Abstract
Microfluidic platforms provide several unique advantages for drug development. In the production of drug carriers, physical properties such as size and shape, and chemical properties such as drug composition and pharmacokinetic parameters, can be modified simply and effectively by tuning the flow rate and geometries. Large numbers of carriers can then be fabricated with minimal effort and with little to no batch-to-batch variation. Additionally, cell or tissue culture models in microfluidic systems can be used as in vitro drug screening tools. Compared to in vivo animal models, microfluidic drug screening platforms allow for high-throughput and reproducible screening at a significantly lower cost, and when combined with current advances in tissue engineering, are also capable of mimicking native tissues. In this review, various microfluidic platforms for drug and gene carrier fabrication are reviewed to provide guidelines for designing appropriate carriers. In vitro microfluidic drug screening platforms designed for high-throughput analysis and replication of in vivo conditions are also reviewed to highlight future directions for drug research and development.
Keywords: microfluidics, drug carrier, gene carrier, drug screening, high-throughput
1. Introduction
Self-assembling systems are an attractive class of drug delivery vehicles but physical properties and delivery efficiencies can be strongly dependent on formulation conditions [1]. It can therefore be challenging to generate reproducible and homogenous nanoscale drug carrier formulations by bulk preparation methods. Recent advances in micro/nanofabrication techniques enable more uniform preparation of drug carriers. Microfluidic systems for gene and drug carriers that facilitate controllable preparation of multicomponent systems have addressed several problems by facilitating in situ formulation at the nano/picoliter volume scale. As such, microfluidic systems enable high-throughput and inexpensive carrier production. Furthermore, carriers can be reproducibly prepared with desired sizes, shapes and properties to minimize cytotoxicity and to promote the transport efficiency of target drugs [2]. These techniques facilitate the production of customized drug and gene carriers with patient and drug specificity [3]. By controlling the physical and chemical characteristics of the drug carriers, pharmacokinetic parameters such as drug release, absorption, distribution, and elimination profiles can be modified to improve drug efficacy and safety [4-6].
In addition to carrier production, microfluidic platforms are useful for drug screening. Animal models are commonly used to evaluate drug delivery, efficacy and cytotoxicity but advanced in vitro screening technologies can help to streamline and reduce the number of required animal studies. By integrating 2-dimensional (2D) or 3-dimensional (3D) cell culture techniques with microfluidic methods, in vitro drug screening platforms such as cell-on-a-chip, tissue-on-a-chip and organ-on-a-chip can be created to evaluate drug responses. Further efforts could enable organ-on-a-chip systems to perform the study of systemic drug response and partially replace animal testing. Microfluidic control components such as microvalves and pumps can be integrated into automated platforms to perform high-throughput and multiplexed drug screening that produces physiologically relevant data.
In this review, various microfluidic platforms for producing gene and drug carriers and drug screening tools are introduced. Microfluidic gene and drug carrier fabricators such as diffusion based microfluidic mixers, chaotic mixers, droplet generators and programmable microfluidic platforms are overviewed with a particular focus on the production capability and efficacy of the resulting drug/gene carriers. We then examine microfluidic platforms that integrate cells and engineered tissues with various modes of perfusion into a single device for drug screening applications.
2. Microfluidics-assisted formulation of gene and drug carriers
Recent advances in molecular biology and genetic research have led to the discovery of many promising small molecules with therapeutic benefits. Of increasing importance for drug delivery research is the design of suitable carriers for these molecules, especially for those that have very low bioavailability, are highly toxic, and require protection from rapid degradation and excretion. Such carriers generally need to be stable, biocompatible, biodegradable, and have the ability to target specific tissues. However, the development of these carriers has proven difficult and even those that are proven to be effective are hampered at the fabrication stage by issues with product consistency, fabrication complexity and the prohibitively high cost of materials involved. For example, lipid-based carriers have been relatively successful in achieving high delivery efficacy both in vitro and in vitro. However, the formulation of large numbers of these carriers often requires multistep and labor-intensive processes to properly control size distribution [7]. Furthermore, conventional protocols for lipid carriers can sometimes call for large volumes (on the order of milliliters) of expensive drugs or nucleic acids to ensure that encapsulation is sufficiently high enough to have any therapeutic effect.
Microfluidics have thus been proposed as a method to alleviate this particular bottleneck in the drug development process. The versatility of microfluidic devices allows researchers to precisely design specific device geometries that can easily and consistently create homogenous carriers composed of a wide variety of materials and with high encapsulation efficiencies. Additionally, the small scale of these devices minimizes the quantities of reagents required and can therefore facilitate high-throughput production at a significantly lower cost compared to conventional bulk mixing techniques. In this section, general considerations for designing the proper device for carrier formulation are reviewed, and innovative microfluidic techniques for gene and drug carrier fabrication are highlighted and evaluated based on the encapsulation or transfection efficacy of the produced carriers (Table 1).
Table 1. Devices for microfluidics-assisted gene and drug carrier production.
| Device Type | Carrier Composition | Encapsulated Molecule |
Transfection/Drug Encapsulation Efficiency (%) |
Refs |
|---|---|---|---|---|
|
Diffusion-based
microfluidic mixers |
PEI | GFP plasmid | ~40% | [14, 15] |
| PEI-PCL-PEG | siRNA | ~75.5% | [18] | |
| DSPC-DMG-PEG | siDNA | ~90% | [19] | |
| PLL-DSGPE | pDNA | N/A | [16] | |
| Protamine-PC-DSPE-PEG | Oligonucleotide | ~75% | [17] | |
| PLGA-PEG | Docetaxel | ~51% | [25] | |
| Heparin-folic acid-retinoic acid (HFR) |
Retinoic Acid | 33.4% | [26] | |
| PMMA | Ketoprofen | 53% | [32] | |
| POPC-cholesterol/ POPC-triolein |
Doxorubicin | 60-95% | [33] | |
|
| ||||
|
Droplet
generators |
PEI and PHP | pDNA | ~65% | [21] |
| Lipofectin (DOTMA-DOPE) | EGFP plasmid | ~60% | [22] | |
| DOPC | GFP plasmid | N/A | [27] | |
| DSPC-DSPE-PEG | Doxorubicin | ~28% | [29] | |
| CMC-A/DEX-B (hydrogel) | Bupivacaine-HCl | N/A | [31] | |
|
| ||||
|
Programmable
microfluidics |
CD-PEI and Ad-PAMAM | pDNA | ~70% | [24] |
2.1. Microfluidic device design considerations for carrier production
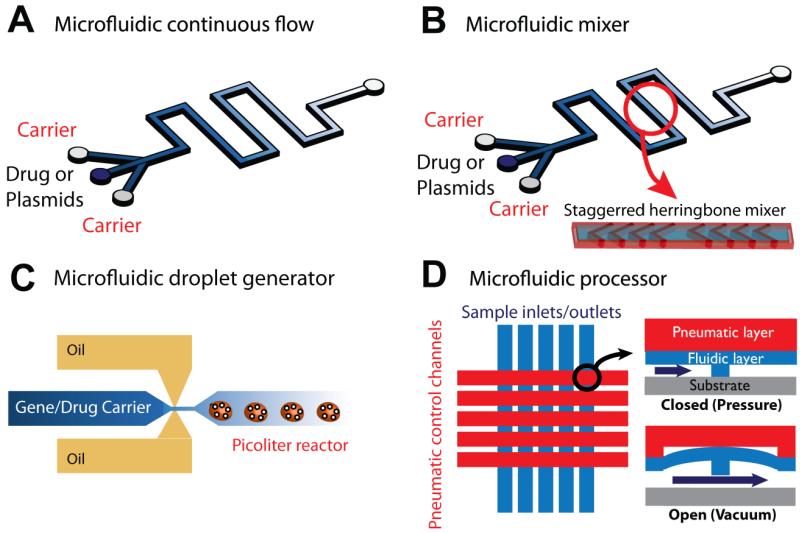
Microfluidic synthesizers for gene/drug carrier fabrication have been developed for the rapid, precise, and effective production of various gene and drug carriers. The most common synthesizer is the microfluidic diffusion-based mixer which consists of multiple inlets and a single outlet and controls the reaction between carrier materials and molecules by flow rate and channel length. These mixers can then be enhanced by incorporating a herringbone pattern in the channel that increases the diffusive boundary area of the samples by at least two orders of magnitude, resulting in rapid and reproducible formulation of drug carriers. However, the mixing that occurs with this method is reliant on the diffusion that occurs in the boundary between the separate flow streams under a continuous flow regime, and this can result in large quantities of unreacted target molecules and carrier materials that can subsequently increase cytotoxicity and decrease the rate of drug transport. The droplet generator technique can eliminate these drawbacks because the reaction of each droplet occurs independently. With the droplet technique, the ratio of carrier materials and target drugs, total volume, the reaction rate and size of the drug carriers can be controlled precisely without contamination issues (Fig. 1).
Figure 1.
General design classes of microfluidic platforms for gene/drug carrier production. (A) Simple diffusion based continuous-flow microfluidic fabricator. (B) Passive micro-mixer, or staggered herringbone mixer, with incorporated synthesizer which enables homogenous and rapid mixing between carrier materials and drugs. (C) Microdroplet generator for precise control of carrier size and composition. (D) Programmable pneumatic microfluidic processor for generating large drug carrier libraries [8].
To ensure that carrier preparation is carried out successfully, however, there are several other factors that must be taken into consideration beyond mode of fabrication, including carrier composition, encapsulated biomolecule, and microfluidic device material. For polyplexes, a cationic polymer is generally used to carry negatively charged plasmids by electrostatic interaction. Selecting a polymer with the proper composition, length and charge is the first step to fabricate the desired gene carrier. By tuning the flow rate in the microfluidic fabricator, desired polyplexes that have the appropriate size, the ratio of compositions, and surface functionality can be obtained. To create siRNA polyplexes, users have to consider the additional factor of minimizing RNA degradation. Alternatively, since lipids are a natural biomaterial, the use of lipoplexes is often advantageous for efficiently transfecting encapsulated genes into cells. Cationic lipids are most commonly used to encapsulate and form complexes with nucleic acids to form organized structures that can be multilamellar, hexagonal, or spherical. The controllability of the final lipoplex structure offered by microfluidics is important, as it has been found that lipoplex morphologies often influence their transfection efficiency to a great degree [9].
When formulating both polyplexes and lipoplexes, various strong solvents are usually involved for dissolving the components. Therefore, the fabrication methods and materials of a microfluidic synthesizer have to be compatible with the various solvents. Soft-lithography techniques using polydimethylsiloxane (PDMS) or poly(methyl methacrylate) (PMMA) as the device material are the most common when it comes to fabricating microfluidic devices. However, swelling of these polymers can occur when it is exposed to strong solvents such as acetone and toluene [10], and this swelling detrimentally affects fluid flow and the controllability of carrier formulation. A method of addressing this issue is to use a hot-embossing technique, in which microfluidic channels can be imprinted on polytetrafluoroethylene (PTFE) and Cyclic Olefin Copolymer (COC), two polymers which have strong chemical resistance [11, 12]. Another simple approach to impart chemical resistance to conventional PDMS devices is to fabricate composite devices in which channels composed of SU-8 polymer films are embedded in PDMS, which provides structural support for the films both during and after fabrication [13]. The devices generated using this method exhibited excellent solvent compatibility and were able to generate both oil-in-water and water-in-oil emulsions in a stable manner, thus expanding the range of possible drug carriers that can be created in a high-throughput manner using microfluidics.
