Abstract
As the scope of potential chemical warfare agents grows rapidly and as the diversity of potential threat scenarios expands with non-state actors, so a need for innovative approaches to countermeasure development has emerged. In the last few years, the utility of the zebrafish as a model organism that is amenable to high-throughput screening has become apparent and this system has been applied to the unbiased discovery of chemical warfare countermeasures. This review summarizes the in vivo screening approach that has been pioneered in the countermeasure discovery arena, and highlights the successes to date as well as the potential challenges in moving the field forward. Importantly, the establishment of a zebrafish platform for countermeasure discovery would offer a rapid response system for the development of antidotes to the continuous stream of new potential chemical warfare agents.
Chemical warfare and modern threat scenarios
In the last two decades multiple factors have conspired to increase the range of potential chemical warfare agents and the scope of potential threat scenarios that must be addressed by national public protection agencies. Globalization of access to information, the ease of international travel, the proliferation of chemicals for industrial or domestic use each contributed to this new reality. Not only are traditional nation states engaged in the development and deployment of new chemical threats, but franchised terror organizations bent on asymmetric warfare are also using the internet to crowdsource the weaponization of common chemicals and to disseminate the information necessary to use such agents1. Countermeasures must be developed not only for domestic or industrial accidents, but also for deliberate mass casualty exposures in a diverse range of public spaces. This rapidly changing landscape of existing and emerging chemical threats has generated a need for a change in the scale and efficiency of countermeasure discovery.
The recent adoption of one model organism, the zebrafish, for high-throughput in vivo disease modeling and chemical biology, has led to its emergence as a tool for drug screening2, 3. In this review we will outline current and potential uses of the zebrafish for the discovery of countermeasures against existing and emerging chemical warfare agents, highlighting some of the most promising advances in this field. We will focus on the potential of the organism to contribute to our understanding of multiple different aspects of the complex interface between chemistry and biology. We will not attempt to be comprehensive, but rather will use specific examples to illustrate the current and future utility of the zebrafish, and to define the hurdles that still must be overcome as the
Contemporary drug discovery
High-throughput screening (HTS) and other high-throughput technologies have begun to have a major impact on biomedical research4. Nowhere has as the extent of implementation been greater than in drug discovery. For well-validated therapeutic targets, HTS based on target binding or function has proven to be an effective mans of identifying compounds that modify the target protein’s activity in vitro4. Unfortunately, many of the compounds discovered in this way have failed clinically for lack of efficacy in vivo or because of toxicity. Such failures suggest that in vitro assays are rarely adequate to predict the therapeutic efficacy of a compound or to predict potential off-target activities causing drug toxicity. This inability of simple in vitro assays to model human disease and toxicology is a fundamental driver for the development of in vivo platforms enabling the use of HTS for the exploration of disease pathways, drug discovery or toxicology4.
Animal models are used widely in countermeasure development, but have not historically been used during early stages of countermeasure discovery. The traditional models used in countermeasure research are not capable of the throughput required for screening large chemical libraries. Ideally, an in vivo model would recapitulate the complexities of integrated human physiology and pharmacology and still be capable of efficient screening of tens or hundreds of thousands of different conditions (chemical or genetic manipulations). Simple invertebrate organisms such as yeast, C. elegans or Drosophila have been used for genetic screening on that scale, but until the zebrafish emerged as a viable model organism, large scale chemical and genetic screens were not feasible with vertebrates5–8.
Zebrafish as a model organism
During the past decade, the zebrafish has become a popular model for phenotype-based screening, in part because it enables in vivo experiments to be performed at the scale and cost of in vitro experiments8. The zebrafish is small for a vertebrate. Adults can grow to 3 cm in length, but embryonic and larval zebrafish are only 1–3 mm long. Larvae can survive for 5–7 days in the wells of standard 96 or 384-well plates, receiving nutrients from their yolks. A pair of adults can lay hundreds of fertilized eggs in a day, so a zebrafish facility can routinely generate the thousands of embryos needed for high-throughput screens 9, 10. The transparency of the zebrafish is another advantage, allowing real time observation of its cell biology and physiology. The transparency also enables effective use of fluorescent markers or molecular reporters to characterize integrative biology across multiple organ systems in a completely native context, at throughputs comparable to those used in in vitro high-throughput screening11. Dozens of transgenic zebrafish lines have been created that express fluorescent proteins in specific cell types or in cells undergoing specific processes, such as apoptosis12. These lines greatly facilitate detection of the physiological and anatomical changes caused by genetic or chemical perturbation.
