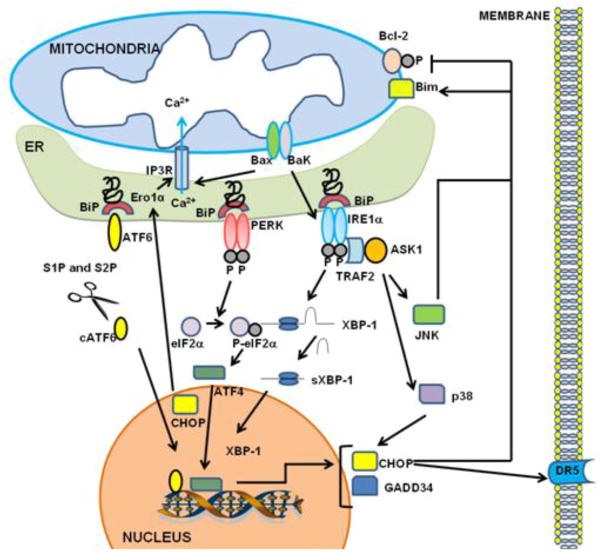
Figure 1. Endoplasmic reticulum (ER) stress signaling.
The ER stress response is mediated by three sensors located at the ER membrane: IRE1α, ATF6 and PERK. Accumulation of unfolded protein recruits BiP to the ER lumen and its dissociation from IRE1α, ATF6 and PERK leads to their activation. Upon dimerization and autophosphorylation, IRE1α splices XBP-1 mRNA, which adjusts the reading frame to allow translation of an active transcriptional factor XBP-1. XBP-1 up-regulates UPR genes encoding ER chaperones and components of the ERAD machinery. IRE1α can also recruit TRAF2 and ASK1, leading to downstream activation of JNK and p38 MAPK. Activated JNK translocates to the mitochondrial membrane and promotes (a) activation of Bim and (b) inhibition of Bcl-2, whereas p38 MAPK phosphorylates and activates CHOP. CHOP can induce transcriptional activation of genes that contribute to cell death, including Ero1α (hyperoxidizes the ER and activates IP3R) and DR5 (promotes caspase 8-dependent cell death). Bax and Bak can also (a) bind to and activate IRE1α and (b) induce release of Ca2+ from the ER. PERK phosphorylates elF2α and attenuates protein translation. However, translation of selected mRNAs is favored under these conditions, including ATF4, which then induces expression of CHOP and GADD34. Activated ATF6 translocates to the Golgi where its cytosolic domain is cleaved by the proteases, S1P and S2P. The cleaved ATF6 fragment forms an active transcriptional factor that mediates expression of several components important for protein folding, degradation, and ER expansion.
Additional ER stress-induced mechanisms contributing to cell death may include activation of the kinase function of IRE1 involving activation of ASK and p38 MAPK (previously discussed in this article), where p38 MAPK activates CHOP via phosphorylation of its transactivation domain [21]. Additionally, both p38MAPK and JNK are reported to promote phosphorylation and activation of pro-apoptotic protein Bax [22]. Among the mechanisms of cell death induced by pro-apoptotic proteins Bax and Bak [22] is their binding and pathological activation of IRE1α [23] (Figure 1).
3. UPR independent ER stress–signaling and cell death
3.1. Calcium
The ER lumen is the major storage of intracellular Ca2+ and Ca2+-binding chaperones mediate the proper folding of proteins in the lumen of the ER. It is well established that Ca2+ trafficking in and out of the ER regulates a diversity of cellular responses and signaling transduction pathways relevant to stress response, modulation of transcriptional processes, and development. For instance, acute release of Ca2+ from the ER can trigger a variety of signaling mechanisms that promote cell death mainly by Ca2+-mediated mitochondrial cell death [24]. Conversely, pulses of Ca2+ delivered via IP3Rs at contact sites of ER and mitochondria promote oxidative phosphorylation, which sustains ATP levels and cell survival [25] [26]. Other proteins involved in ER Ca2+-mediated apoptosis are Bax and Bak. Transient over-expression of Bax results in release of ER Ca2+, with a subsequent increase in mitochondrial Ca2+ and enhanced cytochrome c release. In contract, cells with deficiency of both Bax and Bak have reduced Ca2+ release from ER upon stimulation with IP3 and other ER Ca2+-mobilizing agents (Figure 1) [26]. Calreticulin, a major Ca2+ binding ER chaperone is also a key component for folding of newly synthesized proteins and for other quality control pathways of the ER. Therefore, fluctuations of the levels of Ca2+ in the ER can severely impact folding capacity and trigger cell death. For instance, fibroblasts from calreticulin-deficient embryos have impaired agonist-induced Ca2+ release and deletion of this gene in embryos is lethal [27]. In summary, alterations in Ca2+ dynamics seem to play an essential role not only in the ER but also some ER stress-associated mechanisms of cell death.
3.2. MEKK1 (MAP3K4)
Recently, Kang et al [28] have identified a new signaling pathway where Cdk5 and MEKK1 induce apoptosis in a Drosophila model of retinitis pigmentosa, independently of the three canonical UPR branches, IRE1α, PERK and ATF6. In vivo studies in the fly showed that Cdk5 phosphorylates MEKK1 (human ortholog = MAP3K4) to activate the JNK signaling pathway in response to ER stress. Ablation of this pathway delayed age-associated retinal degeneration in the Drosophila model of retinitis pigmentosa, where a rhodopsin mutant mimicking the autosomal dominant allele of affected humans was expressed in the fly eye.
3.3. ER membrane re-organization
Interesting studies from Varadarajanv, et al. [29] have uncovered a new cellular stress response characterized by a striking, but reversible, reorganization of ER membranes that occurs independently of the UPR, resulting in impaired ER transport and function that culminates in cell death. ER membrane aggregation was regulated, in part, by anti-apoptotic Bcl-2 family members, particularly Mcl-1. Using connectivity mapping, they reported the widespread occurrence of this stress response by identifying several structurally diverse chemicals from different pharmacological classes, including antihistamines, antimalarials, and antipsychotics, which induce ER membrane reorganization. Furthermore, they demonstrated that ER membrane aggregation can result in pathological consequences.
4. Suppressors of ER-stress induced apoptosis
4.1 Bax-inhibitor 1 (BI-1/Tmbim6)
BI-1 (Tmbim6) is a highly conserved multi-transmembrane protein that resides predominantly in the ER. BI-1 has been characterized as a pro-survival protein in the context of ER stress. This pro-survival property has been demonstrated against a plethora of cellular insults such as Ca2+ fluctuations, reactive oxygen species, cytosolic acidification, accumulation of unfolded proteins, oxygen deprivation, and alterations in metabolism [30]. The physiological relevance of BI-1 was first described by Chae, et al. who demonstrated that BI-1 suppresses an apoptosis pathway linked to ER stress in diverse types of cultured cells and in mice in vivo [31]. In contrast, BI-1-deficient cells displayed high sensitivity to apoptosis induced by ER stress agents such as thapsigargin and tunicamycin, but not to stimulators of the extrinsic and intrinsic mitochondrial pathways of cell death. In the same study, it was also shown that BI-1 reduces releasable Ca2+ from the ER. BI-1 has been shown to associate with Bcl-2 family members (Bcl-2/Bcl-XL) and operate downstream of these proteins to regulate ER Ca2+ homeostasis [32]. Cells over-expressing BI-1 have reduced luminal concentrations of Ca2+ in the ER, whereas cells in which BI-1 is ablated have higher steady-state levels of free Ca2++ in the ER. Reconstitution of a C-terminal peptide of BI-1 in lipid-bilayers has suggested that BI-1 may form a Ca2+ permeable pore that accounts for its ability to increase the passive leakage rate of Ca2+ from the ER [33], but other data point to a role for BI-1 in modulating Ca2+ flux via IP3Rs [34]. Recently, our group has demonstrated that BI-1 modulates bioenergetics via an IP3R-dependent manner, presumably by impacting the extent to which Ca2+ is shuttled from ER into mitochondria via IP3Rs at sites of contact of these organelles [25].
It has been reported that BI-1 suppresses IRE1α activity in both fly and mouse models of ER stress by forming a complex with IRE1α, which then nullifies its endoribonuclease activity [35]. For instance, knocking out the gene encoding BI-1 consistently led to hyperactivity of the IRE1α pathway. Conversely, cells over-expressing BI-1 showed reduced IRE1α kinase and endoribonuclease activity, whereas a C-terminal mutant of BI-1 that fails to bind IRE1α did not suppress IRE1α activity and lacked cytoprotective activity. Stress kinase activation induced by ER stress is also blunted by BI-1 [36], which has implications for control of both cell death and autophagy. Additionally, BI-1 deficiency also increase the ER/Golgi expansion and the secretory activity of primary B cell, functions controlled by XBP-1, a major downstream effector of IRE1α.