2.2. Nucleic acid carrier production in microfluidics
Cationic polymers and lipids comprise the majority of synthetic gene transfer materials. When mixed with anionic nucleic acids, polyplexes (complexes with cationic polymers), lipoplexes (complexes with cationic lipids) and lipopolyplexes (complexes with both polymers and lipids) are formed by electrostatic complexation. The resulting particles may vary in size, charge or shape, depending on reagent concentrations as well as mixing order, speed and solvent. When formulated in bulk, resulting complexes can be polydisperse. Simple and convenient microfluidic diffusion-based synthesizers consisting of a multiple-inlet/single-outlet microchannel have been used to create synthetic nucleic acid carriers using a variety of materials mixed with plasmid or oligonucleotide cargo [14-18]. PEI (polyethylenimine)-pDNA nanocarriers have been fabricated and evaluated as a gene carrier using microfluidic devices [14, 15]. In comparison to analogous carriers prepared by bulk pipette mixing, the PEI polyplex showed more uniform size distribution. In one report, improved cell viability and gene transfection efficiency was achieved from PEI polyplexes fabricated by microfluidic devices compared to bulk preparation [14]. In another example, and siRNA-containing polyplex was fabricated using a simple Y-shaped microfluidic device with an inlet each for siRNA and a PEI-PCL-PEG (block copolymers of polyethylenimine, polycaprolactone, and polyethyleneglycol) [18]. The siRNA nanocarriers from the microfluidic device showed a two-fold improvement in RNA protection and an approximately 10% more effective transfection of siRNA into cultured cells than manually fabricated nanocarriers.
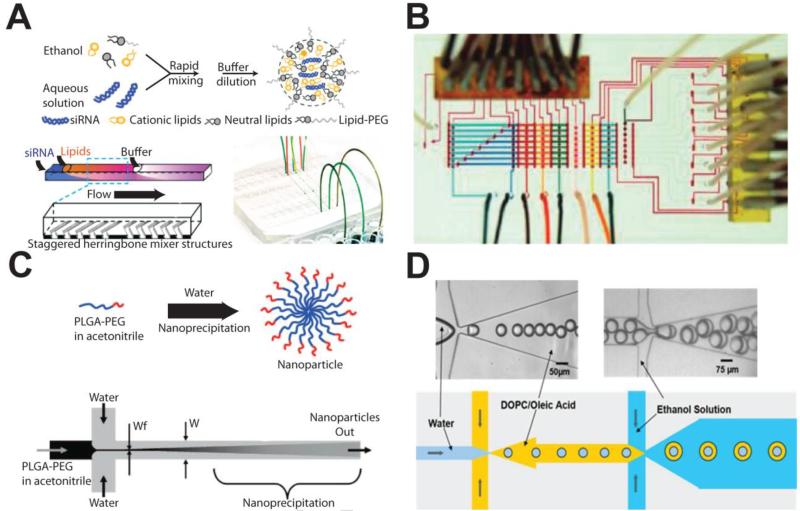
Lipid nanoparticles (LNPs) encapsulating siRNA have also been formulated using a herringbone-patterned chaotic microfluidic [19] (Fig. 2A). Sizes of the LNPs were monodisperse and reproducible, and siRNA encapsulation efficiency was approximately 80%. High quality LNPs containing siRNA were evaluated in an in vivo gene-silencing assay using a mouse model. By controlling the composition of cationic lipid, gene-silencing potencies of greater than 90% were achieved in the in vivo model. In another example, lipopolyplexes composed of a poly(L)lysine (PLL)-pDNA polyplex core encapsulated by a lipid membrane was fabricated by two serial reactions. In the first reaction, PLL and pDNA were injected through the inlets of the microfluidic device to create the polyplex, and in the second reaction, the polyplex and lipid were mixed in the device to create the lipid-encapsulated polyplex [16]. Flow rate of input streams were shown to affect particle size. This study illustrated the feasibility of very rapid (~20 mins.) carrier production through sequential reactions with polymers and lipids. Another sequential reaction study was performed in which a lipopolyplex carrier containing anti-sense oligonucleotide (ODN) was fabricated by interconnecting two 3-inlet/1-outlet microfluidic devices [17]. Three device configurations were tested, with some leading to large or cytotoxic formulations. In the optimized device, polycation/lipid and ODN streams were alternatively squeezed by the other component. It was found that the ODN delivered in carriers produced using the microfluidic synthesizer were more effective at down-regulating target gene expression compared to delivery from carriers produced with bulk fabrication.
Figure 2.
Microfluidic platforms for gene/drug carrier production. (A) Fabrication of lipid nanoparticles containing siRNA by using staggered herringbone mixer [19]. (B) Programmable microfluidic microvalve array for generating a supramolecular nanoparticle library by combinatorial reaction [24]. (C) Anti-cancer drug encapsulation in block copolymer-based nanoparticles in a diffusion based mixer [25]. (D) Cascade droplet generator for double immersion droplet formation [27].
Highly uniform nucleic acid carriers can also be prepared using microfluidic droplet generators [20-22]. By controlling the flow rate of each phase of immiscible fluids in the microfluidic system, the shearing of the fluids enable the generation of droplets with uniform sizes. The most common designs for droplet generators are the T-junction and flow-focusing configurations [23]. With a simple cross-flow microfluidic droplet generator, monodisperse droplets with cationic polymer and pDNA encoding green fluorescence protein (GFP) were synthesized for gene delivery into cultured human cells [21]. Nanocarriers fabricated from the droplet technique were found to induce minimal apoptosis in cells, which is a departure from what is seen with bulk-prepared polyplexes. The carriers generated by the droplet technique presented a continuous uptake profile up to at least 5 hrs, while the nanocarriers from bulk method reached an uptake plateau after approximately 2 hours’ incubation. Overall, droplet- and bulk-generated carriers resulted in ~90% and ~75% cell viability, and ~60% and ~40 % transfection efficiency, respectively. A droplet generator design was also used to create liposomes containing EGFP plasmid [22]. Each droplet functioned as completely localized picoliter reactors and these reactors minimized the reagent aggregation issue which is associated with bulk fabrication. For this reason, the carriers created from the droplet generator showed high dimensional uniformity and low cytotoxicity. By evaluating the transfection efficiency of the plasmid into U2OS cells, it was shown that the droplet generated lipoplexes presented greater transfection rates and with greater reproducibility than products from bulk generation.
A programmable microfluidic processor (Fig. 2B) can be used to optimize gene carrier formulation [24]. In this array, any combinations from various inputs can be generated by programming the microvalve sequence. This processor was used to generate a panel of formulations comprised of multiple components: cyclodextrin-PEI (CD-PEI) and adamantanamine-polyamidoamine (Ad-PAMAM), functional Ad-modified polyethylene glycol for targeting and stabilization, and plasmid DNA. To control the structural and functional properties of the supramolecular nanoparticle based gene carrier, the ratio of each reagent needs to be precisely controlled, and the programmable microfluidic processor enables high-precision automated metering, diluting and mixing of desired reagents. In this case, 250 different combinatorial libraries with minimal size distribution were created within an hour. By selecting an optimal composition of the gene carrier, greater than 70 % transfection efficiency and 96% cell viability in various cancer cell lines was achieved.
2.3. Drug carrier production in microfluidics
Microfluidic synthesizers have also been used to prepare drug-loaded vehicles by encapsulation [25] and chemical conjugation to polymer carriers [26]. By using a simple diffusion-based microfluidic synthesizer, drug-encapsulated polymeric (poly(lactic-co-glycolic acid)-poly(ethylene glycol), or PLGA-PEG) nanoparticles were fabricated by controlling the mixing rate with hydrodynamic focusing (Fig. 2C) [25]. The self-assembly reaction was precisely tuned by the ratio of the flow rates of water and of the solvent containing the copolymer, thereby controlling the degree of mixing within the microfluidic device. Increasing the ratio of flow rates of solvent to water induced slow mixing and resulted in an increase of nanoparticle size, and the chemical properties of the drug carrier were tuned by controlling the carrier composition. Nanoparticles produced in microfluidic devices were smaller in size, encapsulated more drug, and showed slower drug release rates than those prepared by bulk fabrication, especially when hydrophobic PLGA was added to the block copolymer solution. Tran et. al. demonstrated highly efficient preparation of amphiphilic heparin-folic acid-retinoic acid (HFR) bio-conjugates with reduced reaction time and more effective targeted drug delivery [26]. This unique self-assembled nanoparticle drug delivery system was able to selectively deliver drug to folate receptor-positive cancer cells with superior cellular uptake, although the relatively low drug loading efficiency is not ideal.
The production of stable, monodisperse double emulsion-based particles in bulk solution has traditionally been difficult, with the need for multiple fabrication steps. However, the use of microfluidic flow-focusing droplet generators greatly simplifies this process [27-31]. In a study conducted by Teh et. al., DOPC vesicles were synthesized using consecutive flow-focusing channel geometries and solvent extraction (Fig. 2D) [27]. Vesicle diameters could be controlled by adjusting the fluid flow rates of the device and all generated particles were monodisperse in size, with less than 2% variation. Additionally, the produced vesicles were observed to be stable for in solution for up to 3 months, and while longer shelf lives would be ideal for commercially available drug carriers, this is still promising. While this study focused on the development of protein-containing microbioreactor lipid vesicles as artificial cells, the same technique can easily be adapted to create lipid-based microparticles for drug delivery. In a novel approach, a droplet generator was used to reproducibly create monodisperse microbubble liposomes that could carry highly hydrophobic and toxic drugs, such as doxorubicin, at high concentrations [29]. The gas core of these particles makes them more susceptible to disruption and oscillation by acoustic energy, which then leads to enhanced payload delivery. Thus, when used in conjunction with externally applied ultrasound, such vehicles allow for the targeted local delivery of therapeutics. Flow-focusing droplet generators also enable the fabrication of in situ microscale hydrogels containing drugs by sequential reaction. In one example, hydrazide functionalized cellulose (CMC-A) and aldehyde functionalized dextran (DEX-B) were pumped through the droplet generator resulting in the formation of the in situ hydrogels by covalent bonding [31]. By adding oil in the second droplet generator, mono-dispersed and stable hydrogels were obtained. By encapsulating bupivacaine hydrochloride (BPV-HCl) in the hydrogel droplet, the kinetics of the drug release in a physiologically relevant solution was evaluated. The releasing rate of the encapsulated drug was well controlled for 4 days and then slowed down, and released levels of the drug were maintained in the therapeutic ranges for up to 40 days.