Numerous genetic tools have been developed that dramatically increase the utility of the zebrafish as a model organism. These include a largely complete genomic sequence, DNA microarrays, antisense morpholino oligos (MOs) for knocking down any gene, zinc finger nucleases and TALENs for generating targeted mutations in virtually any gene of interest, and transgenic tools for temporally and spatially controlled gene expression13, 14,15, 16. These tools are changing the scope of in vivo models that can be generated and screens that can be conducted.
Conservation of physiology between zebrafish and humans
A central question in the use of the zebrafish in chemical warfare countermeasure discovery is the degree to which the physiological response to a chemical agent is conserved between zebrafish and higher organisms, including humans. Zebrafish develop rapidly, and organogenesis is largely complete within 48 hours post fertilization 17. Physiologic integration is initiated prior to this developmental stage and progresses over the next several days (or weeks) as humoral and neural communication is established18. For example, peripheral innervation by the somatic and autonomic nervous systems begins during early embryogenesis but is not complete until weeks after fertilization. Therefore, care must be taken during assay development to verify that the relevant biology is conserved in the zebrafish at the specific developmental stages being used2, 19.
Prior genetic and chemical screens in zebrafish have demonstrated a high degree of conservation between zebrafish and other organisms, including humans. Genes identified in the numerous zebrafish morphological screens have been consistently validated in mice or humans8, 20. In some instances, zebrafish and human gene discovery have occurred side-by side21, 22. In small molecule screens, too, the correlations between pharmacological effects in zebrafish and humans have proven to strong23, 24. This is true not only for well characterized human drugs, but also for compounds discovered through zebrafish screens18, 25. For example, a zebrafish screen of several thousand compounds identified a novel class of compounds capable of suppressing the vascular defect in gridlock mutants20, 26. Beyond their activity in zebrafish, these compounds promote tubulogenesis in cultured human endothelial cells and improve the collateralization of ischemic mouse hind limbs, confirming that the compounds are vasculogenic not only in embryonic zebrafish but also in adult mammals20, 27. These results support the idea that compounds discovered by in vivo zebrafish screens have relevance beyond the zebrafish and may provide avenues for development of human therapies19, 28.
In vivo screens for chemical warfare countermeasures
To date only two screens have been performed in the zebrafish for chemical warfare countermeasures: one for cyanide antidotes and one for organophosphate antidotes. In each case it was possible to rapidly develop robust assays for the relevant chemical exposures.
Cyanide
On exposure to moderate doses of cyanide in the aqueous media zebrafish undergo a number of rapid physiologic changes. The most dramatic of these is a substantial slowing of the heart rate, presumably due to the sensitivity of this organ’s ion gradients and excitation-contraction coupling to metabolic perturbation. Further indicators of cyanide toxicity include focal neuronal necrosis in specific basal nuclei, abrogation of sensory responses and ultimately death. In addition to these fundamental physiologic effects, it is possible to detect subtle perturbations of higher function in zebrafish, closely paralleling mammalian responses. Studying larvae at 96hpf, once visual neuronal pathways are fully established, it is possible to elicit significant abnormalities in startle latencies within minutes of sublethal cyanide exposure29. At sub lethal doses zebrafish exhibit toxic phenotypes for several hours, but eventually recover. Importantly, the final effective concentration in the fish is likely considerably lower than that in the surrounding aqueous media as a result of penetration and the potential conversion to HCN and loss as a gas30. These phenotypes closely parallel the observed effects with lethal and sublethal doses of cyanide in a wide range of animals ranging from trout to humans3, 31–34. The definition of such stereotypic effects of cyanide on cardiac or neuronal physiology and on survival formed the basis for subsequent automation and screening in the zebrafish model to identify novel countermeasures for cyanide exposure.
High throughput assays for heart rate and simple survival are readily automated and validated at scale using existing countermeasures including known methemoglobin formers (sodium nitrite), known sulfur donors (butanesulfanate and cystamine dihydrochloride) and known cyanide scavengers (hydroxycobalamin and cobinamide)11. Once validated these assays were used to develop a survival screen where lethal doses of cyanide are rescued within 30 minutes. Using this assay libraries of over 46,000 compounds were screened and have led to the discovery of over 20 novel compound classes of cyanide countermeasure35. Derivatives of several of these initial hits exhibit an EC50 in the low nanomolar range and are currently undergoing additional evaluation in other cyanide models36. One of the advantages of direct in vivo screening is the ability to match the screen logic to the specific requirements of prevalent threats. Ongoing screens for cyanide countermeasures are designed to optimize antidotes for specific age ranges or to identify agents that might be adaptable to different threat scenarios.
