Abstract
Purpose of review
The purpose of this review is to highlight the importance of telomeres, the mechanisms implicated in their maintenance, and their role in the etiology as well as the treatment of human esophageal cancer. We will also discuss the role of telomeres in the maintenance/preservation of genomic integrity, the consequences of telomere dysfunction, and the various factors that may affect telomere health in esophageal tissue predisposing it to oncogenesis.
Recent findings
There has been growing evidence that telomeres, which can be affected by various intrinsic and extrinsic factors, contribute to genomic instability, oncogenesis, as well as proliferation of cancer cells.
Summary
Telomeres are the protective DNA-protein complexes at chromosome ends. Telomeric DNA undergoes progressive shortening with age leading to cellular senescence and/or apoptosis. If senescence/apoptosis is prevented as a consequence of specific genomic changes, continued proliferation leads to very short (i.e. dysfunctional) telomeres that can potentially cause genomic instability thus increasing the risk for activation of telomere maintenance mechanisms and oncogenesis. Like many other cancers, esophageal cancer cells have short telomeres and elevated telomerase, the enzyme that maintains telomeres in most cancer cells. Homologous recombination, which is implicated in the alternate pathway of telomere elongation, is also elevated in Barrett’s-associated esophageal adenocarcinoma. Evidence from our laboratory indicates that both telomerase and homologous recombination contribute to telomere maintenance, DNA repair, and the ongoing survival of esophageal cancer cells. This indicates that telomere maintenance mechanisms may potentially be targeted to make esophageal cancer cells static. The rate at which telomeres in healthy cells shorten is determined by a number of intrinsic and extrinsic factors, including those associated with lifestyle. Avoidance of factors that may directly or indirectly injure esophageal tissue including its telomeric and other genomic DNA can not only reduce the risk of development of esophageal cancer but may also have positive impact on overall health and lifespan.
Keywords: Telomere, telomerase, homologous recombination, esophageal, cancer
Introduction
DNA at each chromosome end is comprised of multiple nucleotide repeats of “TTAGGG”. A number of proteins (including TRF1 and TRF2 (1)) interact with these repeats and play specific roles in the maintenance of chromosome ends. It has been proposed that the interaction of TRF2 with terminal “TTAGGG” repeats helps formation of a looped structure at each chromosome end (2). The protective structure formed by association of “TTAGGG” repeats with specific proteins, at each chromosome end, is called a telomere. Telomeres protect chromosomes from degradation by nucleases and unscheduled or unnecessary inter-telomeric recombination and/or fusion (3). Telomeres, therefore, play a critical role in the maintenance of genomic integrity and the preservation of vital genetic information. In the normal cellular environment, the inability of replication machinery to completely synthesize telomeric DNA leads to a gradual shortening of telomeres with age (4, 5). When telomeres shorten beyond a certain length the protective structure falls apart leading to complete or partial loss of the chromosome and subsequently the induction of cellular senescence and/or apoptotic death (6–11). Therefore, the length of telomeric DNA may influence the overall lifespan of a cell in culture and an organism in vivo (12). Telomere shortening can also be expedited by various intrinsic or extrinsic factors which may induce damage to telomeric DNA (13). Excessive telomere shortening is not only associated with reduced lifespan but also with genomic instability that can lead to oncogenesis (14–16).
Telomeres, the DNA-protein complexes at chromosome ends, form a looped structure that caps the chromosomal DNA thus protecting it from degradation, allowing the recognition of DNA damage, and/or preventing interchromosomal fusion (17). However, the length of telomeric DNA in most normal somatic cells shortens with each cell division. When telomere length in a cell reaches the critical length required to support its protective function, the cell undergoes growth arrest and replicative senescence and/or apoptosis (18–20). Short and dysfunctional telomeres can also be recognized as DNA damage leading to p53-dependent apoptosis (21). As a normal cellular process, telomeres undergo a gradual and progressive shortening with age thus limiting the replicative potential and lifespan of normal cells (22, 23). Athough telomere length and the rate of its shortening may vary among different tissues in the body, telomere length negatively correlates with age (5, 22–26). The rate of telomere shortening is also affected by various intrinsic and extrinsic factors including genetic and epigenetic signals, oxidative metabolites, environmental exposures, and individual lifestyle (23, 27–33). For example, smoking, lack of exercise, and consumption of an unhealthy diet (marked by excessive fat and processed meats with the reduced intake of fruits, vegetables, fiber and antioxidants) can accelerate telomere shortening, which in turn can predispose to the early onset of a number of age-related health issues including heart disease, cancer, and reduced lifespan (15, 16, 34–41). In summary, telomeres preserve chromosomal integrity but shorten with age thus limiting the number of doublings a cell can go through in culture or in vivo. The lifespan of normal cells depends on telomere length and the rate of its shortening. Unhealthy diet and lifestyle can increase the rate of telomere shortening leading to early onset of age-associated diseases.