A nanoparticle lipophilic drug carrier containing ketoprofen (KP), a well-known non-steroidal anti-inflammatory drug, was also synthesized using microfluidics [32]. Three inlets for a mixed solution of polymer (poly(methylmethacrylate), or PMMA), drug and Cremophor ELP, and 3 inlets for water were connected to a central outlet that enables the rapid mixing of the six streams of viscous fluids. By using this setup, PMMA-NPs were created with 53% KP encapsulation efficiency and all encapsulated was KP released within 2-4 hrs, which is the time frame necessary for effective KP therapy. By changing the carrier composition, the release rate of drug could be adjusted. For example, with the same drug, a PLGA carrier fabricated using this method takes approximately 70 hrs to release the KP completely. Most important, this work demonstrated nanoparticle production by a continuous rather than batch process.
The herringbone mixer, which provides a higher mixing rate, was utilized to control the size of a lipid nanoparticle consisting of 1-palmitoyl, 2-oleoyl phosphatidycholine (POPC), cholestrerol and the triglyceride triolein by mixing a stream of ethanol-containing lipid with an aqueous system [33]. The physical and chemical properties of the nanoparticles were tuned by controlling the flow rate of aqueous solution and ethanol, pH, temperature, and other environmental factors. As a model drug, doxorubicin was encapsulated in POPC nanoparticles without affecting the nanoparticle size. Since the fluid flow and environmental factors were precisely controlled in the microfluidic platform, more control over nanoparticle size and surface properties could be achieved.
3. Microfluidic platforms for drug screening
Bringing a new drug from initial discovery to commercialization is a lengthy and complex one in which many drugs fail to appear on market before they reach consumers, and even the drugs that make it to the market can cause serious and previously undetected side effects. As such, the determination of the cytotoxicity, potency and efficacy of the new molecule is an important component of the development process. Researchers currently rely on either in vivo animal models or on macroscale in vitro cell culture platforms to study the therapeutic effects and safety of new pharmaceuticals and drug delivery systems, and although these conventional models give important information, they have substantial drawbacks [34]. Drug screening tests with in vivo models are limited by the facts that using living organisms is usually time consuming and expensive, and the reality that insights gained from in vivo models may not be biologically relevant to humans. To counter these shortcomings, in vitro drug screening methods using 2D cell cultures have served as a bridge between in vivo testing and over-simplified biochemical assays. However, as the number of promising drug candidates greatly increases due to advancements in genomics, proteomics, and bioinformatics, it is essential that adequate high-throughput methods of drug screening are established. Additionally, conventional cell culture techniques used for drug screening are hampered by problems associated with the use of relatively large quantities of reagents and of drugs that are often expensive to produce and limited in quantity. Conventional well plate-based assays are also limited by the static nature of the cultures in that continual restoration of nutrients and removal of wastes through perfusion is not possible, requiring regular media changes that increase the risk of sample contamination and experimental errors.
Significant advances in microfluidic technology and techniques have allowed for the development of novel microfluidics-assisted in vitro drug screening methods that are capable of performing high-throughput dynamic assays that can begin to address the issues associated with conventional well plate methods commonly used today [35]. Even with these advances, however, 2D cell cultures often do not fully recapitulate the cell-cell and cell-matrix interactions that occur in organs and tissues. As a result, more attention has been shifted towards developing robust 3D culture methods that better recapitulate what naturally occurs in vivo [36]. Indeed, many of these methods have produced results in drug screening tests that are vastly different from those seen with 2D cultures, but match well with results that are seen clinically. Thus, a rapidly growing approach is to take advantage of recent advances in tissue engineering techniques to create 3D cultures in microfluidic devices for high-throughput assays that will be able to generate biologically relevant data. These 3D cultures have been able to form functional tissue and organ analogs on chips and they represent the future in drug screening, conceivably serving to one day fully replace current 2D culture assays. An overview of 2D and 3D cell-on-a-chip devices can be found in Table 2, and organ-on-a-chip devices can be found in Table 3.
Table 2. Cell-on-a-chip platforms for in vitro drug screening.
| Cultured Cell Type |
Drug | Screening Method | Device Feature(s) | Refs |
|---|---|---|---|---|
| PC3 (prostate cancer) |
TRAIL and doxorubicin/ mitoxantrone |
Live/dead fluorescent assay |
Programmable gradient generation |
[39] |
| BALB/3T3 fibroblasts, bovine endothelial, HeLa |
Digitonin, saponin, CoCl2, NiCl2, acrolein |
Live/dead fluorescent assay |
High-density combinatorial assaying |
[40] |
| HeLa (cervical cancer) |
Etoposide | Lactate dehydrogenase assay |
Passive perfusion pumps and 96-well plate integration |
[41] |
| LCC6/Her-2 (breast cancer) |
Doxorubicin | Live/dead fluorescent assay |
Alginate-supported 3D culture |
[49] |
| HCT-116, HepG2/C3A, Kasumi-1 |
Tegafur, 5-fluorouracil | Live/dead fluorescent assay |
Reproduced multiorgan interactions |
[50] |
| OC-2 (oral cancer) |
Epirubicin | Live/dead fluorescent assay |
Programmable perfusion with integrated pumps |
[51] |
| OEC-M1 (oral cancer) |
Cisplatin | Live/dead fluorescent & lactate dehydrogenase assays |
Integrated temperature control |
[52] |
| B16.F10 (melanoma) |
Sodium fluorescein (dye) | N/A | Gel-free 3D culture | [54] |
| HT-29 (colon cancer) |
5-fluorouracil | Live/dead fluorescent assay & spheroid diameter monitoring |
Long-term spheroid culture |
[57] |
Table 3. Organ-on-a-chip platforms for in vitro drug screening.
| Organ/Tissue | Cultured Cell Type(s) | Replicated Organ Features and Functions |
Refs |
|---|---|---|---|
| Liver | Hepatocytes, fibroblasts |
Albumin and urea secretion, canalicular transport |
[71-74] |
| Kidney | Inner medullary collecting duct (IMCD) cells |
Molecular transport in response to hormonal stimulation |
[75] |
| Lung | Alveolar basal epithelial, microvascular endothelial |
Tight cell junctions, surfactant production, alveolar-capillary microarchitecture |
[76-78] |
| Intestine | Gut epithelial | Villi formation, tight cell junctions, support of native flora |
[79] |
| Heart | Cardiomyocytes | Synchronized contraction | [80] |
| Central nervous system |
Neurons, oligodendrocytes |
Physically separated axons and cell bodies, anisotropic axon growth |
[81] |
3.1. Cell-on-a-chip for in vitro drug screening
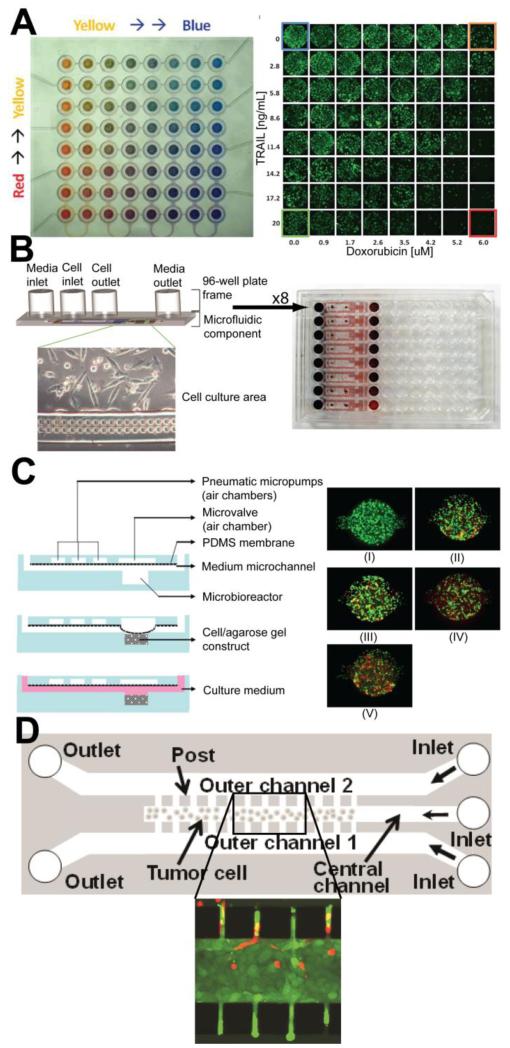
Microfluidic technologies have allowed researches to go beyond the limitations of conventional 2D cell monolayer assay systems by introducing the potential for conducting high-throughput and low-cost screening experiments under physiologically relevant conditions. By using a microfluidic gradient generator and a stable perfusion setup, microfluidic perfusion platforms enabling high-resolution live imaging and dose-dependent drug research were developed using glass etching and standard silicon microfabrication processes [37, 38]. These devices are able to determine cytotoxicity at multiple concentrations in parallel simultaneously, thus saving time and labor, and are also able to do so with a minimal amount of reagents. Furthermore, the ability to accurately and reproducibly create such gradients can serve to recreate physiologically-relevant chemical microenvironments seen in vivo and thus better approximate a drug’s effects in patients. Similarly, by integrating a gradient generator in a programmable and fully automatic microfluidic array, it is possible to determine cancer cell response due to various drug concentrations and mixtures in a pair-wise fashion [39] (Fig. 3A). By using ring-type microvalves, this platform also eliminated the need for constant reagent perfusion and thus minimized the significant shear effect on cultured cells caused by fluid flow, while maximizing the programmability of the assay and the capability for real-time monitoring. Micro-reactors containing PC3 prostate cancer cells were treated with various concentrations of TNF-α related apoptosis induced ligand (TRAIL) in combination with either doxorubicin or mitoxantrone in a sequential or simultaneous dosing method to determine the potential synergistic effects of each combination.
Figure 3.
Cell-on-a-chip devices for in vitro drug screening. (A) Programmable gradient generator for combinatorial drug dosing, with gradients illustrated by red, yellow and blue dye. Representative micrographs of PC3 cells after sequential treatment with TRAIL and doxorubicin [39]. (B) Gravity-driven perfusion pumps for continual and automated perfusion that are compatible with standard 96-well plate dispensing equipment [41]. (C) Multilayer 3D culture device with integrated PDMS membrane pneumatic micropumps for peristaltic perfusion. Representative fluorescent live/dead assays of cultured OC-2 cells exposed to increasing (I-V) concentrations of epirubicin [51]. (D) Gel-free 3D culture system of melanoma cells in which microposts increase cell culture density while enabling small-molecule diffusion to occur. Inset shows sodium fluorescein diffusion from outer chambers into densely-packed melanoma cells [54].
Microfluidic functional units such as microfluidic valves and mixers can also be integrated into microfluidic platforms for high-througput drug screening. For example, by coupling individual reaction chambers with a multi-layer microfluidic control platform, multiplexed cell screening was performed with various cell lines and a panel of toxins [40]. An array of 24 × 24 reactors containing cell capture sieve structures in each reactor was fabricated and controlled by external solenoid pneumatic valves. A series of cell types (BALB/3T3 murine embryonic fibroblast, HeLa and bovine endothelial cells) along with a panel of toxins (digitonin, saponin, CoCl2, NiCl2, acrolein) were loaded into the reactor of each column by opening respective rows of microvalves, and thus extremely high-density toxin-cell combinatorial screening in a multiplexed format was achieved within a single device.