Organophosphates
Organophosphates are small molecule acetylcholinesterase inhibitors used as pesticides and as nerve agents for chemical warfare37–39. Representative organophosphate pesticides include parathion, malathion, and chlorpyrifos, while representative organophosphate nerve agents include sarin, tabun, and VX. Humans exposed to low doses of organophosphates exhibit nausea, vomiting, lachrymation, and bradycardia, while higher-dose exposures cause seizures, cardiovascular and respiratory collapse, and death40, 41.
Organophosphates represent one of the most significant chemical threats today42, 43. Agricultural organophosphates are readily available, making them accessible to terrorists in large quantities. The nerve agents are relatively easy to synthesize and have been used by terrorist groups, including in attacks during the 1990s in Japan. Beyond intentional exposures, the large quantities used for agricultural purposes also raise the possibility of mass exposure due to accidents or frequent, smaller-scale exposure from agricultural or household application of pesticides44, 45.
The current standard antidote for organophosphate exposure is the combined administration of atropine and pralidoxime (2-PAM). Organophosphates covalently inhibit acetylcholinesterase (AChE), preventing breakdown of the neurotransmitter acetylcholine46, 47. Failure to metabolize acetylcholine near cholinergic receptor sites causes continuous stimulation of cholinergic neurons in the central and peripheral nervous systems. Atropine antagonizes cholinergic receptors, and by so doing, it counteracts the effects of acetylcholine accumulation48, 49. Unfortunately, it remains difficult to deliver sufficient atropine to antagonize the excess acetylcholine without causing toxicity from too much cholinergic antagonism. Pralidoxime (2-PAM), an acetylcholinesterase reactivator, exhibits toxicities of its own including dangerous hypertension and tachycardia48. Its use is further limited by the fact that some organophosphates sterically hinder 2-PAM from reactivating the inhibited AChE. Aging of the inhibited AChE also prevents reactivation, meaning that 2-PAM is effective only immediately after organophosphate exposure. Therefore, there is great need for additional organophosphate countermeasures that function via other mechanisms 49–51
An unbiased, screening-based effort to discover additional OP antidotes with diverse and potentially novel mechanisms was recently undertaken. Building upon several studies of AChE function and organophosphate toxicity on zebrafish models52, 53, conditions were identified in which the prototypical organophosphate azinphos-methyl produced consistent behavioral deficits, paralysis, and death in early life stage zebrafish. Using death as an endpoint, a pilot screen of 1200 known drugs was conducted in 96-well format54. Sixteen drugs exhibiting the ability to prevent death in organophosphate-exposed zebrafish were discovered. Biochemical and metabolomic assays performed with the 16 hits confirmed that the compounds function through multiple diverse mechanisms, including reversible AChE antagonism, cholinergic receptor antagonism, and prevention of organophosphate bioactivation. These findings suggest that in vivo screening in zebrafish can discover effective new countermeasures and expand the range of mechanisms by which they function. Therefore, additional screening for novel organophosphate countermeasures is warranted and may enhance our ability to respond to organophosphate exposures.
Organ specific toxicology
Organ specific toxicities remain the most frequent reason for the failure of drugs late in development or for withdrawal of drugs from the market. Countermeasures are usually designed for acute one time use, and so only major acute toxicities are likely to be detected. Countermeasure development rarely has the luxury of extensive clinical trials which may detect less common toxicities. In most instances licensing under the Animal Rule will lead to deployment after only relatively small Phase I trials for safety in volunteer cohorts. New models that might improve our ability to identify countermeasure toxicities earlier in the development process, or in ways that would enable more precise estimation of the risks of toxicity have been a focus of work in pharmacology for decades. In the last few years, several investigators have pursued such modeling in the zebrafish. The toxicology of virtually every organ system from the thyroid to the brain has been investigated18, 55, 56. Cardiotoxicity and hepatotoxicity have been particularly well studied, with extensive conservation of mechanism and outcome having been demonstrated between zebrafish and humans57, 58. It is likely that a publication bias exists in favor of studies that demonstrated strong correlation between zebrafish and human effects, and great care must be taken to understand the parallels between phenotypic assays in fish and other species. Nevertheless, there is reason to believe that many of the compounds that would be toxic in humans will also exhibit comparable toxicities in zebrafish. Therefore, countermeasure screening in intact zebrafish is likely to help eliminate compounds that should not be pursued due to the risk of human toxicity. As the study of toxicity in the zebrafish matures, the correlation between species will become better defined, and the safety and efficacy of novel countermeasure candidates in humans may be predictable based on the candidate compounds’ safety and efficacy in zebrafish.