In summary, BI-1 appears to provide cytoprotection against ER stress through two mechanisms: (a) regulation of the resting ER Ca2+ levels and (b) inhibition of IRE1α signaling (which initiates stress kinase activation) (Figure 2). It remains to be determined whether or not these two roles synergize to confer the cytoprotective benefits of BI-1 against ER stress. To this end, an analysis of BI-1 mutants that differentially abrogate either ER Ca2+ regulation or IRE1α endoribonuclease activity would be highly informative for comparing the relative roles of these two functions as pertains to cytoprotection against ER stress.
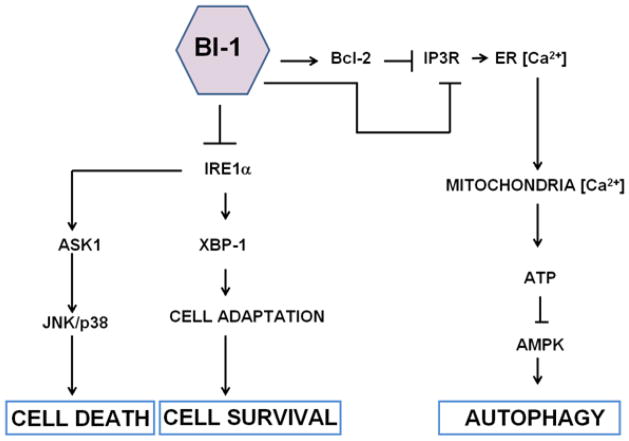
Figure 2. Dual function of BI-1 in ER stress and autophagy.
BI-1 modulates ER stress and autophagy by independent mechanisms. BI-1 suppresses IRE1α by forming a complex with IRE1α, which then nullifies both its endoribonuclease (XBP-1) and kinase activity (JNK). Via an IP3R-dependent mechanism, BI-1 also reduces the steady-state levels of ER Ca2+, which causes a corresponding decline in mitochondrial Ca2+ levels and reduces mitochondria bioenergetics. Reduction in ATP levels (rise in AMP) activates the intracellular energy sensor AMPK, which activates autophagy via effects on Atg1 (Ulk1/Ulk2). BI-1 has also been shown to associate with Bcl-2 to regulate Ca2+ homeostasis, which could also indirectly influence autophagy.
Another member of the Tmbim family, Tmbim-3, has also recently been implicated in regulation of ER Ca2+ but not IRE1α signaling [37]. Thus, redundancy of BI-1 (Tmbim6) and Tmbim3 should be considered in interpreting data where either of these genes was knocked-out or their expression was experimentally silenced.
4.2. Bcl-2/Bcl-XL
In addition to their role in the mitochondrial pathway of apoptosis, Bcl-2 proteins such as Bcl-2 and Bcl-xL also localize at the ER membrane and are protective against ER stress. It is believed that this cytoprotective function is mainly due to the ability of Bcl-2 to lower steady-state levels of ER Ca2+ via IP3Rs. The protective role of Bcl-2 in regulating ER Ca2+ can be inhibited by JNK-mediated phosphorylation [38]. Phosphorylated Bcl-2 loses its anti-apoptotic function by failing to bind to pro-apoptotic “BH3-only” members of the Bcl-2 family and/or increasing Ca2+ release from the ER, which is associated with mitochondria Ca2+ uptake and apoptosis. It has been reported that the pro-apoptotic proteins Bax and Bak also localize at the ER where they antagonize the ER Ca2+ modulating activities of Bcl-2 and Bcl-XL [39]. Additionally, Bax and Bak reportedly bind to IRE1α, resulting in its pathological activation [23], which in turn is neutralized by anti-apoptotic Bcl-2 or Bcl-XL. Finally, given that CHOP induces expression of pro-apoptotic BH3-only protein Bim during ER stress, anti-apoptotic proteins such as Bcl-2 and Bcl-XL are likely to play protective roles against ER stress at other levels, as well.
4.3. MicroRNAs
Emerging data have suggested that microRNAs can either modulate ER stress or be activated by ER stress. For example, PERK-mediated induction of miR30c-2* is stimulated upon ER stress and is correlated with down-regulation of XBP-1 splicing and commitment to cell death [39]. In contrast, miR-211 has also been identified as a PERK target that facilitates pro-survival responses. Chitnis, et al. showed that the microRNAs miR-221 is associated with CHOP-mediated apoptosis during ER stress [40]. In this regard, ER stress-induced apoptosis was reportedly enhanced by miR-221/222 mimics and attenuated by miR-221/222 inhibitors. Promotion of apoptosis by miR-221/222 during ER stress was associated with p27 (Kip1)- and MEK/ERK-mediated cell cycle regulation. Moreover, Behrman, et al. elegantly demonstrated that CHOP regulates miR-708, which plays a homeostatic role in mammalian rod photoreceptor cells, by preventing excessive rhodopsin loads on the ER [41]. Additionally, spliced XBP-1 is necessary for ER stress-associated miR-346 induction. The target genes of miR-346 regulate immune responses and include the major histocompatibility complex (MHC) class I gene products, interferon-induced genes, and the ER antigen peptide transporter 1 (TAP1). Because TAP function is necessary for proper MHC class I-associated antigen presentation, this study provided a novel mechanistic explanation for reduced MHC class I-associated antigen presentation during ER stress. Recently, it was found that sustained IRE1α RNAse activity causes rapid decay of microRNAs (miR-17, -34a, -96 and -125b) that normally repress caspase-2 mRNA [42]. The molecular mechanisms and implications of microRNAs and ER stress response await further investigation.
4.4. Additional suppressors of ER stress-induced apoptosis
Stimulation of cell surface TNF-family death receptors activates caspase-8, which targets a limited number of substrates including Bap31, an integral membrane protein of the ER. The p20 caspase cleavage fragment of Bap31 can direct pro-apoptotic signals between the ER and mitochondria. Adenoviral expression of p20 caused an early release of Ca2+ from the ER, concomitant uptake of Ca2+ into mitochondria, and mitochondrial recruitment of Drp1, a dynamin-related protein that mediates scission of the outer mitochondrial membrane, resulting in dramatic fragmentation and fission of the mitochondrial network. Moreover, prolonged expression of p20 on its own ultimately induced caspase activation and apoptosis through the mitochondria-initiated apoptosome pathway. Therefore, it has been suggested that caspase-8 cleavage of Bap31 at the ER stimulates Ca2+− dependent mitochondrial fission, enhancing the release of cytochrome c [43]. Additional studies demonstrated that tumor cell resistance to ER stress-induced apoptosis was partially mediated by expression levels of calnexin and its association with Bap31, with calnexin suppressing Bap31cleavage [44].
The Bag (Bcl-2 associated athanogene) protein family consists of 6 evolutionary conserved polypeptides (Bag1-Bag6) [45]. They share at least one copy of the BAG domain consisting of three alpha helices that interact with and modulate the activity of the molecular chaperone Hsp70. Several of the Bag proteins have been implicated in the control of apoptosis. Bag5 is overexpressed in malignant prostate tissue and its levels are increased following ER-stress induction. Upon ER stress, Bag5 relocates from the cytoplasm to the ER and interacts with the ER-resident chaperone BiP and enhances its ATPase activity. Bag5 over-expression in prostate cancer cells reportedly inhibits ER-stress induced apoptosis in the unfolded protein response by suppressing PERK-eIF2-ATF4 activity, while enhancing the IRE1α-XBP1 axis of this pathway. Cells expressing high levels of Bag5 showed reduced sensitivity to apoptosis induced by different agents, whereas Bag5 down-regulation resulted in increased stress-induced cell death [46].
Bifunctional Apoptosis Regulator (BAR) is a multi-domain protein that was originally identified as an inhibitor of Bax-mediated cell death. BAR is highly expressed in the brain where it exerts a cytoprotective role against many apoptotic stimuli including ER stress [47]. The mechanistic role of BAR in protecting against ER stress was delineated by Rong at al., who elegantly demonstrated that BAR is an ER-associated E3 ubiquitin ligase that stimulates turnover of the BI-1 protein. Specifically, BAR functions as an ER-associated RING-type E3 ligase, interacts with BI-1, promoting BI-1’s degradation, removing an inhibitory influence on IRE1α signaling. Moreover, the rapid decline of BAR protein levels during ER stress suggests that post-translational modification of BI-1 is dynamic and could contribute to the selective control of IRE1α signaling in cell undergoing prolonged ER stress [48].