These systems, however, still require expensive off-chip pumps that complicate maintenance of sterility during incubation, and the tubing setups required for the controlled long-term perfusion of reagents can sometimes be complex and unwieldy. An approach towards addressing these issues was accomplished by integrating simple microfluidic devices with commercially available 96-well plates. HeLa cells were cultured within the devices and the anti-cancer drug etoposite was flowed into the culture chambers with passive gravity driven perfusion “pumps” (Fig. 3B) [41]. Not only did this technique remove the need for the constant pumping of media and drug solutions throughout the experimental period, the incorporation of the microfluidic devices with a 96-well plate allows for this platform to be used in standard automated dispensing systems that are readily available, thus further enhancing the potential for high-throughput assaying. With further development, these devices could even be scaled up to be integrated with 384-well plate systems to enable even more samples to be tested at once.
Many drug screening assays that are performed to determine the dose-response curves of new drug candidate molecules or drug delivery vehicles are limited in the number of concentrations that can be tested at once due to time and cost considerations. With sometimes fewer than 10 data points, the presence of any data outliers can result in inaccurate or incorrect curve fits. By adapting the droplet generator-type microfluidic devices that are common in carrier formulation applications, dose response curves at significantly higher resolutions than what is normally possible can be attained [42]. Thousands of droplets, each a different concentration, can be easily and rapidly generated using such a device, leading to a chemical library that then is used to create high-precision curves. With flexibility of microfluidic device fabrication, such systems can be easily integrated with many cell-on-a-chip or organ-on-a-chip devices.
All of the previously mentioned cell-on-a-chip devices for in vitro drug screening were used with monolayer cultures of the target cells. However, cells cultured in monolayer conditions have been shown to lose their native phenotypic properties, and due to the multi-cellular resistance and transport phenomena of drugs, the results gained from monolayer cell cultures may not be physiologically relevant [43-45]. To alleviate these issues, microfluidic 3-dimensional (3D) cell culture platforms mimicking in vivo environments have been developed for high-throughput in vitro drug screening [46].
Often times a 3D culture environment is created by seeding cells within a hydrogel network [47-49]. In one such example, two parallel microfluidic channels were fabricated with PDMS and bonded on the linker molecules coated glass and in one of the fluidic channels, alginate hydrogel was immobilized on a linker molecule coated surface (Fig. 3C) [49]. Her-2 breast cancer cells were encapsulated within the alginate hydrogel and were cultured into a 3D tumor spheroid. Doxorubicin was then perfused through the other channel and it was found that compared to a 2D assay of doxorubicin’s effect on cancer cell viability, the drug displayed a reduced effectiveness in 3D culture. These preliminary results illustrate the need for robust 3D culture based in vitro assays to prevent potential discrepancies in drug efficacy when drug candidates are put through animal or clinical studies.
A novel 3D cell culture drug screening device was designed such that the effect of an anti-cancer drug on the metabolism and toxicity of multiple cell/organ types could be investigated simultaneously under medium recirculation conditions that attempted to recreate what is seen in vivo [50]. The device consisted of three different chambers that were specifically designed to mimic the physiological blood retention time of each respective organ type (liver and bone marrow) and of a tumor, and the chambers were interconnected with channels of varying shapes and sizes that mimicked the physiological patterns of blood flow to these tissues. To immobilize the different cancer cell lines, colon cancer cells (HCT-116) and hepatoma cells (HepG2/C3A) were encapsulated in Matrigel and myeloblasts (Kasumi-1) were encapsulated in alginate hydrogel. Tegafur, and oral pro-drug of 5-fluorouracil (5-FU), and 5-FU were tested along a combination of Tegafur and uracil. It was found that when the same assay was conducted in a standard 96-well microplate, there was no impact to cell viability as compared with control, while in the 3D culture microfluidic re-circulating system, significant toxicity was seen in colon cancer cells and an enhanced toxicity could be achieved with the Tegafur-uracil combination. Additionally, liver cells showed much less sensitivity to the drugs while the sensitivity of the myeloblasts was similar to that of the cancer cells. These results match those seen in animal and human clinical studies and illustrate the potential of such 3D culture devices to produce results far more relevant than those seen in conventional static 2D toxicity assays. Furthermore, by designing a chip that contained different organ-specific cell lines, the investigation of organ-specific agonistic and antagonistic effects from various drugs in a multi-organ environment is possible with this platform.
Microfluidic technologies have also enabled the development of devices that combine the advantages offered by 3D cell culture and by programmable reagent perfusion via integrated microvalves and micropumps in a high-throughput manner [51]. A multilayer PDMS device was designed that contained 15 separate microbioreactors, each with their own PDMS membrane pneumatic micropump that generated perfusion through peristaltic flow. Each bioreactor was loaded with oral cancer-2 (OC-2) cells in an agarose suspension, and culture medium with the anti-cancer drug epirubicin was perfused throughout the device with the on-board micropumps with which flow rates across each bioreactor could be uniformly maintained. Huang et. al. proposed a similar pneumatically-driven multiplexed system that serves to address several hurdles with cell-on-a-chip devices, namely temperature control and ease of bioassay analysis, by using a series of PMMA slabs that could be integrated with a transparent indium tin oxide heater chip [52]. Not only could the 3D cell cultures within the device be imaged for toxicity analysis, but the waste media could be collected in a dedicated slab that was designed to be read in standard plate readers. Like most other microfluidic devices, the sheer simplicity and novelty of the integrated perfusion pump system of these devices offers the potential for rapid and large-scale in vitro drug screening for existing and upcoming drugs in a physiologically meaningful manner, and without the need for costly external syringe or peristaltic pumps.
One potential disadvantage of 3D culture systems in which the target cells are encapsulated in hydrogels is that the encapsulation matrix could prevent cells from being cultured at a high enough density to prevent the formation of large interstitial spaces. This would then inadequately simulate small-molecule diffusion in densely packed tumors and therefore limit the model’s effectiveness at predicting clinical results. As a result, several attempts have been made to develop gel-free systems [53, 54]. In a proof-of-concept study of one such system, a device composed of three parallel microfluidic channels separated by narrow posts 50 μm wide and spaced 4μm apart was fabricated to examine small-molecule diffusion and uptake by densely-cultured cancer cells (Fig. 3D) [54]. Melanoma cells were cultured in the center channel, and the posts served as barriers to cell diffusion such that a high cell density could be established. After 12 hrs of culturing, representative membrane-impermeable and membrane-permeable fluorescent small-molecules were flowed through the two outer channels. Cell density in the central channel was found to be comparable to densities seen in tumors in vivo and target densities were achieved in a relatively short amount of time. However, fluorescent dye penetration differed significantly between dyes and further studies with various cancer drugs are necessary to confirm the overall ability of this gel-free system to act as a suitable cell-on-a-chip device for drug screening.
Another method of avoiding the use of hydrogels as a scaffold or matrix for 3D cultures is to control culture conditions such that cell spheroids will form. Tumor spheroids are considered one of the best in vitro models for cancer [55, 56], and methods of culturing large numbers of spheroids at a minimum of labor and cost are highly desired. Ziolkowska et. al. were able to achieve this by using a microfluidic device with incorporated microwells that induced seeded HT-29 human carcinoma cells to aggregate and spontaneously form spheroids [57]. The spheroids maintained high viability over 10-12 days, making these cultures suitable for long-term studies on the effects of drugs. Indeed, using the same device, the spheroids were exposed to repeated doses of 5-fluorouacil and the resulting impact on cell viability could be quantified by measuring spheroid size. This technique and device could conceivably be used to create similar 3D cultures for drug screening experiments with other cell types capable of forming spheroids, such as primary human hepatocytes [58, 59] and cardiomyocytes [60].
The advancement of 3D culture systems in microfluidic devices in recent years has led to the blurring of distinction between cell-on-a-chip and tissue- or organ-on-a-chip devices and it is quite likely that most microfluidics-based in vitro drug screening technologies will eventually move away from a single-cell or cell monolayer model.
3.2. Organ-on-a-chip for in vitro drug screening
While in vitro cell culture models are relatively cheaper and faster than in vivo models, they often do not properly simulate the complex cell-cell and cell-matrix interactions that are critical for regulating cell behavior. In order to effectively and efficiently test drug and chemical effects, more accurate models that mimic human organs and tissues are necessary. Recent developments in tissue engineering, stem cell research, biomaterials, microfluidics and microfabrication enable the development of various types of organ-on-a-chip devices that can conceivably provide more relevant in vitro models for drug screening applications. These organ-on-a-chip devices are created using a combination of microfabrication and tissue engineering techniques [34, 61]. Specifically, these devices closely mimic human organs and tissues by integrating microfluidic reactors and three-dimensional cell cultures to simulate complex cell-cell and cell-matrix interactions while accurately controlling fluid flow within the system [34, 62-65]. In some cases, three dimensional co-cultures of different cells in such devices serve to reproduce the cell-cell interactions that occur between disparate cell types in vivo. What truly distinguishes organs-on-chips from 3D cultures, however, is that these devices are designed to mimic the function of the modeled organ and move beyond static tissue cultures. Finally, as with microfluidic devices and assays in general, the size of organ-on-a-chip devices are small, and each component of the chip can potentially be integrated into a larger human-on-a-chip device to simulate multiple organs for high throughput screening applications in a whole-body model [66-68], and advances in the development of functional capillary networks in microfluidics will not only assist with this integration, but could also enhance the overall functionality of cultured tissues by providing a means for adequate nutrient and waste transportation [69]. Additionally, there have been microfluidic devices that accurately model the diffusion of small molecules between blood vessels and tissues, allowing researchers to paint a more complete picture of systemic drug pharmacokinetics [70]. There are many types of organ-on-a-chip devices that are currently being developed, including liver-on-chips [71-74], kidney-on-chips [75], lung-on-chips [76-78], intestine-on-chips [79], heart-on-chips [80], and central nervous system (CNS)-on-chips [81].