The progressive telomere shortening that occurs as a normal process in most somatic cells is prevented in the germ-line and in a subset of stem cells by telomerase, the enzyme that adds “TTAGGG” repeats to existing telomeres (42, 43). Telomerase activity, which is absent or weakly detected in normal somatic cells, is elevated in the majority of immortal cells and cancer cells (44–46). Telomerase is an enzyme with two distinct components, the protein or catalytic subunit (hTERT) and the RNA subunit (hTR), which carries the telomeric sequence information. The catalytic subunit of the enzyme copies telomeric sequences from the template hTR and reverse transcribes them for incorporation into telomeres (47). Certain cancer cells and immortal cells do not have detectable telomerase activity and elongate their telomeres using an alternative mechanism known as the ALT (alternative lengthening of telomeres) pathway (48). ALT can be defined as homologous recombination-mediated extension of telomeric DNA and requires the activity of several DNA repair and recombination proteins. Unlike telomerase positive cells, ALT cells have long heterogenous telomeres. Electron microscopy has revealed that the 3′ single-stranded part of telomeric DNA undergoes looping and invasion into the adjacent double-stranded DNA. The process is similar to recombinase (RAD51)-mediated homologous pairing and the resulting structure has similarity with Holliday junctions. The invaded DNA strand in this structure is positioned for elongation by the homologous recombination, which is deregulated in cancer. In normal cellular environment homologous recombination is extremely precise and regulated and therefore does not extend telomeres. Moreover, there are mechanisms which prevent unschedualed or unnecessary recombination at telomeric sequences in normal cells; these mechanisms seem to be disrupted in ALT cells.
Shorter telomeres lead to the induction of replicative senescence and/or apoptosis (18, 20). However, in the absence of p53 function or with similar defects in pathways leading to senescence and apoptosis, continued cell division may lead to the production of very short telomeres that contribute to genomic instability by mediating inter-chromosomal fusion (49, 50), and may therefore initiate the carcinogenic process. Activation of mechanisms involved in telomere elongation and/or stabilization in cells with increased genomic instability provides unlimited proliferative potential contributing to development of cancer. Begus-Nahrmann et al. (14) have demonstrated that the induction of telomere dysfunction leads to genomic instability and the development of cancer in the presence of telomerase in vivo. Data from other laboratories also indicate that severe telomere shortening or telomere dysfunction contributes to the development of cancer (49, 51, 52). Individuals whose telomeres are shorter than the average telomere length for their corresponding age group have an increased risk for the development of cancers, including gastrointestinal cancers (36, 40). Dyskeratosis congenita, a genetic disease in which a defect in telomere maintenance leads to excessive telomere shortening is associated with several age-related health problems including a predisposition to cancer and premature death (53, 54). In summary, it seems that excessive or severe telomere shortening can potentially induce genomic instability thus increasing the risk of cancer.
There is growing evidence suggesting a role for telomerase beyond telomeres. As described above, telomerase has been implicated in DNA double strand break (DSB) repair within the genome. There are several interspersed telomeric repeats (ITRs) within our genome. These internal sequences may have evolutionary significance as some of these ITRs are absent in our early ancestors. It is thought that these sequences have been incorporated at sites of DNA breaks at different stages of evolution (55). In cancer cells, simultaneous upregulation of telomerase activity and increased frequency of DSBs may lead to incorporation of “TTAGGG” sequences as part of the DSB repair mechanism. This may be somewhat similar to events during genomic evolution, leaving telomere sequences as scar marks at healed DSB sites. It is yet to be determined if incorporation of “TTAGGG” sequences in the genome involves synthesis of telomeric repeats by telomerase and/or telomeric recombination, which is also upregulated in several cancers. ITRs, however, are not very frequent even within cancer genomes indicating that there must be a natural mechanism to protect the genome from inadvertent addition of telomeric sequences. In yeast, the well-studied protein Pif-1 has been shown to antagonize telomerase activity at telomeres as well as at DSB sites. Pif-1 is an evolutionarily conserved helicase that preferentially unwinds RNA-DNA hybrids and thus displaces telomerase from the telomere or from broken chromosomal ends (56, 57). The relatively less studied human counterpart of yeast pif-1, hpif-1, has been shown to inhibit telomerase and shorten telomere length, however, it is yet to be confirmed if it also has role in inhibiting telomerase activity at DSB site outside telomeres. Interestingly, hpif-1 is found to be upregulated in esophageal cancer as well as other cancer cell lines tested by us (unpublished). The exact role of this upregulation, however, is not yet known. Although the significance of “TTAGGG” sequences in extra-telomeric regions of the genome is not clear, these sequences could be used as landmarks to study genomic evolution in cancer or any other related disease where genomic instability is prevalent such as ataxia telangiectasia, Bloom syndrome, and Fanconi anemia.