5. ER stress and autophagy
ER stress leads to activation of two protein degradation pathways, the ubiquitin-proteasome via ERAD and lysosome-mediated protein degradation via autophagy (reviewed in [49]). ERAD involves retro-translocation of unfolded ER proteins to the cytosol where they are ubiquitinated and degraded by the proteasome. When the buildup of misfolded or unfolded proteins exceeds the ER capacity, autophagy can be induced as a secondary response to degrade accumulated proteins and thus alleviate ER stress. Although ERAD is recognized as the predominant cellular mechanism for removal of unfolded proteins in the context of ER stress, a diversity of studies have shown that autophagy can be potently activated by ER stress (Figure 3).
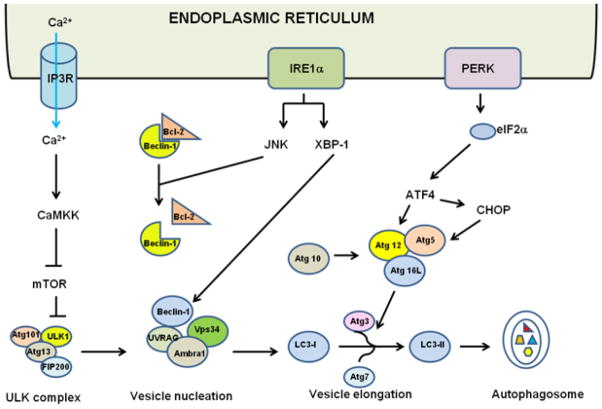
Figure 3. Cross-talk of ER stress and autophagy.
ER stress can induce autophagy, through at least two UPR pathways, PERK-elF2α and IRE1α. Activated IRE1α can recruit TRAF2 and ASK1, which subsequently activates JNK. JNK-mediated phosphorylation of Bcl-2 releases Beclin-1 from its inhibitory interaction with Bcl-2. Free Beclin-1 associates with other members of the ULK1 complex to promote vesicle nucleation. In parallel, spliced XBP-1 can also trigger transcriptional up-regulation of Beclin-1 expression. The elongation process involves two ubiquitin-like conjugation systems that promote the assembly of Atg5-Atg12-Atg16L complex and the LC3 processing (cleavage and lipidation). Activated PERK can induce autophagy through ATF4-driven transcriptional regulation of Atg12 whereas ATF4-mediated CHOP activation can also transcriptionally induce Atg5. Ca2+ release from the ER lumen through the IP3R can activate CaMKK and subsequently relieves mTOR inhibition on the ULK1 complex.
Autophagy is the process by which cellular macromolecules and organellar components are sequestered within double membrane vesicles and delivered to lysosomes for degradation and recycling of bioenergetic substrates. Pioneering studies in yeast delineated many of the essential components of the autophagic machinery, subsequently confirmed and extended in mammalian systems [50]. Formation of the autophagosome membrane requires the sequential action of numerous proteins involved in vesicle nucleation, elongation, fusion with lysosomes and final degradation of engulfed substrates. The first regulatory process involves suppression of the mTOR Ser/Thr kinase, which blocks autophagy by phosphorylation of Atg13, and dissociation of this protein from a complex formed by Atg1 (a protein kinase) and Atg17. Thus, when mTOR becomes inhibited, for instance by rapamycin, autophagy is induced. The primary step of vesicle nucleation is the activation of Vps34, a class III phosphatidylinositol 3-kinase, which associates with a complex formed by Beclin-1, UV irradiation resistance-associated tumor suppressor gene (UVRAG) and the kinase Vps15/p150. Beclin-1 can also interact with the anti-apoptotic protein Bcl-2 at the ER, with Bcl-2 inhibiting starvation-induced autophagy [51]. The next phase of elongation involves two ubiquitin-like conjugation steps. First, the proteins Atg12 and Atg5 are covalently conjugated together, with the cooperation of Atg7 (E1-like) and Atg10 (E2-like). Second, conjugation of Atg8 with phosphatidylethanolamine (PE) in the membranes of autophagic vesicles occurs following its proteolytic cleavage by Atg4, a cysteine protease [52]. The subsequent recruitment of Atg8 and other autophagy-related proteins is believed to trigger vesicle expansion in a concerted manner, presumably by providing the driving force for membrane curvature [53]. The transient conjugation of Atg8 to the membrane lipid PE is essential for phagophore expansion as its mutation leads to defects in autophagosome formation [54]. It is distributed symmetrically on both sides of the autophagosome and it is assumed that there is a quantitative correlation between the amount of Atg8 and the vesicle size [55]. After finishing vesicle expansion, the autophagosome is ready for fusion with the lysosome and Atg8 can either be released from the membrane for recycling or gets degraded in the autolysosome. In humans, some of the orthologs of yeast autophagy genes have been expanded to form gene families, such as Atg1 (represented by the kinases Ulk1 and possibly Ulk2 in humans), Atg4 (represented by the proteases, Autophagin-1, 2, 3, and 4, also known as ATG4A, B, C, and D), and Atg8 (homologs in humans include LC3 and GABARAP). The generation of the PE-conjugated and ATG4-cleaved form of LC3, denominated LC3-II, has been commonly used as an autophagy marker [56].
The molecular mechanisms that link ER stress to autophagy may vary, and various groups have proposed different hypotheses by which these two pathways cross-talk (Figure 2). For instance, various Ca2+ mobilizing agents such as ionomycin, ATP (via purinergic receptors) and thapsigargin (an irreversible inhibitor of the ER Ca2+ ATPase) reportedly inhibit the activity of mTOR, a negative regulator of autophagy, and induce massive accumulation of autophagosomes in a Beclin-1- and Atg7-dependent manner [57]. In this regard, it has been proposed that Ca2+ release from the ER stimulates a CamKK/AMPK-dependent pathway that inhibits mTOR to thus induce autophagy [58]. Additionally, Ogata, et al. demonstrated that ER stress-induced autophagy is regulated by IRE1α interaction with TRAF2 to regulate JNK activation [59]. Recently, it was reported that JNK-mediated phosphorylation of Bcl-2 causes its release of Beclin-1, thereby allowing autophagy to proceed [51]. Of note, the autophagy protein Atg12 can associate with and inhibit Bcl-2 proteins, thereby promoting cell death [60]. In addition, Sakaki, et al. suggested that Ca2+ release from the ER induces protein kinase C (PKC) activation, inducing autophagy via a mTOR-independent mechanism [61]. On the other hand, Kouroku and colleagues showed that the UPR signaling pathway PERK/elF2α induces Atg12 expression [62]. PERK has been shown to mediate autophagy through ATF4-driven transcriptional regulation of ATG genes [63]. Recently, Margariti et al demonstrated that XBP-1 mRNA splicing triggers autophagy through transcriptional activation of Beclin-1 [64]. In view of these data, it is plausible to assume that several mechanisms contribute to functional connections between ER stress and autophagy. Given that autophagy has been linked to protection from several diseases (neurodegeneration, diabetes, infectious diseases) and to promotion of some (cancer) (reviewed in [65]) the linkages between ER stress and autophagy may have relevance to several diseases.
6. ER stress involvement in diseases
ER stress is a hallmark of many common diseases in conditions where (a) the stress is so strong and/or prolonged to the point that cells succumb to death or (b) the ability to overcome ER stress is impaired by a pathological condition. Below, we will discuss some diseases that are either caused or aggravated by components of the ER stress response.
6.1. Neurodegenerative diseases
In neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease, it has been suggested that ER stress evoked by accumulation of β-amyloid plaques and α-synuclein, respectively, plays a causal role in the pathogenesis of these diseases. In Parkinson’s disease, dopaminergic neurons are filled with intracytoplasmic inclusions called “Lewis Bodies”. For instance, phosphorylated PERK and phosphorylated eIF2α were co-localized with α-synuclein, the main component of Lewis bodies, in dopaminergic neurons of Parkinson’s disease patients [66]. Additionally, Parkinson mimetic drugs (such as 6-hydroxydopamine) increased ER stress in dopaminergic neurons whereas a null mutation in CHOP reduced the 6-hydroxydoamine-induced apoptosis in vivo [67]. Histopathological analyses of brains of Alzheimer’s patients have hinted that ER stress may be a component of the pathogenesis of this neurodegenerative disease. For instance, both spliced XBP-1 and phosphorylated IRE1α were found in the brains of individuals with Alzheimer’s disease [68] [69]. Moreover, β-amyloid induces CHOP expression both in cultured cells and animal brains, whereas treatment of cells with CHOP antisense RNA improved neuronal survival after exposure to β-amyloid [70]. Recent studies with autopsied amyotropic lateral sclerosis (ALS) patients and studies using mutant superoxide dismutase (mSOD1) transgenic mice have suggested that ER stress-related toxicity may also be highly relevant to this degenerative disease that destroys spinal motoneurons [71].