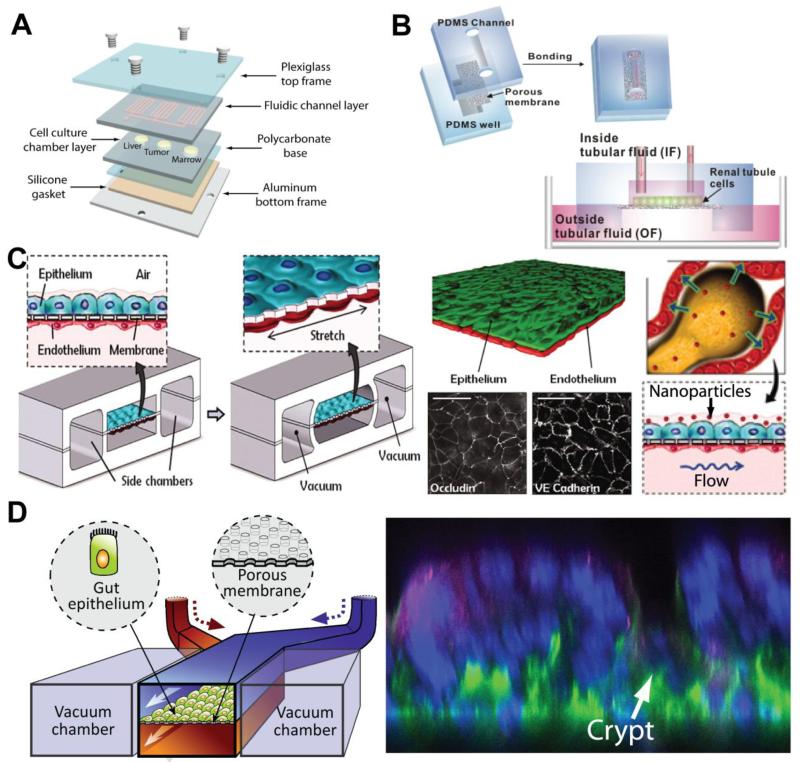
The hepatic clearance of small molecules by the liver is an important parameter that needs to be thoroughly investigated during drug development due to the high rate of failure of drugs both on market and in development due to hepatoxicity. In order to accurately simulate such parameters, many liver-on-chip devices have been created and tested. These microfluidic liver-on-chip devices help investigators estimate pharmacokinetic parameters such as hepatic clearance, cell permeability, bioavailability, and volume distribution without going through time-consuming, labor-intensive, and expensive in vivo animal studies. Such evaluation is demonstrated by Sung et. al., who designed and developed microscale cell culture analog using a novel design and hydrogel-cell culture technique (Fig. 4A) [71]. Using this device, it was demonstrated that it could analyze drug hepatoxicity in combination with a mathematical pharmacokinetic-pharmacodynamic model. A multi-well cell culture system for human liver cells with microfluidic architecture has also demonstrated its precise liver modeling through assessment of gene expression profiles, phase I/II metabolism, canalicular transport, secretion of liver-specific products, and susceptibility to hepatotoxins [72]. In an interesting and novel approach, a microfluidic device was designed with architecture that models the hemodynamic properties of the native human microvascular bed and computational models were used to ensure physiologically-relevant flow and shear forces were maintained in the chip [73]. With this vascular-inspired design, proper nutrient flow and protein transport from the cultured hepatocytes can occur while protecting the cells from excessive shear stresses that have been shown to be deleterious to viability. Compared to static 2D well-plate cultures, cells grown in this device produced significantly greater amounts of albumin and urea, two markers of liver metabolic function. Similar to cell-on-a-chip technology, organ-on-a-chip devices also offer the potential for multiplexed assays that are run in parallel. One example of this is the HepaTox Chip developed by Toh et. al. in which 3D cultures of hepatocytes are cultured in eight parallel channels [74]. The cells grown in 3D culture were found to have maintained the differentiated functions of hepatocytes at levels greater than those seen in multi-well plate controls and the IC50 values of the five model drugs tested correlated well with the LD50 values of the same drugs in vivo, illustrating the advantages 3D cultures offer over their 2D counterparts in recreating and predicting in vivo conditions.
Figure 4.
Organ-on-a-chips for in vitro drug screening. (A) Liver-on-a-chip for pharmacokinetic-pharmacodynamic modeling and analysis [71]. (B) Kidney-on-a-chip that simulates fluid flow within the renal tubules and is able to induce the growth of cells capable of molecular transport [75]. (C) Bioinspired lung-on-a-chip in which a thin, porous, flexible PDMS membrane coated with ECM helps mimic and form the alveolar-capillary barrier, and compartmentalized microchannels simulate lung mechanics during breathing. Long term co-culture of alveolar epithelium (green) and microvascular endothelium (red) in this system causes cells to form tight junctions similar to those seen in vivo. Nanoparticle transport across the alveolar-capillary barrier was modeled and analyzed under both static and breathing conditions [78]. (D) Gut-on-a-chip that forms villi-like structures in response to mechanical stimulation and supports the long-term growth of native flora. Confocal cross-section of tissue grown in device illustrating villi formed by undulating epithelial cells, with basal nuclei (blue) and apical mucin (magenta) separated by a crypt [79].
In addition to the liver, many drugs are also secreted into the kidney and passed out of the body as urine. As such, kidney-on-a-chip devices have been proposed to fully investigate the effects of drugs on this organ system, as drug-induced damage to the renal tubules can often lead to cancer or acute tubule necrosis [82]. Using a multilayered microfluidic device in which renal tubular cells are grown on a permeable membrane that sits over outer tubular fluid (an analog to blood) and are exposed to a continuous stream of inner tubular fluid (mimicking urine precursor fluid), Jang et. al. were able to recapitulate the growing conditions that renal tubular cells experience in vivo, leading to the formation of functional kidney tissue (Fig. 4B) [75]. By utilizing the device to show that hormone stimulation caused a water-transporting protein to move within the cells as it would in native tissue, it was demonstrated that the cells cultured in the device performed their inherent role of regulating water and ion balance via molecular transport and that the device could be used as a drug screening tool. This example also highlights the power of microfluidics for such applications as the ability to create stable and reproducible fluid flow allows for the ability to more accurately mimic the in vivo microenvironment, which is something that is not possible in conventional well plate cultures.
Another organ that has been modeled thoroughly on microscale platforms is the lung. Unlike most organs, lung epithelium is exposed to the air, and a variety of environmental effects such as pathogens and air pollution can cause a response of the lung epithelium, and as such, it is important to simulate the lungs using air-liquid or air-cell interfaces [34, 76]. To simulate such interfaces, a microfluidic platform with suspended membrane culture systems has been developed [76]. This particular lung-on-a-chip system can potentially be adapted for use as a tool to study the paracellular flux of metabolites and drug molecules across different tissue membranes of interest. When designing and fabricating such devices, parameters such as fluid mechanical resistance and oxygen transfer efficiencies are of important concern to simulate the human lungs accurately [77]. Huh et. al. tested their lung-on-a-chip device on toxic and inflammatory responses of the lung to silica nanoparticles (Fig. 4C). In this study, it was found that the mechanical strain of the membrane from simulated breathing enhances the epithelial and endothelial uptake of nanoparticles [78]. The mechanically active lung-on-a-chip device provides a more accurate, low-cost in vitro model for animal and clinical studies for drug screening and toxicology applications and serves as an interesting model for other devices that seek to mimic mechanically active tissues and organs.
A notoriously difficult organ system to simulate in an in vitro environment is the human gut. With many drugs designed to be administered orally, it is critical that the uptake of these drugs through the digestive system and the resulting effects is studied as part of a thorough drug screening regimen. Attempts have been made to recapitulate the architecture of the intestines by culturing intestinal epithelial cells in 3D hydrogel scaffolds that were engineered to mimic the size, shape, and density of the intestinal villi and by using porous membrane inserts in traditional cell culture well plates. However, these constructs have failed to mimic the mechanically active environment of the gut that is critical for organ function and physiology and furthermore, have not allowed for the growth of living flora for an extended period of time. To address these shortcomings, a human gut-on-a-chip was designed and fabricated for simulating intestinal peristalsis-like motions and flow [79]. Using a device design very similar to that used for the lung-on-a-chip designed by Huh et. al., a central microchannel containing a flexible and porous ECM-coated membrane lined by gut epithelial (Caco-2) cells and full height vacuum chambers on both sides of this channel (Fig. 4D). In this case, cyclic application of suction to the vacuum chambers mechanically deformed the Caco-2 monolayer to mimic the peristaltic motions of the intestines. The cultured cells were able to form 3D villi-like structures and quantification of transepithelial electrical resistance (TEER) revealed that 3- to 4-fold higher values were found when compared to static Transwell cultures, indicating that tighter junctions were formed and thereby better mimicking native intestinal tissue. In fact, TEER values continued to improve as culture time increased. Additionally, the dilution rate of the media induced by continual perfusion in the gut-on-a-chip supported long-term co-culture of both epithelial cells and microbes. Given that this device effectively reproduced physiological conditions of the human gut, it could serve as a powerful tool for both drug and toxicity screening and for the study of intestinal pathologies.
The significant healthcare issue of heart disease in many developed countries has led to a multitude of studies on pharmaceuticals for treatment. As such, much work has been devoted to replicating the polarization of cardiomyocytes in culture and effective methods of measuring their biomechanical behavior. While microfluidics have been able to measure the electrophysiological properties of isolated and constrained cardiomyocytes, the applicability of these devices for drug screening is questionable, as single-cell behavior and response to stimuli differs significantly from that of whole tissue. Furthermore, myocyte cultures on standard inflexible tissue culture surfaces make it difficult to ascertain changes in tissue functionality. Based on muscle thin film (MTF) technology that had been developed into a muscle-on-a-chip [83], engineered cardiac tissues assembled in a chip for physiological and pharmaceutical studies as a heart-on-a-chip was designed to efficiently study structure-function relationships of a human heart [80]. By taking advantage of microcontact printing, fibronectin could be patterned on top of the microfluidic substrate to help direct myocyte organization so that an electromechanically coupled anisotropic monolayer could be formed. Heart tissue contractility and architecture under a variety of conditions (healthy, diseased, and exposed to epinephrine) could then be studied and quantified, thus allowing researchers to potentially gain insight into how various drugs affect heart function.
Studies on spinal cord injury are often hampered by the lack of appropriate in vitro models and a simple and efficient method of assaying the effects of therapeutics on axonal regeneration post-injury. A microfluidics platform has been proposed in which micropatterned grooves guide the directional growth of neurites and the resulting isolation of axons from their cell bodies [81]. This allows for the ability to specifically target the injured axons with soluble proteins and drugs and study their effects on axonal regeneration. Additionally, the optical transparency of the material used here in fabricating the device allows for laser-induced axotomy to create precisely localized axonal defects for a significantly improved injury model for studying regeneration at the single axon scale. This platform then embodies a powerful tool in which a single device can generate a robust and accurate in vitro injury model that can easily be analyzed for not only drug screening but for regenerative medicine studies as well.
With more extensive verification of such devices through comparative drug screening and toxicology experiments, these organ-on-a-chip devices will further be advanced and will eventually be used as laboratory and clinical tools for drug screening and toxicological applications due to their unique advantages in scalability, low cost, and accuracy in modeling of human organs.
4. Conclusions and Outlook
Developments in microfluidics technologies and techniques have resulted in major contributions in drug and gene carrier production and toxicological screening. There is tremendous potential for customization due to the flexibility offered by the large range of geometries and materials used in creating microfluidic devices, and the ease with which such devices can be integrated with existing equipment. Most importantly, microtechnology has enabled both the parallelization and sequential integration of many conventional laboratory techniques, thereby significantly increasing the throughput of experimentation.
Microfluidic devices with diffusion mixers, chaotic mixers, and droplet generators enable for the precise control of the mixing rates and compositions of drug carriers. Furthermore, by incorporating programmable microfluidic control systems [8, 84, 85], steps can be automated to find optimal conditions for the fabrication of specific drug or gene carriers. While the widespread adoption of microfluidics-based drug carrier production has been slow despite advances in techniques, it can be expected that as the number of approved nanoparticle-based therapies increases over time, the demand for such high-throughput fabrication technologies will increase as well. However, to make these systems practical for pharmaceutical companies, the scaling factors and device reliability need to be taken into account. Specifically, physical phenomena changes in macroscale production and the ability of such devices to consistently generate drug/gene carriers after long running times should be carefully investigated. Additionally, like any other pharmaceutical-related manufacturing process, care must be taken to ensure that microfluidic production methods meet good manufacturing practices guidelines set forth by regulatory agencies.