Shorter telomeres are associated with esophageal cancer risk
Consistent with the role of telomere dysfunction in genomic instability (50) and carcinogenesis (52), telomeres are shorter in the majority of cancer cells relative to corresponding control cells, whereas telomerase activity or the ability to elongate telomeres is elevated. A semi-quantitative study using quantitative fluorescent in situ hybridization (QFISH) in tissue sections showed that telomeres of certain chromosomes are shorter than normal, in Barrett’s esophageal adenocarcinoma as well as in early Barrett’s neoplastic lesions (58). We have also shown that telomere length is shorter in primary Barrett’s esophageal adenocarcinoma cells purified by laser capture microdissection (9) as compared to normal esophageal epithelial cells. Shorter telomeres have also been reported in esophageal squamous cell carcinoma, and in these cases, telomere shortening has been shown to associate with genomic instability (59). Consistently, the shorter telomeres in precancerous lesions of esophageal carcinoma and other epithelial cancers have been shown to correlate with progression to cancer (60). Other independent studies have also confirmed the association of shorter telomeres with the etiology of esophageal cancer. In a study in which telomere length was measured by quantitative PCR, short telomeres in the leukocytes of Barrett’s esophagus patients with premalignant disease were significantly associated with progression to esophageal cancer (61). Similarly, another study demonstrated that reduction of both the average telomere length as well the telomere length of chromosomes 17p and 12q as compared to control individuals were associated with a significantly elevated risk of esophageal cancer (62). These studies indicate that excessive telomere shortening can potentially contribute to development and/or progression of esophageal cancer.
Impact of esophageal cancer risk factors on telomeres may contribute to the oncogenic process
Risk factors for esophageal cancer include exposure of esophageal tissue to acid (as a consequence of gastroesophageal reflux), tobacco smoke, alcohol consumption, unhealthy diet, and possibly hot liquids (63). Since many of these factors are potentially DNA damaging, they may pose a special threat to telomeric DNA, which already undergoes shortening as a normal cellular process. Exposure of esophageal tissue to acid is particularly mutagenic. Data from our laboratory show that even a brief exposure to acid leads to a marked induction of homologous recombination activity and to RAD51 expression in human cells (64). Acid exposure is probably the reason that certain types of mutations are observed at higher frequency in carcinomas of esophagus compared to other solid tumors (65). Similarly cigarette smoke, which contains a number of DNA damaging carcinogenic agents, is associated with a dose-dependent increase in telomere length reduction (26, 36). It has also been proposed that telomere length may indicate the extent of smoke-induced oxidative DNA damage (66). Consumption of alcohol and an unhealthy diet are also associated with damage to DNA and telomeres. Genotoxic metabolites of alcohol (acetaldehyde) (67) as well as the consumption of certain foods or the use of certain food preparation methods can threaten telomeres and genomic integrity. For example, a negative correlation of telomere length with the intake of polyunsaturated fatty acids has been reported (68). Although the consumption of very hot liquids does not appear to have a definitive association with telomere shortening, it has been indicated as a risk fatcor for esophageal squamous cell carcinoma. Nevertheless, since heat can lead to the production of abasic sites in DNA, consumption of hot liquids over a long time period may not only cause esophageal tissue injury but may also contribute to damage in genomic as well as telomeric DNA. Chronic inflammation, whether caused by these or other exposures, also has been linked to the carcinogenic process in many neoplastic and pre-neoplastic processes including Barrett’s esophagus (69). Inflammation is associated with production of reactive oxygen species and may therefore contribute to oncogenic process by compromising the stability of the genome and telomeres (70). In summary, lifestyle and dietary factors may negatively impact telomere length in esophageal tissue contributing to etiology of cancer.
Telomere maintenance and DNA repair cooperate in conferring unlimited proliferative potential to esophageal cancer cells
We have found that inhibitors of homologous recombination (HR) reduce telomere length in telomerase positive Barrett’s esophageal adenocarcinoma cells (71). This suggests that recombinational repair is closely connected to telomere maintenance. Telomerase inhibition in esophageal cancer cells also causes induction of PML bodies, induction of H2AX phosphorylation, an increased number of DNA breaks as detected by comet assay, elevation of HR activity, and increased genomic instability as assessed by genome-wide SNP analyses. Genomic instability is further increased upon restriction endonuclease-induced DSBs in hTERT-knockdown BAC cells. These data indicate that telomerase is also involved in the repair of spontaneous and induced DNA breaks in esophageal cancer cells. The induction of HR and PML bodies seen following telomerase inhibition increases the possibility of telomere elongation through the ALT pathway. Consistent with this, Wu et al. (72) reported that treatment with a telomerase inhibitor was associated with increased levels of 53BP1 and γ-H2AX foci as well as an increase in the number and size of 53BP1 ionizing radiation-induced foci in esophageal cancer cells. These data indicate a close relationship between telomerase-mediated telomere maintenance and HR-mediated DNA repair in Barrett’s esophageal cancer cells. Our data showing that a telomerase inhibitor impacts DNA repair as well as telomere length suggests that this strategy might be utilized as a means of sensitizing tumor cells to radiotherapy, and we are currently exploring this idea.