Future directions
The zebrafish combines many of the best attributes of traditional in vitro and in vivo systems. The organism offers much of the physiological complexity and relevance of mammalian model systems, while also allowing experimentation at the scale and cost of in vitro screening. To maximize the utility of the zebrafish in discovery mode, much greater understanding of the parallels between the fish and higher organisms will be required, both in health and in disease. It will also be important to develop adaptable screening assays capable not only of addressing previously encountered chemical threats, but also of rapidly modeling new threats as they emerge and screening efficiently for candidate countermeasures for these nascent threats. An unbiased strategy to evaluating toxicity (of both chemical threats and potential countermeasures) may be feasible in the near future combining multi-channel organ specific reporters, functional genomics and automated image analysis56. The prospect of systematic exploration of threat-countermeasure interactions across a wide range of ages and background susceptibilities is also highly attractive. Importantly, these efforts in countermeasure development also have the potential to directly impact drug discovery not only through technology innovation, but also at the level of specific pharmacologic tools59.
As these goals are attained, the role of the zebrafish as a tool for identifying chemical warfare countermeasures is likely to expand. It is conceivable with parallel efforts in zebrafish disease modeling that the annotation of a chemical class as a potential threat and the discovery of matched countermeasures might eventually occur in parallel60. This approach would complement current low-throughput toxicity studies in other animal models. A robust zebrafish screening platform may be one of the few technologies able to deliver on the promise of threat prediction and neutralization at the scale necessary to combat the breadth of potential chemical warfare agents1.
Figure.
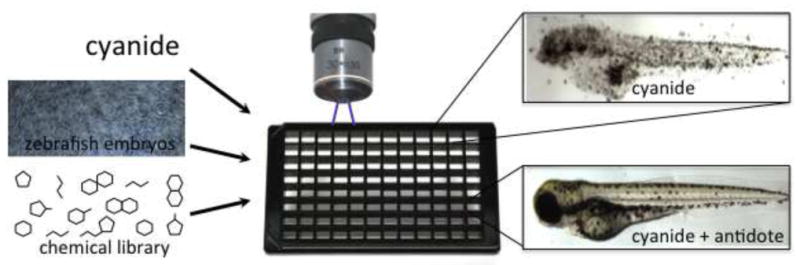
Hundreds or thousands of drugs can be added in 96 well plate format, to zebrafish exposed to lethal doses of agents such as cyanide. Using automated screening microscopy it is then possible to identify the compounds that prevent the lethal or sub-lethal effects of cyanide or any other agent that has a response in this system.
Acknowledgments
This work was supported by the NIH/NINDS Chemical Warfare Countermeasure Program.