The development of microfluidic cell-on-chips, tissue-on-chips, and organ-on-chips for drug screening applications provide high-throughput and more physiologically accurate drug screening tools that could eventually largely supplant animal models in use today which are often expensive, ethically problematic, and do not always produce results that translate well clinically. In taking these technologies to the next level of biological mimicry, more complex organ-on-a-chip devices that integrate multiple simulated organs joined by microfluidic channel networks have been proposed to serve as a human-on-a-chip [66-68], and this approach has generated tremendous interest in recent years, as shown by the infusion of significant funds into projects that seek to develop these multi-organ-simulating devices [86]. The greatest limiting factor at this point in regards to this technology, as with other in vitro culture models, has been the ability to properly order cells in their native hierarchy in a thick living structure such that they form the proper tissues. Continued advances in tissue engineering and regenerative medicine through the use of microfluidic bioreactors and nanopatterning that can easily be incorporated into devices may help alleviate this [87-89]. Additionally, developments in the ability to reliably study and control the differentiation of stem cells using microfluidics will allow for the creation of even more accurate tissue and organ analogs, as well as increase the potential for the development of personalized screening in which patient-derived stem cells are used [90]. Devices could also be integrated with nanoelectronics to obtain accurate real-time readings of cellular and tissue responses to drugs, a capability that is beyond what is currently possible with conventional screening techniques [91].
Recently, there has been significant progress made towards bringing microfluidics-based cell cultures for high-throughput drug screening to the market, with several devices and technologies currently available for purchase. An example of this is the 96-well format passive perfusion cell culture system highlighted previously in this review [41, 92]. Further development of this technology now allows for the generation of perfused 3D cell culture arrays [93] and for the culturing of primary hepatocytes under biomimetic conditions [94]. There are also tubeless microfluidic systems capable of 3D cell co-cultures that are designed to be compatible with automated high content analysis equipment and standard microscopes for assaying cell viability and chemotaxis in response to administered drug candidates [95, 96]. Soon to be available will be a microfluidics-based platform that features an innovative valve and channel design that improves uniformity in reagent delivery and allows for periodic dosing of one or more drugs. Increased spatial and temporal resolution of assays can be achieved with this system, and it has been shown to be an effective tool for high-throughput studies on cell signaling dynamics and epigenetics, even at the single-cell level [97, 98]. To date, there have been no major regulatory issues raised in regards to the use of microfluidic cell- and organ-on-chips for drug screening and in fact, the United States Food and Drug Administration (FDA) is actively involved in fostering the development of this technology.
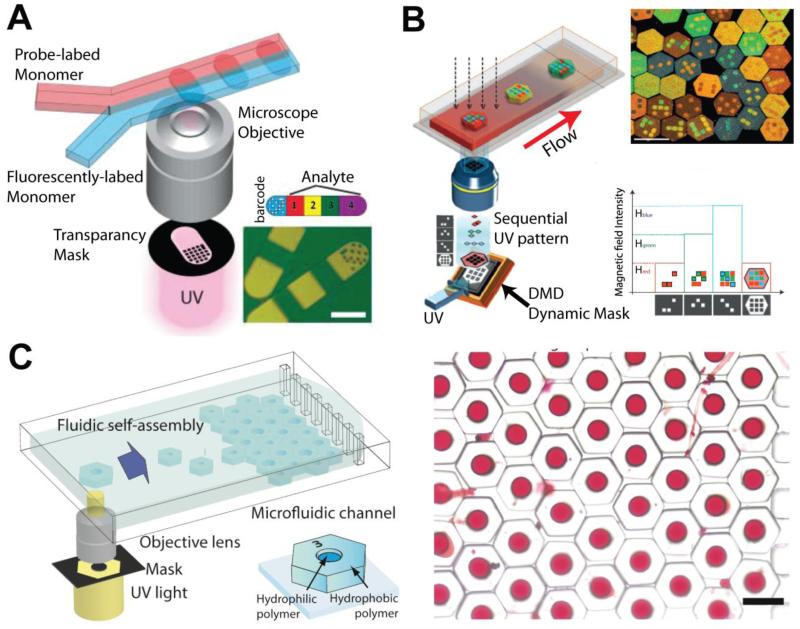
Continued advancements for microfluidic drug screening platforms could include integration with sortable microfluidic drug or gene carrier production devices that utilize photopolymerized polymers with unique barcodes and shapes [99-101] (Fig. 5). With these techniques, a library consisting of a large quantity of carriers with different drug content and concentrations could be generated and quickly sorted and diverted to their respective linked cell-on-a-chip or tissue-on-a-chip screening modules. This would then allow for production and testing to occur in a single device. Coupled with automation and a flexible system in which modules could easily be swapped in an out, this can significantly increase the efficiency and speed of in vitro drug evaluation.
Figure 5.
Microfluidic-assisted in situ micropatterning techniques for creating various types of sortable microparticles and microcarriers. (A) Dot-patterned barcoded biomolecules generated by microfluidic in situ photo-polymerization technique [90]. (B) Color-barcoded microparticles created by electromagnetic system, dynamic mask, and in situ polymerization [91]. (C) Amphiphilic microcarriers fabricated by in situ UV polymerization filled with red dye as a model drug. Droplet wells can be formed in a variety of shapes and sizes, which allows for sorting and dose control [92].
The highlighted advances in microfluidics have been widely touted as prime examples of next generation in drug discovery technology. However, much work remains to be done to address the many hurdles that stand in the way of taking these technologies to the next step, especially in regards to improving ease of fabrication and use, and increasing assay analysis efficiency. With continued research efforts to make microfluidic platforms for drug manufacture and screening more practical, such devices could have a significant impact on both basic research and the pharmaceutical industry by providing precise, high-throughput and effective tools.
Acknowledgements
D. H. Kim thanks the Department of Bioengineering at the University of Washington for the new faculty startup fund. D. H. Kim is also supported by the Wallace H. Coulter Foundation Translational Research Partnership Award, a Muscular Dystrophy Association (MDA) Research Grant (MDA 255907), and an American Heart Association (AHA) Scientist Development Grant (AHA 13SDG14560076). J. Kim thanks the Department of Mechanical Engineering and Office of Vice President for Research at Texas Tech University for the new faculty start-up fund. S. H. Pun is supported by a National Institutes of Health (NIH) grant (NIH 1R01NS064404).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sun Q, Radosz M, Shen Y. Challenges in design of translational nanocarriers. J. Control. Release. 2012;164:156–169. doi: 10.1016/j.jconrel.2012.05.042. [DOI] [PubMed] [Google Scholar]
- [2].Fahmy TM, Fong PM, Park J, Constable T, Saltzman WM. Nanosystems for simultaneous imaging and drug delivery to T cells. AAPS J. 2007;9:E171–180. doi: 10.1208/aapsj0902019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim J, Hwang I, Britain D, Chung TD, Sun Y, Kim DH. Microfluidic approaches for gene delivery and gene therapy. Lab Chip. 2011;11:3941–3948. doi: 10.1039/c1lc20766k. [DOI] [PubMed] [Google Scholar]
- [4].Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- [5].Edel JB, Fortt R, DeMello JC, DeMello AJ. Microfluidic routes to the controlled production of nanoparticles. Chem. Commun. 2002:1136–1137. doi: 10.1039/b202998g. [DOI] [PubMed] [Google Scholar]
- [6].Takakura Y, Hashida M. Macromolecular carrier systems for targeted drug delivery: pharmacokinetic considerations on biodistribution. Pharm. Res. 1996;13:820–831. doi: 10.1023/a:1016084508097. [DOI] [PubMed] [Google Scholar]
- [7].Wagner A, Vorauer-Uhl K. Liposome technology for industrial purposes. Journal of Drug Delivery. 2011;2011:591325. doi: 10.1155/2011/591325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim J, Kang M, Jensen EC, Mathies RA. Lifting gate PDMS microvalves and pumps for microfluidic control. Anal. Chem. 2012;84:2067–2071. doi: 10.1021/ac202934x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ma B, Zhang S, Jiang H, Zhao B, Lv H. Lipoplex morphologies and their influences on transfection efficiency in gene delivery. J. Control. Release. 2007;123:184–194. doi: 10.1016/j.jconrel.2007.08.022. [DOI] [PubMed] [Google Scholar]
- [10].Lee JN, Park C, Whitesides GM. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal. Chem. 2003;75:6544–6554. doi: 10.1021/ac0346712. [DOI] [PubMed] [Google Scholar]
- [11].Nunes PS, Ohlsson PD, Ordeig O, Kutter JP. Cyclic olefin polymers: emerging materials for lab-on-a-chip applications. Microfluid. Nanofluid. 2010;9:145–161. [Google Scholar]
- [12].Ren KN, Dai W, Zhou JH, Su J, Wu HK. Whole-Teflon microfluidic chips. Proc. Natl. Acad. Sci. USA. 2011;108:8162–8166. doi: 10.1073/pnas.1100356108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hashimoto M, Langer R, D S. Kohane, Benchtop fabrication of microfluidic systems based on curable polymers with improved solvent compatibility. Lab Chip. 2013;13:252–259. doi: 10.1039/c2lc40888k. [DOI] [PubMed] [Google Scholar]
- [14].Koh CG, Kang X, Xie Y, Fei Z, Guan J, Yu B, Zhang X, Lee LJ. Delivery of polyethylenimine/DNA complexes assembled in a microfluidics device. Mol. Pharm. 2009;6:1333–1342. doi: 10.1021/mp900016q. [DOI] [PubMed] [Google Scholar]
- [15].Debus H, Beck-Broichsitter M, Kissel T. Optimized preparation of pDNA/poly(ethylene imine) polyplexes using a microfluidic system. Lab Chip. 2012;12:2498–2506. doi: 10.1039/c2lc40176b. [DOI] [PubMed] [Google Scholar]
- [16].Kuramoto H, Park YS, Kaji N, Tokeshi M, Kogure K, Shinohara Y, Harashima H, Baba Y. On-chip fabrication of mutifunctional envelope-type nanodevices for gene delivery. Anal. Bioanal. Chem. 2008;391:2729–2733. doi: 10.1007/s00216-008-2124-7. [DOI] [PubMed] [Google Scholar]