6.2. Ophthalmology disorders
A growing number of reports have suggested that accumulation of misfolded proteins plays an important role in the pathogenesis of several eye diseases such as retinitis pigmentosa, glaucoma, and macular degeneration. Retinitis pigmentosa (RP) is an age-related retinal degenerative disease caused by mutant rhodopsins. In a rat model of RP expressing rhodopsin-P23H, levels of ATF6, phosphorylated elF2α and CHOP were elevated when compared to control animals. Over-expression of BiP and components of the ERAD machinery in this mouse model reduced CHOP expression and ameliorated the electrical response of photoreceptors, suggesting that ER stress plays a major pathological role in this disease [72]. In wet age-related macular degeneration (AMD), choroidal neovascularization promoted by increased VEGF and capillary leakage cause fluid exudation, hemorrhages, and fibrotic scars, thereby damaging photoreceptors and causing vision loss. Oxidative stress, inflammation and ER stress have been shown to play a combinatory effect in the pathogenesis of AMD. As an example, high levels of oxidized proteins, lipids, and nucleic acids in AMD lesions can activate a local inflammatory response, which in turn can stimulate ER stress. In fact, AMD lesions, also named drusens, contain high levels of oxidated LDLs, which stimulate VEGF expression via the PERK/ATF4 signaling pathway [73]. Moreover, therapy with chemical chaperones such as TMAO (trimethylamide-N-oxide) has been demonstrated to alleviate protein aggregation in AMD [74]. Last, in a mouse model of primary open angle glaucoma harboring the most common mutation in Myocilin, reduction of ER stress with chemical chaperones prevented cell death by promoting secretion of mutant Myocilin in the aqueous humor and by decreasing intracellular accumulation of Myocilin in the ER [75].
6.3. Inflammation
ER stress pathways are closely linked to mechanisms involved in immunity and inflammation. IRE1α can be phosphorylated by signals downstream of Toll-like receptors (TLRs) to induce XBP-1 mRNA splicing and support production of proinflammatory cytokines in macrophages [76]. On the other hand, TLR signaling can also inhibit ATF6 and CHOP in macrophages [77]. Unfolded protein accumulation has also been described in many autoimmune diseases, including inflammatory bowel disease (IBD), multiple sclerosis, and rheumatoid arthritis [78]. With respect to the UPR machinery, Kaser et al [79] reported that XBP-1 deletion in intestinal epithelial cells (IEC) results in spontaneous enteritis and overactive responses of IECs to IBD inducers, TNFα and flagellin. This phenotype was attributed to the inability of XBP-1-deficient IEC to generate antimicrobial activity and respond properly to inflammatory signals in the local milieu. Additionally, this study also identified several single nucleotide polymorphisms (SNPs) within the XBP-1 gene locus that conferred high risk for both types of IBD, Crohn’s disease and ulcerative colitis.
Though better known for its role in sensing viral RNA in the context of innate immune responses that stimulate interferon production, the double-strand RNA-dependent protein kinase (PKR) is also reportedly activated by ER stress [80]. When activated, PKR, similarly to PERK, phosphorylates elF2α, which leads to shutdown of protein synthesis and inhibition of viral replication [81]. Moreover, PKR has also been shown to participate in inflammasome activation and the cytokine HMGB1 release. Studies from Lu et al [82] reported that PKR broadly regulates inflammasome activation by phosphorylating members of the NLR (Nucleotide binding, Leucine rich repeat Receptors), including NLRP3 (NLR family pyrin domain-containing 3), NLRP1, and NLRC4 (NLR family CARD domain-containing protein 4).
Using a combination of genetic, pharmacological, and biochemical approaches, Lee et al [83]convincingly demonstrated that murine calcium-sensing receptor (CASR) has a major role in NLRP3 inflammasome activation through its effects on ER Ca2+ (IP3R-mediated) and cAMP. Briefly, Ca2+ and other CASR agonists activated the NLRP3 inflammasome whereas knockdown of CASR reduced inflammasome activation in response to NLRP3 activators. CASR activates the inflammasome via an IP3-mediated mechanism that releases ER Ca2+ stores. Understanding the precise mechanisms linking ER Ca2+ release to inflammasome activation is a topic that merits further elucidation.
6.4. Viral Infections
In the course of viral infection a large number of viral proteins, especially glycoproteins, are synthesized allowing viral replication and maturation. This high demand for proteins triggers ER stress leading to both cell survival and cell death. Expect for BiP, which seems to be induced in most of the viral infections [84], different viruses may induce a specific UPR response. For instance, cytomegalovirus (CMV) infection inhibits ATF6 pathways but activates the IRE1α pathway as an alternative mechanism to upregulate the expression of chaperones. The transcriptional activation of XBP-1 target genes is inhibited in order to prevent viral proteins in the ER from being degraded [85]. On the other hand, viruses such as the herpes simplex virus 1 (HSV1) are known for disarming the activation of PERK. In fact, the γ134.5, a virulence factor encoded by herpes simplex viruses, plays an important role in inhibiting elF2α phosphorylation and alleviating the translation arrest in mammalian cells treated with thapsigargin and DTT [86]. Additionally, replication of hepatitis C virus (HCV) has been shown to stimulate the ATF6 pathway but inhibit the IRE1α/XBP-1 pathway. Interestingly, several viruses have been shown to induce ER stress-mediated apoptosis. Infection of Japanese encephalitis virus triggers p38 MAPK, CHOP activation, and cell death and is inhibited by over-expression of Bcl-2 [84]. Similarly, a cytopathic strain of bovine diarrhea virus induces apoptosis via CHOP and phosphorylation of PERK and elF2α [84].
6.5. Cancer
The tumor microenvironment is characterized by poor vascularization, low oxygen supply, nutrient deprivation, and acidic pH -- all of which are activators of ER stress. In rapidly growing cancers, UPR has been shown to exert an important cytoprotective role that assists folding of newly synthesized proteins necessary for tumor growth. Among the UPR branches, IRE1α and PERK are important for tumor cell survival and growth under hypoxic conditions. Tumors derived from PERK-deficient transformed mouse embryonic fibroblasts (MEFs) show evidence of increased cell death rates and impaired ability to stimulate angiogenesis. More convincingly, the importance of PERK/elF2α-mediated UPR signaling was supported by a study with MEFs that expressed a non-phosphorylated form of elF2α that impaired cell survival under extreme hypoxia [87]. The IRE1α/XBP-1 pathway has been shown to be crucial for angiogenesis in the early stages of tumor development, as evidenced by studies where the expression of a dominant-negative from of IRE1α or inhibition of XBP-1 splicing by RNAi reduced blood vessel formation in a human tumor xenograft model [88]. Inhibition of the IRE1α/XBP-1 pathway has been explored recently as a target for anticancer therapy. For instance, small molecules that inhibit IRE1α-mediated XBP-1 splicing significantly suppressed multiple myeloma cell growth in an animal model [89]. Moreover, the transcriptional activity of XBP-1, which has been previously shown to be required for plasma cell differentiation [90], is reportedly suppressed by proteasome inhibitors in myeloma cells [91]. XBP1 inhibition was associated with high levels of apoptosis, suggesting a role of this transcription factor in perpetuating this malignancy. Furthermore, over-expression of BI-1, another modulator of IRE1α, has been shown to promote tumor growth in a tumor xenograft model [92, 93]. Finally, elevated levels of the ER chaperone BiP in cancer cell lines and clinical tumor specimens are frequently present in a diverse variety of malignancies, with expression correlated with pro-survival mechanisms, chemoresistance to therapy, and poor prognosis [94].
6.6. Metabolic diseases
ER stress plays an important role in the capacity of cells to deal with nutrient fluctuations and additionally some nutrients such as saturated fats induce ER stress. For example, high fat diet (HFD) is a well-known inducer of UPR and pathological ER stress signaling (reviewed in [95]). In fact, mice fed a HFD and the severe ob/ob mouse model of genetic obesity show elevated phosphorylated PERK and IRE1α and JNK activation in both adipose tissue and liver [96, 97]. Moreover, studies from Teodoro-Morrison, et al. showed that in a mouse model that over-produces BiP, β-cell were protected against glucose intolerance and insulin resistance caused by obesity. Liver and muscle tissues from leptin-receptor deficient (db/db) mice have reduced BI-1 mRNA expression. Conversely, restoration of hepatic BI-1 protected mice from obesity-associated insulin resistance and glucose intolerance due to suppression of the downstream IRE1α signaling [98]. ER stress caused by HFD has also been implicated in insulin resistance mechanisms of relevance to type 2 diabetes (T2DM). Genetic ablation of XBP-1 caused increased sensitivity to ER stress, insulin resistance, and degradation of the insulin receptor substrate-1 (IRS-1), uncoupling it from the insulin receptor signaling [99]. Additionally, stress kinase activation as a result of ER stress (in the context of HFD and obesity) results in serine phosphorylation of IRS-1, uncoupling it from insulin receptor signaling and promoting insulin resistance [98, 100].