Telomere maintenance mechanisms as therapeutic targets in esophageal cancer
Expression of telomerase, both the catalytic subunit (hTERT) and the RNA component (hTR), has been shown to be elevated in Barrett’s esophagus and even further elevated in high grade dysplasia and esophageal adenocarcinoma (46, 73). These studies indicate that telomerase is activated in esophageal cancer and thus maintains telomere length, providing unlimited proliferative potential to these cancer cells.
Investigating epithelial cells purified from esophagi by laser capture microdissection (LCM), we demonstrated that telomerase activity is elevated in Barrett’s adenocarcinoma (BAC), whereas telomeres are shorter (9). These data identified telomerase as a promising target in BAC (9). We have pre-clinically evaluated different classes of telomerase inhibitors including G-quadruplex intercalating agents (6, 8, 74), oligonucleotides targeting the catalytic subunit of telomerase (9, 75), and siRNAs targeting the RNA component of telomerase (7) in esophageal as well as other cancer cells. We have shown that telomerase inhibition induces senescence and apoptosis in cancer cells. Besides telomere maintenance, telomerase also seems to play a role in the repair of DNA breaks. As reported by Wu et al. (72), treatment with a telomerase inhibitor was associated with increased levels of 53BP1 and increased γ-H2AX foci as well as increased ionizing radiation-induced number and size of 53BP1 foci in esophageal cancer cells.
Data from our laboratory show that RAD51, which is important in both telomere maintenance and genomic instability, is upregulated in BAC (64). Consistently, the homologous recombination (HR) activity is also elevated in BAC as well as other cancer and immortal cells (64, 76, 77). Inhibition of telomerase in BAC further stimulates the HR pathway contributing to the maintenance of telomere length and proliferation (71). Consistently, simultaneous inhibition of the telomerase and HR pathways is more effective in the prevention of telomere maintenance and growth of BAC tumors in mice (71).
In summary, telomere maintenance mechanisms, telomerase itself, and HR have been identified as promising targets in BAC. A therapeutic strategy targeting both telomerase and HR has the potential to inhibit proliferation and prevent tumor growth in esophageal cancer and probably other malignancies.
Conclusions
It has been demonstrated that telomeres are shorter than normal in precancerous lesions of the esophagus as well as other sites. The association of these short telomeres with genomic instability and progression to cancer indicates that excessive telomere shortening contributes to etiology of esophageal cancer. Lifestyle factors such as alcohol consumption, consumption of very hot liquids, an unhealthy diet, and factors that may cause or worsen acid reflux have the potential to expose esophageal tissue to genotoxic agents having the potential to expedite telomere erosion. Although telomeres are shorter in esophageal cancer, telomerase is activated providing unlimited proliferation potential to esophageal cancer cells. Homologous recombination, which is involved in the alternate pathway of telomere maintenance as well as genomic instability, is also elevated in Barrett’s associated esophageal adenocarcinoma cells and contributes to telomere stabilization. There is also evidence that both the telomerase and homologous recombination pathways contribute to telomere maintenance, DNA repair, and survival of esophageal cancer cells indicating that telomere maintenance mechanisms may be a potential therapeutic target for Barrett’s associated adenocarcinoma and probably other cancers (Figure 1).
Figure 1. Telomeres as targets in esophageal cancer.
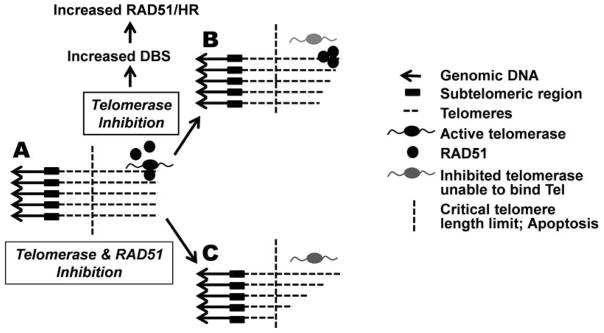
(A) Shortening of telomeres (dashed lines) is prevented by telomerase. In Barrett’s esophageal adenocarcinoma cells homologous recombination (HR) and RAD51 also contribute to telomere stabilization71. (B) Inhibition of telomerase in these cells increases DNA breaks (DBS) as well as RAD51 expression and HR activity71 indicating that telomerase has a role in DNA repair. Telomerase inhibition leads to gradual telomere shortening and apoptosis. (C) Suppression of both telomerase and HR expedite telomere erosion and apoptosis in these cells71.