- [17].Koh CG, Zhang X, Liu S, Golan S, Yu B, Yang X, Guan J, Jin Y, Talmon Y, Muthusamy N, Chan KK, Byrd JC, Lee RJ, Marcucci G, Lee LJ. Delivery of antisense oligodeoxyribonucleotide lipopolyplex nanoparticles assembled by microfluidic hydrodynamic focusing. J. Control. Release. 2010;141:62–69. doi: 10.1016/j.jconrel.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Endres T, Zheng M, Beck-Broichsitter M, Samsonova O, Debus H, Kissel T. Optimising the self-assembly of siRNA loaded PEG-PCL-lPEI nano-carriers employing different preparation techniques. J. Control. Release. 2012;160:583–591. doi: 10.1016/j.jconrel.2012.04.013. [DOI] [PubMed] [Google Scholar]
- [19].Chen D, Love KT, Chen Y, Eltoukhy AA, Kastrup C, Sahay G, Jeon A, Dong Y, Whitehead KA, Anderson DG. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled microfluidic formulation. J. Am. Chem. Soc. 2012;134:6948–6951. doi: 10.1021/ja301621z. [DOI] [PubMed] [Google Scholar]
- [20].Wang J-T, Wang J, Han J-J. Fabrication of advanced particles and particle-based materials assisted by droplet-based microfluidics. Small. 2011;7:1728–1754. doi: 10.1002/smll.201001913. [DOI] [PubMed] [Google Scholar]
- [21].Ho Y-P, Grigsby CL, Zhao F, Leong KW. Tuning Physical properties of nanocomplexes through microfluidics-assisted confinement. Nano Lett. 2011;11:2178–2182. doi: 10.1021/nl200862n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hsieh AT, Hori N, Massoudi R, Pan PJ, Sasaki H, Lin YA, Lee AP. Nonviral gene vector formation in monodispersed picolitre incubator for consistent gene delivery. Lab Chip. 2009;9:2638–2643. doi: 10.1039/b823191e. [DOI] [PubMed] [Google Scholar]
- [23].Teh SY, Lin R, Hung LH, Lee AP. Droplet microfluidics. Lab Chip. 2008;8:198–220. doi: 10.1039/b715524g. [DOI] [PubMed] [Google Scholar]
- [24].Wang H, Liu K, Chen KJ, Lu Y, Wang S, Lin WY, Guo F, Kamei K, Chen YC, Ohashi M, Wang M, Garcia MA, Zhao XZ, Shen CK, Tseng HR. A rapid pathway toward a superb gene delivery system: programming structural and functional diversity into a supramolecular nanoparticle library. ACS Nano. 2010;4:6235–6243. doi: 10.1021/nn101908e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Karnik R, Gu F, Basto P, Cannizzaro C, Dean L, Kyei-Manu W, Langer R, Farokhzad OC. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett. 2008;8:2906–2912. doi: 10.1021/nl801736q. [DOI] [PubMed] [Google Scholar]
- [26].Tran TH, Nguyen CT, Kim D-P, Lee Y.-k., Huh KM. Microfluidic approach for highly efficient synthesis of heparin-based bioconjugates for drug delivery. Lab Chip. 2012;12:589–594. doi: 10.1039/c1lc20769e. [DOI] [PubMed] [Google Scholar]
- [27].Teh S-Y, Khnouf R, Fan H, Lee AP. Stable, biocompatible lipid vesicle generation by solvent extraction-based droplet microfluidics. Biomicrofluidics. 2011;5:044113. doi: 10.1063/1.3665221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fisher JS, Lee AP. Cell encapsulation on a microfluidic platform. Proc. MicroTAS. 2004 [Google Scholar]
- [29].Hettiarachchi K, Zhang S, Feingold S, Lee AP, Dayton PA. Controllable microfluidic synthesis of multiphase drug-carrying lipospheres for site-targeted therapy. Biotechnol. Prog. 2009;25:938–945. doi: 10.1002/btpr.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rondeau E, Cooper-White JJ. Biopolymer microparticle and nanoparticle formation within a microfluidic device. Langmuir. 2008;24:6937–6945. doi: 10.1021/la703339u. [DOI] [PubMed] [Google Scholar]
- [31].Kesselman LRB, Shinwary S, Selvaganapathy PR, Hoare T. Synthesis of monodisperse, covalently cross-linked, degradable “smart” microgels using microfluidics. Small. 2012;8:1092–1098. doi: 10.1002/smll.201102113. [DOI] [PubMed] [Google Scholar]
- [32].Anton N, Bally F, Serra CA, Ali A, Arntz Y, Mely Y, Zhao M, Marchioni E, Jakhmola A, Vandamme TF. A new microfluidic setup for precise control of the polymer nanoprecipitation process and lipophilic drug encapsulation. Soft Matter. 2012;8:10628–10635. [Google Scholar]
- [33].Zhigaltsev IV, Belliveau N, Hafez I, Leung AKK, Huft J, Hansen C, Cullis PR. Bottom-up design and synthesis of limit size lipid nanoparticle systems with aqueous and triglyceride cores using millisecond microfluidic mixing. Langmuir. 2012;28:3633–3640. doi: 10.1021/la204833h. [DOI] [PubMed] [Google Scholar]
- [34].Ghaemmaghami AM, Hancock MJ, Harrington H, Kaji H, Khademhosseini A. Biomimetic tissues on a chip for drug discovery. Drug Discov. Today. 2012;17:173–181. doi: 10.1016/j.drudis.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wu M-H, Huang S-B, Lee G-B. Microfluidic cell culture systems for drug research. Lab Chip. 2010;10:939–956. doi: 10.1039/b921695b. [DOI] [PubMed] [Google Scholar]
- [36].Breslin S, O’Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug. Discov. Today. 2012 doi: 10.1016/j.drudis.2012.10.003. http://dx.doi.org/10.1016/j.drudis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- [37].Liu C, Wang L, Xu Z, Li J, Ding X, Wang Q, Chunyu L. A multilayer microdevice for cell-based high-throughput drug screening. J. Micromech. Microeng. 2012;22:065008. [Google Scholar]
- [38].Park J, Kim DH, Kim G, Kim Y, Choi E, Levchenko A. Simple haptotactic gradient generation within a triangular microfluidic channel. Lab Chip. 2010;10:2130–2138. doi: 10.1039/b924222h. [DOI] [PubMed] [Google Scholar]
- [39].Kim J, Taylor D, Agrawal N, Wang H, Kim H, Han A, Rege K, Jayaraman A. A programmable microfluidic cell array for combinatorial drug screening. Lab Chip. 2012;12:1813–1822. doi: 10.1039/c2lc21202a. [DOI] [PubMed] [Google Scholar]
- [40].Wang Z, Kim M-C, Marquez M, Thorsen T. High-density microfluidic arrays for cell cytotoxicity analysis. Lab Chip. 2007;7:740–745. doi: 10.1039/b618734j. [DOI] [PubMed] [Google Scholar]
- [41].Lee P, Ghorashian N, Gaige T, Hung P. Microfluidic system for automated cell-based assays. J. Lab. Autom. 2007;12:363–367. doi: 10.1016/j.jala.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Oliver AEH, Miller J, Thomas Mangeat, Jean-Christophe Baret, Lucas Frenz, Bachir El Debs, Estelle Mayot, Samuels Michael L., Rooney Eamonn K., Pierre Dieu, Martin Galvan, Link Darren R., Griffiths Andrew D. High-resolution dose–response screening using droplet-based microfluidics. Proc. Natl. Acad. Sci. USA. 2012;109:378–383. doi: 10.1073/pnas.1113324109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vinci M, Gowan S, Boxall F, Patterson L, Zimmermann M, Court W, Lomas C, Mendiola M, Hardisson D, Eccles SA. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012;10:29. doi: 10.1186/1741-7007-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pampaloni F, Reynaud EG, Stelzer EHK. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Bio. 2007;8:839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- [45].Lin R-Z, Chang H-Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008;3:1172–1184. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- [46].Rimann M, Graf-Hausner U. Synthetic 3D multicellular systems for drug development. Curr. Opin. Biotechnol. 2012;23:803–809. doi: 10.1016/j.copbio.2012.01.011. [DOI] [PubMed] [Google Scholar]
- [47].Shin Y, Han S, Jeon JS, Yamamoto K, Zervantonakis IK, Sudo R, Kamm RD, Chung S. Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Nat. Protoc. 2012;7:1247–1259. doi: 10.1038/nprot.2012.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kim MS, Yeon JH, Park JK. A microfluidic platform for 3-dimensional cell culture and cell-based assays. Biomed. Microdev. 2007;9:25–34. doi: 10.1007/s10544-006-9016-4. [DOI] [PubMed] [Google Scholar]
- [49].Chen MCW, Gupta M, Cheung KC. Alginate-based microfluidic system for tumor spheroid formation and anticancer agent screening. Biomed. Microdev. 2010;12:647–654. doi: 10.1007/s10544-010-9417-2. [DOI] [PubMed] [Google Scholar]
- [50].Sung JH, Shuler ML. A micro cell culture analog (μCCA) with 3-D hydrogel culture of multiple cell lines to assess metabolism-dependent cytotoxicity of anti-cancer drugs. Lab Chip. 2009;9:1385–1394. doi: 10.1039/b901377f. [DOI] [PubMed] [Google Scholar]
- [51].Wu M-H, Huang S-B, Cui Z, Cui Z, Lee G-B. Development of perfusion-based micro 3-D cell culture platform and its application for high throughput drug testing. Sensor. Actuat. B-Chem. 2008;129:231–240. [Google Scholar]
- [52].Huang SB, Wang SS, Hsieh CH, Lin YC, Lai CS, Wu MH. An integrated microfluidic cell culture system for high-throughput perfusion three-dimensional cell culture-based assays: effect of cell culture model on the results of chemosensitivity assays. Lab Chip. 2013;13:1133–1143. doi: 10.1039/c2lc41264k. [DOI] [PubMed] [Google Scholar]
- [53].Ong SM, Zhang C, Toh YC, Kim SH, Foo HL, Tan CH, van Noort D, Park S, Yu H. A gel-free 3D microfluidic cell culture system. Biomaterials. 2008;29:3237–3244. doi: 10.1016/j.biomaterials.2008.04.022. [DOI] [PubMed] [Google Scholar]
- [54].Elliott NT, Yuan F. A microfluidic system for investigation of extravascular transport and cellular uptake of drugs in tumors. Biotechnol. Bioeng. 2012;109:1326–1335. doi: 10.1002/bit.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. Multicellular tumor spheroids: an underestimated tool is catching up again. J. Biotechnol. 2010;148:3–15. doi: 10.1016/j.jbiotec.2010.01.012. [DOI] [PubMed] [Google Scholar]
- [56].Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008;3:1172–1184. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- [57].Ziolkowska K, Stelmachowska A, Kwapiszewski R, Chudy M, Dybko A, Brzozka Z. Long-term three-dimensional cell culture and anticancer drug activity evaluation in a microfluidic chip. Biosens. Bioelectron. 2013;40:68–74. doi: 10.1016/j.bios.2012.06.017. [DOI] [PubMed] [Google Scholar]
- [58].Meng Q. Three-dimensional culture of hepatocytes for prediction of drug-induced hepatotoxicity. Expert Opin. Drug Met. 2010;6:733–746. doi: 10.1517/17425251003674356. [DOI] [PubMed] [Google Scholar]