UPR has also been suggested to participate in carbohydrate metabolism, which has been evidenced in studies of T2DM. For instance, ER stress can inhibit STAT-3-dependent suppression of gluconeogenic enzymes and play an important role in the increase of hepatic glucose production in obesity and diabetes [101]. Work from Wang, et al. [102] has also shown that acute ER stress triggered the phosphorylation and nuclear entry of CRTC2, which in turn promoted the expression of ATF6 and activated gluconeogenesis. Yoshizawa, et al. [103] have also revealed that ATF4 alters glucose metabolism by increasing insulin sensitivity in liver, adipose, and muscle tissues.
Recent studies in T2DM have demonstrated a direct involvement of UPR in the death of pancreatic β-cells. Due to high insulin demand, β-cells greatly rely on the ER to ensure synthesis and proper folding of pro-insulin. The involvement of UPR and, more specifically the PERK-eIF2α pathway, was revealed by studies in PERK-null mice and in the human disease Wolcott-Rallison syndrome, a rare infantile-onset insulin-requiring diabetes caused by a loss-of-function mutation in the PERK gene [104]. For instance, PERK-deficient pancreatic β-cells are more sensitive to ER-stress induced apoptosis and PERK deficient mice develop neonatal hyperglycemia caused by islet proliferation defects and increased apoptosis [63]. Additionally, mice defective in the elF2α phosphorylation develop diabetes due to unregulated proinsulin translation, increased oxidative damage, reduced expression of β-cell-specific genes, and apoptosis [105].
Advanced glycation endproducts (AGEs) are caused under conditions of oxidative stress and hyperglycemia, typical features of T2DM. Glycated proteins function abnormally and have been reported to induce ER stress as evidenced by increasing levels of Grp78 and apoptosis in adipocytes treated with glycated serum albumin. In addition, the ER stress inhibitor taurine conjugated ursodeoxycholic acid (TUDCA), which acts as a chaperone that promotes the folding and trafficking of unfolded or malfolded proteins, prevents AGE-induced apoptosis [106]. Like glycated albumin, extracellular matrix is frequently modified by advanced glycation in the skin of diabetic patients. The advanced-glycated type I collagen also causes pro-apoptotic UPR-mediated apoptosis via CHOP activation in dermal fibroblasts, suggesting a pathophysiological role for the link between advanced glycation and ER stress in diabetic wounds [107].
In a diabetes context, Oslowski, et al. reported that thioredoxin-interacting protein (TXNIP) was induced by ER stress through the action of both IRE1α and PERK. In turn, TXNIP promoted IL-1β production by the NLRP3 inflammasome and in pancreatic β-cells [108]. Simultaneously, Lerner, et al. showed that IRE1α increases TXNIP mRNA stability by reducing levels of TXNIP destabilizing microRNA, miR-17, thus activating the NLRP3 inflammasome, causing procapase-1 cleavage, and IL-1β secretion [109]. It has also been shown that mice with XBP-1 deficiency in β-cells have hyperglycemia and glucose intolerance, with features of diabetes. Moreover it has been demonstrated that PKR can be activated by ER stress. PKR coordinates the activity of inflammatory cytokines and insulin receptor signaling through mechanisms that are only partly understood [82].
6.7. Atherosclerosis
In atherogenesis, arterial wall stressors such as oxidative stress and subendothelial retention of apolipoprotein B-containing lipoproteins trigger the expression of adhesion molecules, cytokines and chemokines in endothelial cells, leading to attraction of inflammatory cells, mainly macrophages. The macrophages internalize lipoproteins and undergo apoptosis, which in turn leads to secondary necrosis. Recently, ER stress has emerged as an important component that influences the course of atherosclerosis, particularly in the advanced stages of the disease. Analyses of human atherosclerotic plaques and animal studies have shown clear evidence of ER stress in macrophages and endothelial cells, the major cells implicated in atherogenesis. For instance, both free cholesterol and 7-ketocholesterol cause ER stress-mediated macrophage apoptosis though activation of CHOP. Conversely, both in vivo and in vitro models demonstrated that genetic silencing of CHOP leads to a reduction in macrophage death with decreases in plaque rupture [110, 111]. The IRE1α/ASK1/JNK pathway contributes to CHOP-mediated macrophage death. For instance, Western diet-fed Apolipoprotein E null mice treated with an IRE1α small interfering RNA or with a JNK inhibitor showed reduced cholesterol-induced macrophage death. The involvement of ER stress in atherosclerosis pathogenesis was also supported by chemical studies using chaperones. In a genetic model of hypercholesterolemic atherosclerosis associated with apolipoprotein E deficiency, administration of a chemical chaperone (phenyl butyric acid) reduced vascular lesion damage and macrophage apoptosis in vivo and in vitro. Furthermore, the same study also showed that mitigation of ER stress prevented macrophagy fatty-acid-binding protein-4 expression, an important component of lipid toxicity in macrophages [112].
ER stress mechanisms may also be relevant to heart disease in the context of both ischemia-reperfusion injury (myocardial infarction) and heart failure. For example, in models of cardiac hypertrophy (heart failure) caused by aortic banding, ER stress has been implicated as a mechanism responsible for cardiomyocyte apoptosis [113]. Chemical chaperones that relieve ER stress also reduce cardiomyocyte apoptosis in the aortic banding model in rodents [114]. Interestingly, β-adrenergic receptor blockers have been reported to ameliorate ER stress in heart failure models, probably through their effects on ER Ca2+ dynamics [115]. Ask1−/− mice are protected from heart failure caused by aortic banding, showing less cardiomyocyte apoptosis in vivo [116], which further suggests the likely involvement of ER stress in heart failure given the prominent role of this apoptotic kinase downstream of IRE1α activation.
ER stress is also clearly activated during myocardial infarction (reviewed in [117]). ATF6 plays a cardioprotective role in this context [13], while IRE1α pathway-mediated ER stress seems to be destructive. For example, the apical kinase in the IRE1α stress kinase pathway ASK1 plays a prominent role, in that Ask1−/− mice showed preservation of left ventricular function compared to wild-type controls after coronary arterial ligation [116].
Conclusions and Future perspectives
Cellular stress responses are a fundamental part of a normal cell physiology. In fact, the adaptation to the “adversity” has greatly shaped the evolution of multi-cellular organisms and has likely promoted the emergence of diverse cellular resilience mechanisms. Whether or not stress culminates in cell death survival or death is determined by multiple different factors, such as intensity and longevity of stress, cell type, and other environmental factors.
The ER is a highly dynamic organelle that exerts a major role in coordinating signaling pathways that ensure cell adaptation, cellular resilience, and survival. Although increasing evidence has suggested that numerous diseases are caused by abnormal cellular responses to ER stress, many questions remain unanswered regarding the roles of ER stress in health and disease. What level of UPR signaling is ideal for enabling cellular adaptation and survival? To what extent are the adaptive versus destructive UPR and non-UPR responses to ER stress involved in the pathophysiology of diseases? How can we selectively modulate the ER stress responses in sick but not healthy cells? And finally, how does the ER interconnect with other cellular organelles to modulate cell death?
Evidence for a role of ER stress-mediated cell death in a variety of diseases make this process an attractive target for therapy. In particular, small molecule inhibitors of the kinase-components of the UPR, PERK and IRE1α, are potential druggable candidates for cancer. On the other hand, players in the IRE1α-TRAF2-ASK1 pathway could also be targets for preventing death of neurons and cardiomyocytes. However, targeting only one branch of the ER stress pathway might not be sufficient to prevent cell death. Thus, a better understanding of the mechanisms that orchestrate the ER stress responses may help to devise future strategies of safely modulating this process for therapeutic benefit.
Highlights.
ER-mediated cell death mechanisms
Cross-talk of ER stress and autophagy
Involvement of ER stress in diseases
Acknowledgments
We thank the NIH (AG-15393). R. Sano was supported by the Tobacco-Related Disease Research Foundation (TRDRP).