Acknowledgments
Funding:
Research work conducted in our laboratory and some of the work discussed here is supported in part by grants from National Cancer Institute R01CA125711 to MAS, from the Dept. of Veterans Affairs Merit Review Awards I01- BX001584 (to NCM) and from the National Institutes of Health Grants RO1-124929, PO1-155258, P50-100007 and PO1-78378 to NCM. JSG is supported by a Career Development Award from the Dept. of Veterans Affairs.
Footnotes
Disclosures:
The authors have no conflict of interest to declare; all authors have read the journal’s policy on disclosure of potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matulic M, Sopta M, Rubelj I. Telomere dynamics: the means to an end. Cell Prolif. 2007 Aug;40(4):462–74. doi: 10.1111/j.1365-2184.2007.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, et al. Mammalian telomeres end in a large duplex loop. Cell. 1999;97(4):503–14. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 3.Day JP, Marder BA, Morgan WF. Telomeres and their possible role in chromosome stabilization. Environ Mol Mutagen. 1993;22(4):245–9. doi: 10.1002/em.2850220411. [DOI] [PubMed] [Google Scholar]
- 4.Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev. 2002 Sep;66(3):407–25. doi: 10.1128/MMBR.66.3.407-425.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–60. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 6.Shammas MA, Shmookler Reis RJ, Li C, Koley H, Hurley LH, Anderson KC, Munshi NC. Telomerase Inhibition and Cell Growth Arrest Following Telomestatin Treatment in Multiple Myeloma. Clinical Cancer Research. 2004;10(2):770–6. doi: 10.1158/1078-0432.ccr-0793-03. [DOI] [PubMed] [Google Scholar]
- 7.Shammas MA, Koley H, Batchu RB, Bertheau RC, Protopopov A, Munshi NC, et al. Telomerase inhibition by siRNA causes senescence and apoptosis in Barrett’s adenocarcinoma cells: mechanism and therapeutic potential. Mol Cancer. 2005 Jul 15;4:24. doi: 10.1186/1476-4598-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shammas MA, Koley H, Beer DG, Li C, Goyal RK, Munshi NC. Growth arrest, apoptosis, and telomere shortening of Barrett’s-associated adenocarcinoma cells by a telomerase inhibitor. Gastroenterology. 2004 May;126(5):1337–46. doi: 10.1053/j.gastro.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 9.Shammas MA, Qazi A, Batchu RB, Bertheau RC, Wong JY, Rao MY, et al. Telomere maintenance in laser capture microdissection-purified Barrett’s adenocarcinoma cells and effect of telomerase inhibition in vivo. Clin Cancer Res. 2008 Aug 1;14(15):4971–80. doi: 10.1158/1078-0432.CCR-08-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shammas MA, Koley H, Bertheau RC, Neri P, Fulciniti M, Tassone P, et al. Telomerase inhibitor GRN163L inhibits myeloma cell growth in vitro and in vivo. Leukemia. 2008 Jul;22(7):1410–8. doi: 10.1038/leu.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shammas MA, Reis RJ, Li C, Koley H, Hurley LH, Anderson KC, et al. Telomerase inhibition and cell growth arrest after telomestatin treatment in multiple myeloma. Clin Cancer Res. 2004 Jan 15;10(2):770–6. doi: 10.1158/1078-0432.ccr-0793-03. [DOI] [PubMed] [Google Scholar]
- 12.Harley CB. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991 Mar-Nov;256(2–6):271–82. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- 13.Shammas MA. Telomeres, lifestyle, cancer, and aging. Curr Opin Clin Nutr Metab Care. Jan;14(1):28–34. doi: 10.1097/MCO.0b013e32834121b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Begus-Nahrmann Y, Hartmann D, Kraus J, Eshraghi P, Scheffold A, Grieb M, et al. Transient telomere dysfunction induces chromosomal instability and promotes carcinogenesis. J Clin Invest. Jun 1;122(6):2283–8. doi: 10.1172/JCI61745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003 Feb 1;361(9355):393–5. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 16.Farzaneh-Far R, Cawthon RM, Na B, Browner WS, Schiller NB, Whooley MA. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arterioscler Thromb Vasc Biol. 2008 Jul;28(7):1379–84. doi: 10.1161/ATVBAHA.108.167049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClintock B. The Stability of Broken Ends of Chromosomes in Zea Mays. Genetics. 1941 Mar;26(2):234–82. doi: 10.1093/genetics/26.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong JG, Costanzo A, Yang HQ, Melino G, Kaelin WG, Jr, Levrero M, et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999 Jun 24;399(6738):806–9. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- 19.Shin JS, Hong A, Solomon MJ, Lee CS. The role of telomeres and telomerase in the pathology of human cancer and aging. Pathology. 2006 Apr;38(2):103–13. doi: 10.1080/00313020600580468. [DOI] [PubMed] [Google Scholar]