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kosal ME. Near Term Threats of Chemical Weapons Terrorism. Monterey: Center on Contemporary Conflict, Naval Postgraduate School; 2006. [Google Scholar]
- 2.MacRae CA, Peterson RT. Zebrafish-based small molecule discovery. Chem Biol. 2003;10(10):901–8. doi: 10.1016/j.chembiol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov. 2005;4(1):35–44. doi: 10.1038/nrd1606. [DOI] [PubMed] [Google Scholar]
- 4.Bleicher KH, Bohm HJ, Muller K, Alanine AI. Hit and lead generation: beyond high-throughput screening. Nat Rev Drug Discov. 2003;2(5):369–78. doi: 10.1038/nrd1086. [DOI] [PubMed] [Google Scholar]
- 5.Yeh JR, Crews CM. Chemical genetics: adding to the developmental biology toolbox. Dev Cell. 2003;5(1):11–9. doi: 10.1016/s1534-5807(03)00200-4. [DOI] [PubMed] [Google Scholar]
- 6.Stockwell BR. Chemical genetics: ligand-based discovery of gene function. Nat Rev Genet. 2000;1(2):116–25. doi: 10.1038/35038557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kokel D, Bryan J, Laggner C, White R, Cheung CY, Mateus R, et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nature chemical biology. 2010;6(3):231–7. doi: 10.1038/nchembio.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patton EE, Zon LI. The art and design of genetic screens: zebrafish. Nat Rev Genet. 2001;2(12):956–66. doi: 10.1038/35103567. [DOI] [PubMed] [Google Scholar]
- 9.Anderson RH, Webb S, Brown NA, Lamers W, Moorman A. Development of the heart: (3) formation of the ventricular outflow tracts, arterial valves, and intrapericardial arterial trunks. Heart. 2003;89(9):1110–8. doi: 10.1136/heart.89.9.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacRae CA, Fishman MC. Zebrafish: the complete cardiovascular compendium. Cold Spring Harb Symp Quant Biol. 2002;67:301–7. doi: 10.1101/sqb.2002.67.301. [DOI] [PubMed] [Google Scholar]
- 11.Burns CG, Milan DJ, Grande EJ, Rottbauer W, MacRae CA, Fishman MC. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat Chem Biol. 2005;1(5):263–4. doi: 10.1038/nchembio732. [DOI] [PubMed] [Google Scholar]
- 12.Liu NA, Huang H, Yang Z, Herzog W, Hammerschmidt M, Lin S, et al. Pituitary corticotroph ontogeny and regulation in transgenic zebrafish. Mol Endocrinol. 2003;17(5):959–66. doi: 10.1210/me.2002-0392. [DOI] [PubMed] [Google Scholar]
- 13.Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26(2):216–20. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- 14.Clark KJ, Voytas DF, Ekker SC. A TALE of two nucleases: gene targeting for the masses? Zebrafish. 2011;8(3):147–9. doi: 10.1089/zeb.2011.9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng X, Noyes MB, Zhu LJ, Lawson ND, Wolfe SA. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol. 2008;26(6):695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foley JE, Maeder ML, Pearlberg J, Joung JK, Peterson RT, Yeh JR. Targeted mutagenesis in zebrafish using customized zinc-finger nucleases. Nat Protoc. 2009;4(12):1855–67. doi: 10.1038/nprot.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haffter P, Nusslein-Volhard C. Large scale genetics in a small vertebrate, the zebrafish. Int J Dev Biol. 1996;40(1):221–7. [PubMed] [Google Scholar]
- 18.Milan DJ, Peterson TA, Ruskin JN, Peterson RT, MacRae CA. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation. 2003;107(10):1355–8. doi: 10.1161/01.cir.0000061912.88753.87. [DOI] [PubMed] [Google Scholar]
- 19.Macrae CA. Cardiac Arrhythmia: In vivo screening in the zebrafish to overcome complexity in drug discovery. Expert Opin Drug Discov. 2010;5(7):619–32. doi: 10.1517/17460441.2010.492826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson RT, Shaw SY, Peterson TA, Milan DJ, Zhong TP, Schreiber SL, et al. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat Biotechnol. 2004;22(5):595–9. doi: 10.1038/nbt963. [DOI] [PubMed] [Google Scholar]
- 21.Xu X, Meiler SE, Zhong TP, Mohideen M, Crossley DA, Burggren WW, et al. Cardiomyopathy in zebrafish due to mutation in an alternatively spliced exon of titin. Nat Genet. 2002;30(2):205–9. doi: 10.1038/ng816. [DOI] [PubMed] [Google Scholar]