Abbreviations
- AGE
advanced glycated end-products
- ALS
amyotropic lateral sclerosis
- AMD
age-related macular degeneration
- ARE
antioxidant response elements
- ASK1
apoptotic-signaling kinase-1
- ATF4
activating transcription factor 4
- ATF6
activating transcription factor 6
- BAG
Bcl-2 associated athanogene
- BAR
bifunctional apoptosis regulator
- BI-1
bax-inhibitor 1
- BiP
binding immunoglobulin protein
- CMV
cytomegalovirus
- CASR
calcium-sensing receptor
- CHOP
transcriptional factor C/EBP homologous protein
- CRE
ATF/cAMP response elements
- DRP-1
dynamin-related protein
- elF2α
eukaryotic translation initiation factor
- ER
endoplasmic reticulum
- ERAD
ER-assisted degradation
- ERO1α
endoplasmic reticulum oxidoreductase-1
- ERSE
ER stress-response element
- GADD34
growth arrest and DNA damage-inducible 34
- HCV
hepatitis C virus
- HFD
high fat diet
- HO-1
heme oxygenase 1
- HSV
herpes simplex virus
- IBD
inflammatory bowel disease
- IEC
intestinal epithelial cells
- IP3R
inositol triphosphate receptor
- IRE1α
inositol-requiring protein-1
- JNK
Jun-N-terminal kinase
- MEF
mouse embryonic fibroblast
- MHC
major histocompatibility complex
- NLRP
NOD-like receptor (NLR) family pyrin domain-containing
- NRF2
nuclear erythroid 2 p45-related factor 2
- PDIA6
protein disulfide isomerase associated 6
- PERK
protein kinase RNA, PKR)-like ER kinase
- PKC
protein kinase C
- RIDD
regulated IRE1-dependent decay of mRNA
- RP
retinitis pigmentosa
- SNP
single nucleotide polymorphism
- TAP1
ER antigen peptide transporter 1
- T2DM
type 2 diabetes
- TLR
toll-like receptor
- TXNIP
thioredoxin-interacting protein
- UPR
unfolded protein response
- VEGF
vascular endothelial growth factor
- XBP-1
X box-binding protein-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gardner BM, Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science. 2011;333:1891–1894. doi: 10.1126/science.1209126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pincus D, Chevalier MW, Aragon T, van Anken E, Vidal SE, El-Samad H, Walter P. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 2010;8:e1000415. doi: 10.1371/journal.pbio.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shamu CE, Walter P. Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J. 1996;15:3028–3039. [PMC free article] [PubMed] [Google Scholar]
- 4.Hassler J, Cao SS, Kaufman RJ. IRE1, a double-edged sword in pre-miRNA slicing and cell death. Developmental cell. 2012;23:921–923. doi: 10.1016/j.devcel.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ron D, Hubbard SR. How IRE1 reacts to ER stress. Cell. 2008;132:24–26. doi: 10.1016/j.cell.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng X, Xiao L, Lang W, Gao F, Ruvolo P, May WS., Jr Novel role for JNK as a stress-activated Bcl2 kinase. J Biol Chem. 2001;276:23681–23688. doi: 10.1074/jbc.M100279200. [DOI] [PubMed] [Google Scholar]
- 8.Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279:45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 10.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey D, O’Hare P. Transmembrane bZIP transcription factors in ER stress signaling and the unfolded protein response. Antioxid Redox Signal. 2007;9:2305–2321. doi: 10.1089/ars.2007.1796. [DOI] [PubMed] [Google Scholar]
- 13.Vekich JA, Belmont PJ, Thuerauf DJ, Glembotski CC. Protein disulfide isomerase-associated 6 is an ATF6-inducible ER stress response protein that protects cardiac myocytes from ischemia/reperfusion-mediated cell death. Journal of molecular and cellular cardiology. 2012;53:259–267. doi: 10.1016/j.yjmcc.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belmont PJ, Chen WJ, Thuerauf DJ, Glembotski CC. Regulation of microRNA expression in the heart by the ATF6 branch of the ER stress response. Journal of molecular and cellular cardiology. 2012;52:1176–1182. doi: 10.1016/j.yjmcc.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belmont PJ, Chen WJ, Thuerauf DJ, Glembotski CC. Regulation of microRNA expression in the heart by the ATF6 branch of the ER stress response. J Mol Cell Cardiol. 52:1176–1182. doi: 10.1016/j.yjmcc.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- 17.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 18.Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang XZ, Ron D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP Kinase. Science. 1996;272:1347–1349. doi: 10.1126/science.272.5266.1347. [DOI] [PubMed] [Google Scholar]
- 22.Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem. 2006;281:21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- 23.Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Glimcher LH, Korsmeyer SJ. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 24.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 25.Sano R, Hou YC, Hedvat M, Correa RG, Shu CW, Krajewska M, Diaz PW, Tamble CM, Quarato G, Gottlieb RA, Yamaguchi M, Nizet V, Dahl R, Thomas DD, Tait SW, Green DR, Fisher PB, Matsuzawa S, Reed JC. Endoplasmic reticulum protein BI-1 regulates Ca(2)(+)-mediated bioenergetics to promote autophagy. Genes & development. 2012;26:1041–1054. doi: 10.1101/gad.184325.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 27.Lim S, Chang W, Lee BK, Song H, Hong JH, Lee S, Song BW, Kim HJ, Cha MJ, Jang Y, Chung N, Choi SY, Hwang KC. Enhanced calreticulin expression promotes calcium-dependent apoptosis in postnatal cardiomyocytes. Mol Cells. 2008;25:390–396. [PubMed] [Google Scholar]
- 28.Kang MJ, Chung J, Ryoo HD. CDK5 and MEKK1 mediate pro-apoptotic signalling following endoplasmic reticulum stress in an autosomal dominant retinitis pigmentosa model. Nature cell biology. 2012;14:409–415. doi: 10.1038/ncb2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varadarajan S, Bampton ET, Smalley JL, Tanaka K, Caves RE, Butterworth M, Wei J, Pellecchia M, Mitcheson J, Gant TW, Dinsdale D, Cohen GM. A novel cellular stress response characterised by a rapid reorganisation of membranes of the endoplasmic reticulum. Cell death and differentiation. 2012;19:1896–1907. doi: 10.1038/cdd.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishikawa T, Watanabe N, Nagano M, Kawai-Yamada M, Lam E. Bax inhibitor-1: a highly conserved endoplasmic reticulum-resident cell death suppressor. Cell death and differentiation. 2011;18:1271–1278. doi: 10.1038/cdd.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chae HJ, Kim HR, Xu C, Bailly-Maitre B, Krajewska M, Krajewski S, Banares S, Cui J, Digicaylioglu M, Ke N, Kitada S, Monosov E, Thomas M, Kress CL, Babendure JR, Tsien RY, Lipton SA, Reed JC. BI-1 regulates an apoptosis pathway linked to endoplasmic reticulum stress. Molecular cell. 2004;15:355–366. doi: 10.1016/j.molcel.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 32.Xu C, Xu W, Palmer AE, Reed JC. BI-1 regulates endoplasmic reticulum Ca2+ homeostasis downstream of Bcl-2 family proteins. J Biol Chem. 2008;283:11477–11484. doi: 10.1074/jbc.M708385200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bultynck G, Kiviluoto S, Henke N, Ivanova H, Schneider L, Rybalchenko V, Luyten T, Nuyts K, De Borggraeve W, Bezprozvanny I, Parys JB, De Smedt H, Missiaen L, Methner A. The C terminus of Bax inhibitor-1 forms a Ca2+-permeable channel pore. The Journal of biological chemistry. 2012;287:2544–2557. doi: 10.1074/jbc.M111.275354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiviluoto S, Schneider L, Luyten T, Vervliet T, Missiaen L, De Smedt H, Parys JB, Methner A, Bultynck G. Bax inhibitor-1 is a novel IP(3) receptor-interacting and -sensitizing protein. Cell death & disease. 2012;3:e367. doi: 10.1038/cddis.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lisbona F, Rojas-Rivera D, Thielen P, Zamorano S, Todd D, Martinon F, Glavic A, Kress C, Lin JH, Walter P, Reed JC, Glimcher LH, Hetz C. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell. 2009;33:679–691. doi: 10.1016/j.molcel.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castillo K, Rojas-Rivera D, Lisbona F, Caballero B, Nassif M, Court FA, Schuck S, Ibar C, Walter P, Sierralta J, Glavic A, Hetz C. BAX inhibitor-1 regulates autophagy by controlling the IRE1alpha branch of the unfolded protein response. EMBO J. 2011;30:4465–4478. doi: 10.1038/emboj.2011.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rojas-Rivera D, Armisen R, Colombo A, Martinez G, Eguiguren AL, Diaz A, Kiviluoto S, Rodriguez D, Patron M, Rizzuto R, Bultynck G, Concha ML, Sierralta J, Stutzin A, Hetz C. TMBIM3/GRINA is a novel unfolded protein response (UPR) target gene that controls apoptosis through the modulation of ER calcium homeostasis. Cell death and differentiation. 2012;19:1013–1026. doi: 10.1038/cdd.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Molecular cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 39.Byrd AE, Aragon IV, Brewer JW. MicroRNA-30c-2* limits expression of proadaptive factor XBP1 in the unfolded protein response. The Journal of cell biology. 