- 20.Stiewe T, Putzer BM. p73 in apoptosis. Apoptosis. 2001 Dec;6(6):447–52. doi: 10.1023/a:1012433522902. [DOI] [PubMed] [Google Scholar]
- 21.Hayflick L. The cellular basis for biological aging. In: Finch CE, Hayflick L, editors. Handbook of the biology of aging. N.Y: Van Nostrand Reinhold Co; 1977. pp. 159–86. [Google Scholar]
- 22.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003 May 1;23(5):842–6. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 23.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005 Aug 20–26;366(9486):662–4. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 24.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008 Apr;88(2):557–79. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 25.Jiang H, Schiffer E, Song Z, Wang J, Zurbig P, Thedieck K, et al. Proteins induced by telomere dysfunction and DNA damage represent biomarkers of human aging and disease. Proc Natl Acad Sci U S A. 2008 Aug 12;105(32):11299–304. doi: 10.1073/pnas.0801457105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song Z, von Figura G, Liu Y, Kraus JM, Torrice C, Dillon P, et al. Lifestyle impacts on the aging-associated expression of biomarkers of DNA damage and telomere dysfunction in human blood. Aging Cell. Aug;9(4):607–15. doi: 10.1111/j.1474-9726.2010.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benetti R, Garcia-Cao M, Blasco MA. Telomere length regulates the epigenetic status of mammalian telomeres and subtelomeres. Nat Genet. 2007 Feb;39(2):243–50. doi: 10.1038/ng1952. [DOI] [PubMed] [Google Scholar]
- 28.Celli GB, de Lange T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat Cell Biol. 2005 Jul;7(7):712–8. doi: 10.1038/ncb1275. [DOI] [PubMed] [Google Scholar]
- 29.Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008 Jan 28;168(2):154–8. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- 30.Munoz P, Blanco R, Flores JM, Blasco MA. XPF nuclease-dependent telomere loss and increased DNA damage in mice overexpressing TRF2 result in premature aging and cancer. Nat Genet. 2005 Oct;37(10):1063–71. doi: 10.1038/ng1633. [DOI] [PubMed] [Google Scholar]
- 31.Nawrot TS, Staessen JA, Gardner JP, Aviv A. Telomere length and possible link to X chromosome. Lancet. 2004 Feb 14;363(9408):507–10. doi: 10.1016/S0140-6736(04)15535-9. [DOI] [PubMed] [Google Scholar]
- 32.Nordfjall K, Eliasson M, Stegmayr B, Melander O, Nilsson P, Roos G. Telomere length is associated with obesity parameters but with a gender difference. Obesity (Silver Spring) 2008 Dec;16(12):2682–9. doi: 10.1038/oby.2008.413. [DOI] [PubMed] [Google Scholar]
- 33.Steinert S, Shay JW, Wright WE. Modification of subtelomeric DNA. Mol Cell Biol. 2004 May;24(10):4571–80. doi: 10.1128/MCB.24.10.4571-4580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007 Jan 13;369(9556):107–14. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 35.Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007 Jan 1;165(1):14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 36.McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007 Apr;16(4):815–9. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- 37.Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care. 2006 Feb;29(2):283–9. doi: 10.2337/diacare.29.02.06.dc05-1715. [DOI] [PubMed] [Google Scholar]
- 38.Valdes AM, Richards JB, Gardner JP, Swaminathan R, Kimura M, Xiaobin L, et al. Telomere length in leukocytes correlates with bone mineral density and is shorter in women with osteoporosis. Osteoporos Int. 2007 Sep;18(9):1203–10. doi: 10.1007/s00198-007-0357-5. [DOI] [PubMed] [Google Scholar]
- 39.van der Harst P, van der Steege G, de Boer RA, Voors AA, Hall AS, Mulder MJ, et al. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007 Apr 3;49(13):1459–64. doi: 10.1016/j.jacc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 40.Wu X, Amos CI, Zhu Y, Zhao H, Grossman BH, Shay JW, et al. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst. 2003 Aug 20;95(16):1211–8. doi: 10.1093/jnci/djg011. [DOI] [PubMed] [Google Scholar]