- [59].Tostoes RM, Leite SB, Serra M, Jensen J, Bjorquist P, Carrondo MJ, Brito C, Alves PM. Human liver cell spheroids in extended perfusion bioreactor culture for repeated-dose drug testing. Hepatology. 2012;55:1227–1236. doi: 10.1002/hep.24760. [DOI] [PubMed] [Google Scholar]
- [60].Bartholoma P, Gorjup E, Monz D, Reininger-Mack A, Thielecke H, Robitzki A. Three-dimensional in vitro reaggregates of embryonic cardiomyocytes: a potential model system for monitoring effects of bioactive agents. J. Biomol. Screen. 2005;10:814–822. doi: 10.1177/1087057105280070. [DOI] [PubMed] [Google Scholar]
- [61].Moraes C, Mehta G, Lesher-Perez SC, Takayama S. Organs-on-a-chip: a focus on compartmentalized microdevices. Ann. Biomed. Eng. 2012;40:1211–1227. doi: 10.1007/s10439-011-0455-6. [DOI] [PubMed] [Google Scholar]
- [62].Baker M. Tissue models: A living system on a chip. Nature. 2011;471:661–665. doi: 10.1038/471661a. [DOI] [PubMed] [Google Scholar]
- [63].Huh D, Torisawa Y.-s., Hamilton GA, Kim HJ, Ingber DE. Microengineered physiological biomimicry: Organs-on-Chips. Lab Chip. 2012;12:2156–2164. doi: 10.1039/c2lc40089h. [DOI] [PubMed] [Google Scholar]
- [64].Elliott NT, Yuan F. A review of three-dimensional in vitro tissue models for drug discovery and transport studies. J. Pharm. Sci. 2011;100:59–74. doi: 10.1002/jps.22257. [DOI] [PubMed] [Google Scholar]
- [65].Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011;21:745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Esch MB, King TL, Shuler ML. The role of body-on-a-chip devices in drug and toxicity studies. Annu. Rev. Biomed. Eng. 2011;13:55–72. doi: 10.1146/annurev-bioeng-071910-124629. [DOI] [PubMed] [Google Scholar]
- [67].Zhang C, Zhao Z, Rahim NAA, Noort D.v., Yu H. Towards a human-on-chip: Culturing multiple cell types on a chip with compartmentalized microenvironments. Lab Chip. 2009;9:3185–3192. doi: 10.1039/b915147h. [DOI] [PubMed] [Google Scholar]
- [68].Sonntag F, Schilling N, Mader K, Gruchow M, Klotzbach U, Lindner G, Horland R, Wagner I, Lauster R, Howitz S, Hoffmann S, Marx U. Design and prototyping of a chip-based multi-micro-organoid culture system for substance testing, predictive to human (substance) exposure. J. Biotechnol. 2010;148:70–75. doi: 10.1016/j.jbiotec.2010.02.001. [DOI] [PubMed] [Google Scholar]
- [69].Moya ML, Hsu YH, Lee A, Hughes CC, George S. In vitro perfused human capillary networks. Tissue Eng Part C Methods. 2013 doi: 10.1089/ten.tec.2012.0430. doi: 10.1089/ten.tec.2012.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wu J, Chen Q, Liu W, Zhang Y, Lin JM. Cytotoxicity of quantum dots assay on a microfluidic 3D-culture device based on modeling diffusion process between blood vessels and tissues. Lab Chip. 2012;12:3474–3480. doi: 10.1039/c2lc40502d. [DOI] [PubMed] [Google Scholar]
- [71].Sung JH, Kam C, Shuler ML. A microfluidic device for a pharmacokinetic-pharmacodynamic (PK-PD) model on a chip. Lab Chip. 2010;10:446–455. doi: 10.1039/b917763a. [DOI] [PubMed] [Google Scholar]
- [72].Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008;26:120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- [73].Carraro A, Hsu WM, Kulig KM, Cheung WS, Miller ML, Weinberg EJ, Swart EF, Kaazempur-Mofrad M, Borenstein JT, Vacanti JP, Neville C. In vitro analysis of a hepatic device with intrinsic microvascular-based channels. Biomed. Microdev. 2008;10:795–805. doi: 10.1007/s10544-008-9194-3. [DOI] [PubMed] [Google Scholar]
- [74].Toh YC, Lim TC, Tai D, Xiao G, van Noort D, Yu H. A microfluidic 3D hepatocyte chip for drug toxicity testing. Lab Chip. 2009;9:2026–2035. doi: 10.1039/b900912d. [DOI] [PubMed] [Google Scholar]
- [75].Jang KJ, Suh KY. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip. 2010;10:36–42. doi: 10.1039/b907515a. [DOI] [PubMed] [Google Scholar]
- [76].Nalayanda DD, Puleo C, Fulton WB, Sharpe LM, Wang T-H, Abdullah F. An open-access microfluidic model for lung-specific functional studies at an air-liquid interface. Biomed. Microdev. 2009;11:1081–0189. doi: 10.1007/s10544-009-9325-5. [DOI] [PubMed] [Google Scholar]
- [77].Kniazeva T, Epshteyn AA, Hsiao JC, Kim ES, Kolachalama VB, Charest JL, Borenstein JT. Performance and scaling effects in a multilayer microfluidic extracorporeal lung oxygenation device. Lab Chip. 2012;12:1686–1695. doi: 10.1039/c2lc21156d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting Organ-level Lung Functions on a Chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kim HJ, Huh D, Hamilton G, Ingber DE. Human gut-on-a-chip inhabited by microbial flora that expriences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12:2165–2174. doi: 10.1039/c2lc40074j. [DOI] [PubMed] [Google Scholar]
- [80].Grosberg A, Alford PW, McCain ML, Parker KK. Ensembles of engineered cardiac tissue for physiological and pharmacological study: Heart on a chip. Lab Chip. 2011;11:4165–4173. doi: 10.1039/c1lc20557a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kim HJ, Park JW, Byun JH, Vahidi B, Rhee SW, Jeon NL. Integrated microfluidics platforms for investigating injury and regeneration of CNS axons. Ann. Biomed. Eng. 2012;40:1268–1276. doi: 10.1007/s10439-012-0515-6. [DOI] [PubMed] [Google Scholar]
- [82].Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin. Nephrol. 2003;23:460–464. doi: 10.1016/s0270-9295(03)00089-5. [DOI] [PubMed] [Google Scholar]
- [83].Feinberg AW, Feigel A, Shevkoplyas SS, Sheehy S, Whitesides GM, Parker KK. Muscular thin films for building actuators and powering devices. Science. 2007;317:1366–1370. doi: 10.1126/science.1146885. [DOI] [PubMed] [Google Scholar]
- [84].Jensen EC, Zeng Y, Kim J, Mathies RA. Microvalve enabled digital microfluidic systems for high performance biochemical and genetic analysis. J. Lab. Autom. 2010;5:455–463. doi: 10.1016/j.jala.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kim J, Jensen EC, Stockton AM, Mathies RA. Universal microfluidic automaton for autonomous sample processing: application to the Mars organic analyzer. Anal. Chem. 2013 doi: 10.1021/ac303767m. doi: 10.1021/ac303767m. [DOI] [PubMed] [Google Scholar]
- [86].Ingber DE. The Wyss Institute at Harvard University. IEEE Pulse. 2011;2:43–46. doi: 10.1109/MPUL.2011.941455. [DOI] [PubMed] [Google Scholar]
- [87].Andersson H, van den Berg A. Microfabrication and microfluidics for tissue engineering: state of the art and future opportunities. Lab Chip. 2004;4:98–103. doi: 10.1039/b314469k. [DOI] [PubMed] [Google Scholar]
- [88].Kim HN, Jiao A, Hwang NS, Kim MS, Kang DH, Kim DH, Suh KY. Nanotopography-guided tissue engineering and regenerative medicine. Adv. Drug Deliv. Rev. 2012 doi: 10.1016/j.addr.2012.07.014. http://dx.doi.org/10.1016/j.addr.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kim DH, Kshitiz RR, Smith P, Kim EH, Ahn HN, Kim E, Marban KY, Suh A. Levchenko, Nanopatterned cardiac cell patches promote stem cell niche formation and myocardial regeneration. Integr. Biol. 2012;4:1019–1033. doi: 10.1039/c2ib20067h. [DOI] [PubMed] [Google Scholar]
- [90].Gupta K, Kim DH, Ellison D, Smith C, Kundu A, Tuan J, Suh KY, Levchenko A. Lab-on-a-chip devices as an emerging platform for stem cell biology. Lab Chip. 2010;10:2019–2031. doi: 10.1039/c004689b. [DOI] [PubMed] [Google Scholar]
- [91].Tian B, Liu J, Dvir T, Jin L, Tsui JH, Qing Q, Suo Z, Langer R, Kohane DS, Lieber CM. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat. Mater. 2012;11:986–994. doi: 10.1038/nmat3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Hung PJ, Lee PJ, Sabounchi P, Lin R, Lee LP. Continuous perfusion microfluidic cell culture array for high-throughput cell-based assays. Biotechnol. Bioeng. 2005;89:1–8. doi: 10.1002/bit.20289. [DOI] [PubMed] [Google Scholar]
- [93].Chen SYC, Hung PJ, Lee PJ. Microfluidic array for three-dimensional perfusion culture of human mammary epithelial cells. Biomed. Microdev. 2011;13:753–758. doi: 10.1007/s10544-011-9545-3. [DOI] [PubMed] [Google Scholar]
- [94].Zhang MY, Lee PJ, Hung PJ, Johnson T, Lee LP, Mofrad MRK. Microfluidic environment for high density hepatocyte culture. Biomed. Microdev. 2008;10:117–121. doi: 10.1007/s10544-007-9116-9. [DOI] [PubMed] [Google Scholar]
- [95].Montanez-Sauri SI, Sung KE, Berthier E, Beebe DJ. Enabling screening in 3D microenvironments: probing matrix and stromal effects on the morphology and proliferation of T47D breast carcinoma cells. Integr. Biol. 2013;5:631–640. doi: 10.1039/c3ib20225a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Bauer M, Su G, Beebe DJ, Friedl A. 3D microchannel co-culture: method and biological validation. Integr. Biol. 2010;2:371–378. doi: 10.1039/c0ib00001a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Cheong R, Wang CJ, Levchenko A. High content cell screening in a microfluidic device. Mol. Cell. Proteomics. 2009;8:433–442. doi: 10.1074/mcp.M800291-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Cheong R, Wang CJ, Levchenko A. Using a microfluidic device for high-content analysis of cell signaling. Sci. Signal. 2009;2:pl2. doi: 10.1126/scisignal.275pl2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Pregibon DC, Toner M, Doyle PS. Multifunctional encoded particles for high-throughput biomolecule analysis. Science. 2007;315:1393–1396. doi: 10.1126/science.1134929. [DOI] [PubMed] [Google Scholar]
- [100].Lee H, Kim J, Kim H, Kim J, Kwon S. Colour-barcoded magnetic microparticles for multiplexed bioassays. Nat. Mater. 2010;9:745–749. doi: 10.1038/nmat2815. [DOI] [PubMed] [Google Scholar]
- [101].Park W, Han S, Lee H, Kwon S. Free-floating amphiphilic picoliter droplet carriers for multiplexed liquid loading in a microfluidic channel. Microfluid. Nanofluid. 2012;13:511–518. [Google Scholar]