2012;196:689–698. doi: 10.1083/jcb.201201077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chitnis NS, Pytel D, Bobrovnikova-Marjon E, Pant D, Zheng H, Maas NL, Frederick B, Kushner JA, Chodosh LA, Koumenis C, Fuchs SY, Diehl JA. miR-211 is a prosurvival microRNA that regulates chop expression in a PERK-dependent manner. Molecular cell. 2012;48:353–364. doi: 10.1016/j.molcel.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Behrman S, Acosta-Alvear D, Walter P. A CHOP-regulated microRNA controls rhodopsin expression. The Journal of cell biology. 2011;192:919–927. doi: 10.1083/jcb.201010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Upton JP, Wang L, Han D, Wang ES, Huskey NE, Lim L, Truitt M, McManus MT, Ruggero D, Goga A, Papa FR, Oakes SA. IRE1alpha cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science. 2012;338:818–822. doi: 10.1126/science.1226191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breckenridge DG, Stojanovic M, Marcellus RC, Shore GC. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol. 2003;160:1115–1127. doi: 10.1083/jcb.200212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delom F, Emadali A, Cocolakis E, Lebrun JJ, Nantel A, Chevet E. Calnexin-dependent regulation of tunicamycin-induced apoptosis in breast carcinoma MCF-7 cells. Cell death and differentiation. 2007;14:586–596. doi: 10.1038/sj.cdd.4402012. [DOI] [PubMed] [Google Scholar]
- 45.Kabbage M, Dickman MB. The BAG proteins: a ubiquitous family of chaperone regulators. Cell Mol Life Sci. 2008;65:1390–1402. doi: 10.1007/s00018-008-7535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruchmann A, Roller C, Walther TV, Schafer G, Lehmusvaara S, Visakorpi T, Klocker H, Cato AC, Maddalo D. Bcl-2 associated athanogene 5 (Bag5) is overexpressed in prostate cancer and inhibits ER-stress induced apoptosis. BMC cancer. 2013;13:96. doi: 10.1186/1471-2407-13-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee GH, Yan C, Shin SJ, Hong SC, Ahn T, Moon A, Park SJ, Lee YC, Yoo WH, Kim HT, Kim DS, Chae SW, Kim HR, Chae HJ. BAX inhibitor-1 enhances cancer metastasis by altering glucose metabolism and activating the sodium-hydrogen exchanger: the alteration of mitochondrial function. Oncogene. 2010;29:2130–2141. doi: 10.1038/onc.2009.491. [DOI] [PubMed] [Google Scholar]
- 48.Rong J, Chen L, Toth JI, Tcherpakov M, Petroski MD, Reed JC. Bifunctional apoptosis regulator (BAR), an endoplasmic reticulum (ER)-associated E3 ubiquitin ligase, modulates BI-1 protein stability and function in ER Stress. The Journal of biological chemistry. 2011;286:1453–1463. doi: 10.1074/jbc.M110.175232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4:e423. doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 51.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Tanida I, Sou YS, Ezaki J, Minematsu-Ikeguchi N, Ueno T, Kominami E. HsAtg4B/HsApg4B/autophagin-1 cleaves the carboxyl termini of three human Atg8 homologues and delipidates microtubule-associated protein light chain 3- and GABAA receptor-associated protein-phospholipid conjugates. J Biol Chem. 2004;279:36268–36276. doi: 10.1074/jbc.M401461200. [DOI] [PubMed] [Google Scholar]
- 53.Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, Yoshimori T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol. 2001;152:657–668. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 55.Xie Z, Nair U, Klionsky DJ. Atg8 controls phagophore expansion during autophagosome formation. Mol Biol Cell. 2008;19:3290–3298. doi: 10.1091/mbc.E07-12-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Satoo K, Noda NN, Kumeta H, Fujioka Y, Mizushima N, Ohsumi Y, Inagaki F. The structure of Atg4B-LC3 complex reveals the mechanism of LC3 processing and delipidation during autophagy. EMBO J. 2009;28:1341–1350. doi: 10.1038/emboj.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoyer-Hansen M, Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14:1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- 58.Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jaattela M. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 59.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rubinstein AD, Eisenstein M, Ber Y, Bialik S, Kimchi A. The autophagy protein Atg12 associates with antiapoptotic Bcl-2 family members to promote mitochondrial apoptosis. Mol Cell. 2011;44:698–709. doi: 10.1016/j.molcel.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 61.Sakaki K, Kaufman RJ. Regulation of ER stress-induced macroautophagy by protein kinase C. Autophagy. 2008;4:841–843. doi: 10.4161/auto.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E, Momoi T. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- 63.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 64.Margariti A, Li H, Chen T, Martin D, Vizcay-Barrena G, Alam S, Karamariti E, Xiao Q, Zampetaki A, Zhang Z, Wang W, Jiang Z, Gao C, Ma B, Chen YG, Cockerill G, Hu Y, Xu Q, Zeng L. XBP1 mRNA splicing triggers an autophagic response in endothelial cells through BECLIN-1 transcriptional activation. The Journal of biological chemistry. 2013;288:859–872. doi: 10.1074/jbc.M112.412783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sugeno N, Takeda A, Hasegawa T, Kobayashi M, Kikuchi A, Mori F, Wakabayashi K, Itoyama Y. Serine 129 phosphorylation of alpha-synuclein induces unfolded protein response-mediated cell death. J Biol Chem. 2008;283:23179–23188. doi: 10.1074/jbc.M802223200. [DOI] [PubMed] [Google Scholar]
- 67.Silva RM, Ries V, Oo TF, Yarygina O, Jackson-Lewis V, Ryu EJ, Lu PD, Marciniak SJ, Ron D, Przedborski S, Kholodilov N, Greene LA, Burke RE. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J Neurochem. 2005;95:974–986. doi: 10.1111/j.1471-4159.2005.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee JH, Won SM, Suh J, Son SJ, Moon GJ, Park UJ, Gwag BJ. Induction of the unfolded protein response and cell death pathway in Alzheimer’s disease, but not in aged Tg2576 mice. Experimental & molecular medicine. 2010;42:386–394. doi: 10.3858/emm.2010.42.5.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. Journal of neuroinflammation. 2009;6:41. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prasanthi JR, Larson T, Schommer J, Ghribi O. Silencing GADD153/CHOP gene expression protects against Alzheimer’s disease-like pathology induced by 27-hydroxycholesterol in rabbit hippocampus. PloS one. 2011;6:e26420. doi: 10.1371/journal.pone.0026420. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Kanekura K, Suzuki H, Aiso S, Matsuoka M. ER stress and unfolded protein response in amyotrophic lateral sclerosis. Mol Neurobiol. 2009;39:81–89. doi: 10.1007/s12035-009-8054-3. [DOI] [PubMed] [Google Scholar]
- 72.Kang MJ, Ryoo HD. Suppression of retinal degeneration in Drosophila by stimulation of ER-associated degradation. Proc Natl Acad Sci U S A. 2009;106:17043–17048. doi: 10.1073/pnas.0905566106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oskolkova OV, Afonyushkin T, Leitner A, von Schlieffen E, Gargalovic PS, Lusis AJ, Binder BR, Bochkov VN. ATF4-dependent transcription is a key mechanism in VEGF up-regulation by oxidized phospholipids: critical role of oxidized sn-2 residues in activation of unfolded protein response. Blood. 2008;112:330–339. doi: 10.1182/blood-2007-09-112870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sauer T, Patel M, Chan CC, Tuo J. Unfolding the Therapeutic Potential of Chemical Chaperones for Age-related Macular Degeneration. Expert Rev Ophthalmol. 2008;3:29–42. doi: 10.1586/17469899.3.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zode GS, Kuehn MH, Nishimura DY, Searby CC, Mohan K, Grozdanic SD, Bugge K, Anderson MG, Clark AF, Stone EM, Sheffield VC. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. The Journal of clinical investigation. 2011;121:3542–3553. doi: 10.1172/JCI58183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nature immunology. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woo CW, Kutzler L, Kimball SR, Tabas I. Toll-like receptor activation suppresses ER stress factor CHOP and translation inhibition through activation of eIF2B. Nature cell biology. 2012;14:192–200. doi: 10.1038/ncb2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garg AD, Kaczmarek A, Krysko O, Vandenabeele P, Krysko DV, Agostinis P. ER stress-induced inflammation: does it aid or impede disease progression? Trends in molecular medicine. 2012;18:589–598. doi: 10.1016/j.molmed.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 79.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Der SD, Lau AS. Involvement of the double-stranded-RNA-dependent kinase PKR in interferon expression and interferon-mediated antiviral activity. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:8841–8845. doi: 10.1073/pnas.92.19.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Proud CG, Colthurst DR, Ferrari S, Pinna LA. The substrate specificity of protein kinases which phosphorylate the alpha subunit of eukaryotic initiation factor 2. Eur J Biochem. 1991;195:771–779. doi: 10.1111/j.1432-1033.1991.tb15765.x. [DOI] [PubMed] [Google Scholar]