- 41.Zee RY, Michaud SE, Germer S, Ridker PM. Association of shorter mean telomere length with risk of incident myocardial infarction: a prospective, nested case-control approach. Clin Chim Acta. 2009 May;403(1–2):139–41. doi: 10.1016/j.cca.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Broccoli D, Young JW, de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci U S A. 1995;92(20):9082–6. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tzukerman M, Selig S, Skorecki K. Telomeres and telomerase in human health and disease. J Pediatr Endocrinol Metab. 2002 Mar;15(3):229–40. doi: 10.1515/jpem.2002.15.3.229. [DOI] [PubMed] [Google Scholar]
- 44.Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, et al. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. Embo J. 1992;11(5):1921–9. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer [see comments] Science. 1994;266(5193):2011–5. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 46.Lord RV, Salonga D, Danenberg KD, Peters JH, DeMeester TR, Park JM, et al. Telomerase reverse transcriptase expression is increased early in the Barrett’s metaplasia, dysplasia, adenocarcinoma sequence. J Gastrointest Surg. 2000 Mar-Apr;4(2):135–42. doi: 10.1016/s1091-255x(00)80049-9. [DOI] [PubMed] [Google Scholar]
- 47.Blackburn EH. Telomerases. Annu Rev Biochem. 1992;61:113–29. doi: 10.1146/annurev.bi.61.070192.000553. [DOI] [PubMed] [Google Scholar]
- 48.Dunham MA, Neumann AA, Fasching CL, Reddel RR. Telomere maintenance by recombination in human cells.[see comment] Nature Genetics. 2000 Dec;26(4):447–50. doi: 10.1038/82586. [DOI] [PubMed] [Google Scholar]
- 49.Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97(4):527–38. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- 50.De Lange T. Telomere-related genome instability in cancer. Cold Spring Harb Symp Quant Biol. 2005;70:197–204. doi: 10.1101/sqb.2005.70.032. [DOI] [PubMed] [Google Scholar]
- 51.Frias C, Pampalona J, Genesca A, Tusell L. Telomere dysfunction and genome instability. Front Biosci. 17:2181–96. doi: 10.2741/4044. [DOI] [PubMed] [Google Scholar]
- 52.Meeker AK. Telomeres and telomerase in prostatic intraepithelial neoplasia and prostate cancer biology. Urol Oncol. 2006 Mar-Apr;24(2):122–30. doi: 10.1016/j.urolonc.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 53.Dokal I, Vulliamy T, Mason P, Bessler M. Clinical utility gene card for: dyskeratosis congenita. Eur J Hum Genet. Nov;19(11) doi: 10.1038/ejhg.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001 Sep 27;413(6854):432–5. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 55.Nergadze SG, Rocchi M, Azzalin CM, Mondello C, Giulotto E. Insertion of telomeric repeats at intrachromosomal break sites during primate evolution. Genome Res. 2004 Sep;14(9):1704–10. doi: 10.1101/gr.2778904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boule JB, Vega LR, Zakian VA. The yeast Pif1p helicase removes telomerase from telomeric DNA. Nature. 2005 Nov 3;438(7064):57–61. doi: 10.1038/nature04091. [DOI] [PubMed] [Google Scholar]
- 57.Makovets S, Blackburn EH. DNA damage signalling prevents deleterious telomere addition at DNA breaks. Nat Cell Biol. 2009 Nov;11(11):1383–6. doi: 10.1038/ncb1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Finley JC, Reid BJ, Odze RD, Sanchez CA, Galipeau P, Li X, et al. Chromosomal instability in Barrett’s esophagus is related to telomere shortening. Cancer Epidemiol Biomarkers Prev. 2006 Aug;15(8):1451–7. doi: 10.1158/1055-9965.EPI-05-0837. [DOI] [PubMed] [Google Scholar]
- 59.Zheng YL, Hu N, Sun Q, Wang C, Taylor PR. Telomere attrition in cancer cells and telomere length in tumor stroma cells predict chromosome instability in esophageal squamous cell carcinoma: a genome-wide analysis. Cancer Res. 2009 Feb 15;69(4):1604–14. doi: 10.1158/0008-5472.CAN-08-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meeker AK, Hicks JL, Iacobuzio-Donahue CA, Montgomery EA, Westra WH, Chan TY, et al. Telomere length abnormalities occur early in the initiation of epithelial carcinogenesis. Clin Cancer Res. 2004 May 15;10(10):3317–26. doi: 10.1158/1078-0432.CCR-0984-03. [DOI] [PubMed] [Google Scholar]