- 22.Gerull B, Gramlich M, Atherton J, McNabb M, Trombitas K, Sasse-Klaassen S, et al. Mutations of TTN, encoding the giant muscle filament titin, cause familial dilated cardiomyopathy. Nat Genet. 2002;30(2):201–4. doi: 10.1038/ng815. [DOI] [PubMed] [Google Scholar]
- 23.Peterson RT, Link BA, Dowling JE, Schreiber SL. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc Natl Acad Sci U S A. 2000;97(24):12965–9. doi: 10.1073/pnas.97.24.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khersonsky SM, Jung DW, Kang TW, Walsh DP, Moon HS, Jo H, et al. Facilitated forward chemical genetics using a tagged triazine library and zebrafish embryo screening. J Am Chem Soc. 2003;125(39):11804–5. doi: 10.1021/ja035334d. [DOI] [PubMed] [Google Scholar]
- 25.Langheinrich U. Zebrafish: a new model on the pharmaceutical catwalk. Bioessays. 2003;25(9):904–12. doi: 10.1002/bies.10326. [DOI] [PubMed] [Google Scholar]
- 26.Weinstein BM, Stemple DL, Driever W, Fishman MC. Gridlock, a localized heritable vascular patterning defect in the zebrafish. Nat Med. 1995;1(11):1143–7. doi: 10.1038/nm1195-1143. [DOI] [PubMed] [Google Scholar]
- 27.Ren B, Deng Y, Mukhopadhyay A, Lanahan AA, Zhuang ZW, Moodie KL, et al. ERK1/2-Akt1 crosstalk regulates arteriogenesis in mice and zebrafish. J Clin Invest. 2010;120(4):1217–28. doi: 10.1172/JCI39837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deo RC, Macrae CA. The zebrafish:scalable in vivo modeling for systems biology. Wiley Interdiscip Rev Syst Biol Med. 2010 doi: 10.1002/wsbm.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kokel D, Bryan J, Laggner C, White R, Cheung CY, Mateus R, et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat Chem Biol. 2010;6(3):231–7. doi: 10.1038/nchembio.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson CP, Baskin SI, Groff WA, Franz DR. Cyanide loss from tissue baths in the presence and absence of tissue. Toxicol Lett. 1984;21(3):305–8. doi: 10.1016/0378-4274(84)90088-2. [DOI] [PubMed] [Google Scholar]
- 31.Kerger H, Dodidou P, Passani-Kruppa D, Gruttner J, Birmelin M, Volz A, et al. Excessive methaemoglobinaemia and multi-organ failure following 4-DMAP antidote therapy. Resuscitation. 2005;66(2):231–5. doi: 10.1016/j.resuscitation.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Hall AH, Kulig KW, Rumack BH. Suspected cyanide poisoning in smoke inhalation: complications of sodium nitrite therapy. J Toxicol Clin Exp. 1989;9(1):3–9. [PubMed] [Google Scholar]
- 33.Gracia R, Shepherd G. Cyanide poisoning and its treatment. Pharmacotherapy. 2004;24(10):1358–65. doi: 10.1592/phco.24.14.1358.43149. [DOI] [PubMed] [Google Scholar]
- 34.Baskin SI, Horowitz AM, Nealley EW. The antidotal action of sodium nitrite and sodium thiosulfate against cyanide poisoning. J Clin Pharmacol. 1992;32(4):368–75. doi: 10.1002/j.1552-4604.1992.tb03849.x. [DOI] [PubMed] [Google Scholar]
- 35.Nath AK, Roberts LD, Liu Y, Mahon SB, Kim S, Ryu JH, et al. Chemical and metabolomic screens identify novel biomarkers and antidotes for cyanide exposure. Faseb J. 2013;27(5):1928–38. doi: 10.1096/fj.12-225037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baskin SI, Porter DW, Rockwood GA, Romano JA, Jr, Patel HC, Kiser RC, et al. In vitro and in vivo comparison of sulfur donors as antidotes to acute cyanide intoxication. J Appl Toxicol. 1999;19(3):173–83. doi: 10.1002/(sici)1099-1263(199905/06)19:3<173::aid-jat556>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366(1–2):1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Worek F, Koller M, Thiermann H, Szinicz L. Diagnostic aspects of organophosphate poisoning. Toxicology. 2005;214(3):182–9. doi: 10.1016/j.tox.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Peter JV, Moran JL, Graham PL. Advances in the management of organophosphate poisoning. Expert Opin Pharmacother. 2007;8(10):1451–64. doi: 10.1517/14656566.8.10.1451. [DOI] [PubMed] [Google Scholar]
- 40.Costa LG, Giordano G, Guizzetti M, Vitalone A. Neurotoxicity of pesticides: a brief review. Front Biosci. 2008;13:1240–9. doi: 10.2741/2758. [DOI] [PubMed] [Google Scholar]