- 82.Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, Zou Y, Erlandsson-Harris H, Yang H, Ting JP, Wang H, Andersson U, Antoine DJ, Chavan SS, Hotamisligil GS, Tracey KJ. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.He B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 2006;13:393–403. doi: 10.1038/sj.cdd.4401833. [DOI] [PubMed] [Google Scholar]
- 85.Tirosh B, Iwakoshi NN, Lilley BN, Lee AH, Glimcher LH, Ploegh HL. Human cytomegalovirus protein US11 provokes an unfolded protein response that may facilitate the degradation of class I major histocompatibility complex products. J Virol. 2005;79:2768–2779. doi: 10.1128/JVI.79.5.2768-2779.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng G, Feng Z, He B. Herpes simplex virus 1 infection activates the endoplasmic reticulum resident kinase PERK and mediates eIF-2alpha dephosphorylation by the gamma(1)34.5 protein. J Virol. 2005;79:1379–1388. doi: 10.1128/JVI.79.3.1379-1388.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, Koromilas A, Wouters BG. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol. 2002;22:7405–7416. doi: 10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Romero-Ramirez L, Cao H, Regalado MP, Kambham N, Siemann D, Kim JJ, Le QT, Koong AC. X box-binding protein 1 regulates angiogenesis in human pancreatic adenocarcinomas. Transl Oncol. 2009;2:31–38. doi: 10.1593/tlo.08211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ri M, Tashiro E, Oikawa D, Shinjo S, Tokuda M, Yokouchi Y, Narita T, Masaki A, Ito A, Ding J, Kusumoto S, Ishida T, Komatsu H, Shiotsu Y, Ueda R, Iwawaki T, Imoto M, Iida S. Identification of Toyocamycin, an agent cytotoxic for multiple myeloma cells, as a potent inhibitor of ER stress-induced XBP1 mRNA splicing. Blood cancer journal. 2012;2:e79. doi: 10.1038/bcj.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, Friend D, Grusby MJ, Alt F, Glimcher LH. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 91.Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc Natl Acad Sci U S A. 2003;100:9946–9951. doi: 10.1073/pnas.1334037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grzmil M, Kaulfuss S, Thelen P, Hemmerlein B, Schweyer S, Obenauer S, Kang TW, Burfeind P. Expression and functional analysis of Bax inhibitor-1 in human breast cancer cells. J Pathol. 2006;208:340–349. doi: 10.1002/path.1902. [DOI] [PubMed] [Google Scholar]
- 93.Grzmil M, Thelen P, Hemmerlein B, Schweyer S, Voigt S, Mury D, Burfeind P. Bax inhibitor-1 is overexpressed in prostate cancer and its specific down-regulation by RNA interference leads to cell death in human prostate carcinoma cells. Am J Pathol. 2003;163:543–552. doi: 10.1016/S0002-9440(10)63682-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li X, Zhang K, Li Z. Unfolded protein response in cancer: the physician’s perspective. Journal of hematology & oncology. 2011;4:8. doi: 10.1186/1756-8722-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends in molecular medicine. 2012;18:59–68. doi: 10.1016/j.molmed.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 96.Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 97.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 98.Bailly-Maitre B, Belgardt BF, Jordan SD, Coornaert B, von Freyend MJ, Kleinridders A, Mauer J, Cuddy M, Kress CL, Willmes D, Essig M, Hampel B, Protzer U, Reed JC, Bruning JC. Hepatic Bax inhibitor-1 inhibits IRE1alpha and protects from obesity-associated insulin resistance and glucose intolerance. J Biol Chem. 2010;285:6198–6207. doi: 10.1074/jbc.M109.056648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hage Hassan R, Hainault I, Vilquin JT, Samama C, Lasnier F, Ferre P, Foufelle F, Hajduch E. Endoplasmic reticulum stress does not mediate palmitate-induced insulin resistance in mouse and human muscle cells. Diabetologia. 2012;55:204–214. doi: 10.1007/s00125-011-2328-9. [DOI] [PubMed] [Google Scholar]
- 100.Kawasaki N, Asada R, Saito A, Kanemoto S, Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep. 2012;2:799. doi: 10.1038/srep00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kimura K, Yamada T, Matsumoto M, Kido Y, Hosooka T, Asahara S, Matsuda T, Ota T, Watanabe H, Sai Y, Miyamoto K, Kaneko S, Kasuga M, Inoue H. Endoplasmic reticulum stress inhibits STAT3-dependent suppression of hepatic gluconeogenesis via dephosphorylation and deacetylation. Diabetes. 2012;61:61–73. doi: 10.2337/db10-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Y, Vera L, Fischer WH, Montminy M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature. 2009;460:534–537. doi: 10.1038/nature08111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yoshizawa T, Hinoi E, Jung DY, Kajimura D, Ferron M, Seo J, Graff JM, Kim JK, Karsenty G. The transcription factor ATF4 regulates glucose metabolism in mice through its expression in osteoblasts. J Clin Invest. 2009;119:2807–2817. doi: 10.1172/JCI39366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saito A, Ochiai K, Kondo S, Tsumagari K, Murakami T, Cavener DR, Imaizumi K. Endoplasmic reticulum stress response mediated by the PERK-eIF2(alpha)-ATF4 pathway is involved in osteoblast differentiation induced by BMP2. The Journal of biological chemistry. 2011;286:4809–4818. doi: 10.1074/jbc.M110.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, Gildersleeve RD, Pennathur S, Kaufman RJ. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10:13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamabe S, Hirose J, Uehara Y, Okada T, Okamoto N, Oka K, Taniwaki T, Mizuta H. Intracellular accumulation of advanced glycation end products induces apoptosis via endoplasmic reticulum stress in chondrocytes. The FEBS journal. 2013;280:1617–1629. doi: 10.1111/febs.12170. [DOI] [PubMed] [Google Scholar]
- 107.Loughlin DT, Artlett CM. Modification of collagen by 3-deoxyglucosone alters wound healing through differential regulation of p38 MAP kinase. PloS one. 2011;6:e18676. doi: 10.1371/journal.pone.0018676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oslowski CM, Hara T, O’Sullivan-Murphy B, Kanekura K, Lu S, Hara M, Ishigaki S, Zhu LJ, Hayashi E, Hui ST, Greiner D, Kaufman RJ, Bortell R, Urano F. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell metabolism. 2012;16:265–273. doi: 10.1016/j.cmet.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lerner AG, Upton JP, Praveen PV, Ghosh R, Nakagawa Y, Igbaria A, Shen S, Nguyen V, Backes BJ, Heiman M, Heintz N, Greengard P, Hui S, Tang Q, Trusina A, Oakes SA, Papa FR. IRE1alpha induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell metabolism. 2012;16:250–264. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N, Gorgun CZ, Hotamisligil GS. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140:338–348. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsukano H, Gotoh T, Endo M, Miyata K, Tazume H, Kadomatsu T, Yano M, Iwawaki T, Kohno K, Araki K, Mizuta H, Oike Y. The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:1925–1932. doi: 10.1161/ATVBAHA.110.206094. [DOI] [PubMed] [Google Scholar]
- 112.Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, Hotamisligil GS. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Guan HS, Shangguan HJ, Shang Z, Yang L, Meng XM, Qiao SB. Endoplasmic reticulum stress caused by left ventricular hypertrophy in rats: effects of telmisartan. The American journal of the medical sciences. 2011;342:318–323. doi: 10.1097/MAJ.0b013e3182112baf. [DOI] [PubMed] [Google Scholar]
- 114.Park CS, Cha H, Kwon EJ, Sreenivasaiah PK, Kim do H. The chemical chaperone 4-phenylbutyric acid attenuates pressure-overload cardiac hypertrophy by alleviating endoplasmic reticulum stress. Biochem Biophys Res Commun. 2012;421:578–584. doi: 10.1016/j.bbrc.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 115.Dalal S, Foster CR, Das BC, Singh M, Singh K. Beta-adrenergic receptor stimulation induces endoplasmic reticulum stress in adult cardiac myocytes: role in apoptosis. Molecular and cellular biochemistry. 2012;364:59–70. doi: 10.1007/s11010-011-1205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yamaguchi O, Higuchi Y, Hirotani S, Kashiwase K, Nakayama H, Hikoso S, Takeda T, Watanabe T, Asahi M, Taniike M, Matsumura Y, Tsujimoto I, Hongo K, Kusakari Y, Kurihara S, Nishida K, Ichijo H, Hori M, Otsu K. Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci U S A. 2003;100:15883–15888. doi: 10.1073/pnas.2136717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tabas I. Macrophage apoptosis in atherosclerosis: consequences on plaque progression and the role of endoplasmic reticulum stress. Antioxid Redox Signal. 2009;11:2333–2339. doi: 10.1089/ars.2009.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]