- 61.Risques RA, Vaughan TL, Li X, Odze RD, Blount PL, Ayub K, et al. Leukocyte telomere length predicts cancer risk in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2007 Dec;16(12):2649–55. doi: 10.1158/1055-9965.EPI-07-0624. [DOI] [PubMed] [Google Scholar]
- 62.Xing J, Ajani JA, Chen M, Izzo J, Lin J, Chen Z, et al. Constitutive short telomere length of chromosome 17p and 12q but not 11q and 2p is associated with an increased risk for esophageal cancer. Cancer Prev Res (Phila) 2009 May;2(5):459–65. doi: 10.1158/1940-6207.CAPR-08-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghadirian P, Vobecky J, Vobecky JS. Factors associated with cancer of the oesophagus: an overview. Cancer Detect Prev. 1988;11(3–6):225–34. [PubMed] [Google Scholar]
- 64.Pal J, Bertheau R, Buon L, Qazi A, Batchu RB, Bandyopadhyay S, et al. Genomic evolution in Barrett’s adenocarcinoma cells: critical roles of elevated hsRAD51, homologous recombination and Alu sequences in the genome. Oncogene. Aug 18;30(33):3585–98. doi: 10.1038/onc.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 66.Babizhayev MA, Savel’yeva EL, Moskvina SN, Yegorov YE. Telomere length is a biomarker of cumulative oxidative stress, biologic age, and an independent predictor of survival and therapeutic treatment requirement associated with smoking behavior. Am J Ther. Nov;18(6):e209–26. doi: 10.1097/MJT.0b013e3181cf8ebb. [DOI] [PubMed] [Google Scholar]
- 67.Haas SL, Ye W, Lohr JM. Alcohol consumption and digestive tract cancer. Curr Opin Clin Nutr Metab Care. Sep;15(5):457–67. doi: 10.1097/MCO.0b013e3283566699. [DOI] [PubMed] [Google Scholar]
- 68.Cassidy A, De Vivo I, Liu Y, Han J, Prescott J, Hunter DJ, et al. Associations between diet, lifestyle factors, and telomere length in women. Am J Clin Nutr. May;91(5):1273–80. doi: 10.3945/ajcn.2009.28947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Poehlmann A, Kuester D, Malfertheiner P, Guenther T, Roessner A. Inflammation and Barrett’s carcinogenesis. Pathol Res Pract. May 15;208(5):269–80. doi: 10.1016/j.prp.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 70.Farinati F, Piciocchi M, Lavezzo E, Bortolami M, Cardin R. Oxidative stress and inducible nitric oxide synthase induction in carcinogenesis. Dig Dis. 28(4–5):579–84. doi: 10.1159/000320052. [DOI] [PubMed] [Google Scholar]
- 71.Lu R, Pal J, Buon L, Nanjappa P, Shi J, Fulciniti M, et al. Targeting homologous recombination and telomerase in Barrett’s adenocarcinoma: impact on telomere maintenance, genomic instability and tumor growth. Oncogene. Apr 22; doi: 10.1038/onc.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu X, Smavadati S, Nordfjall K, Karlsson K, Qvarnstrom F, Simonsson M, et al. Telomerase antagonist imetelstat inhibits esophageal cancer cell growth and increases radiation-induced DNA breaks. Biochim Biophys Acta. Dec;1823(12):2130–5. doi: 10.1016/j.bbamcr.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Morales CP, Lee EL, Shay JW. In situ hybridization for the detection of telomerase RNA in the progression from Barrett’s esophagus to esophageal adenocarcinoma. Cancer. 1998 Aug 15;83(4):652–9. [PubMed] [Google Scholar]
- 74.Shammas MA, Shmookler Reis RJ, Akiyama M, Koley H, Chauhan D, Hideshima T, et al. Telomerase inhibition and cell growth arrest by G-quadruplex interactive agent in multiple myeloma. Molecular Cancer Therapeutics. 2003 Sep;2(9):825–33. [PubMed] [Google Scholar]
- 75.Akiyama M, Hideshima T, Shammas MA, Hayashi T, Hamasaki M, Tai YT, et al. Effects of oligonucleotide N3′-->P5′ thio-phosphoramidate (GRN163) targeting telomerase RNA in human multiple myeloma cells. Cancer Research. 2003 Oct 1;63(19):6187–94. [PubMed] [Google Scholar]
- 76.Xia SJ, Shammas MA, Shmookler Reis RJ. Elevated recombination in immortal human cells is mediated by HsRAD51 recombinase. Molecular & Cellular Biology. 1997 Dec;17(12):7151–8. doi: 10.1128/mcb.17.12.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shammas MA, Shmookler Reis RJ, Koley H, Batchu RB, Li C, Munshi NC. Dysfunctional homologous recombination mediates genomic instability and progression in myeloma. Blood. 2009 Mar 5;113(10):2290–7. doi: 10.1182/blood-2007-05-089193. [DOI] [PMC free article] [PubMed] [Google Scholar]