- 41.Weinbroum AA. Pathophysiological and clinical aspects of combat anticholinesterase poisoning. Br Med Bull. 2004;72:119–33. doi: 10.1093/bmb/ldh038. [DOI] [PubMed] [Google Scholar]
- 42.Newmark J. Nerve agents. Neurologist. 2007;13(1):20–32. doi: 10.1097/01.nrl.0000252923.04894.53. [DOI] [PubMed] [Google Scholar]
- 43.Lawrence DT, Kirk MA. Chemical terrorism attacks: update on antidotes. Emerg Med Clin North Am. 2007;25(2):567–95. abstract xi. doi: 10.1016/j.emc.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Karr CJ, Solomon GM, Brock-Utne AC. Health effects of common home, lawn, and garden pesticides. Pediatr Clin North Am. 2007;54(1):63–80. viii. doi: 10.1016/j.pcl.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Jaga K, Dharmani C. Sources of exposure to and public health implications of organophosphate pesticides. Rev Panam Salud Publica. 2003;14(3):171–85. doi: 10.1590/s1020-49892003000800004. [DOI] [PubMed] [Google Scholar]
- 46.Costa LG, Giordano G, Guizzetti M, Vitalone A. Neurotoxicity of pesticides: a brief review. Front Biosci. 2008;13:1240–9. doi: 10.2741/2758. [DOI] [PubMed] [Google Scholar]
- 47.Weinbroum AA. Pathophysiological and clinical aspects of combat anticholinesterase poisoning. Br Med Bull. 2004;72:119–33. doi: 10.1093/bmb/ldh038. [DOI] [PubMed] [Google Scholar]
- 48.Munro NB, Watson AP, Ambrose KR, Griffin GD. Treating exposure to chemical warfare agents: implications for health care providers and community emergency planning. Environ Health Perspect. 1990;89:205–15. doi: 10.1289/ehp.9089205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peter JV, Moran JL, Graham PL. Advances in the management of organophosphate poisoning. Expert Opin Pharmacother. 2007;8:1451–64. doi: 10.1517/14656566.8.10.1451. [DOI] [PubMed] [Google Scholar]
- 50.Albuquerque EX, Pereira EF, Aracava Y, Fawcett WP, Oliveira M, Randall WR, et al. Effective countermeasure against poisoning by organophosphorus insecticides and nerve agents. Proc Natl Acad Sci USA. 2006;103:13220–5. doi: 10.1073/pnas.0605370103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Newmark J. Therapy for nerve agent poisoning. Arch Neurol. 2004;61:649–52. doi: 10.1001/archneur.61.5.649. [DOI] [PubMed] [Google Scholar]
- 52.Behra M, Cousin X, Bertrand C, Vonesch J, Biellmann D, Chatonnet A, et al. Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo. Nat Neurosci. 2005;5(2):111–8. doi: 10.1038/nn788. [DOI] [PubMed] [Google Scholar]
- 53.Kuester E, Altenburger R. Comparison of cholin- and carboxylesterase enzyme inhibition and visible effects in the zebrafish embryo bioassay under short-term paraoxon-methyl exposure. Biomarkers. 2006;11(4):341–54. doi: 10.1080/13547500600742136. [DOI] [PubMed] [Google Scholar]
- 54.Jin S, Sarkar KS, Jin YN, Liu Y, Kokel D, Van Ham TJ, et al. An in vivo zebrafish screen identifies organophosphate antidotes with diverse mechanisms of action. Journal of biomolecular screening. 2013;18(1):108–15. doi: 10.1177/1087057112458153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strahle U, Grabher C. The zebrafish embryo as a model for assessing off-target drug effects. Dis Model Mech. 2010;3(11–12):689–92. doi: 10.1242/dmm.006312. [DOI] [PubMed] [Google Scholar]
- 56.Peterson RT, Macrae CA. Systematic Approaches to Toxicology in the Zebrafish. Annu Rev Pharmacol Toxicol. 2011 doi: 10.1146/annurev-pharmtox-010611-134751. [DOI] [PubMed] [Google Scholar]
- 57.Ung CY, Lam SH, Hlaing MM, Winata CL, Korzh S, Mathavan S, et al. Mercury-induced hepatotoxicity in zebrafish: in vivo mechanistic insights from transcriptome analysis, phenotype anchoring and targeted gene expression validation. BMC Genomics. 2010;11:212. doi: 10.1186/1471-2164-11-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sukardi H, Chng HT, Chan EC, Gong Z, Lam SH. Zebrafish for drug toxicity screening: bridging the in vitro cell-based models and in vivo mammalian models. Expert Opin Drug Metab Toxicol. 2011;7(5):579–89. doi: 10.1517/17425255.2011.562197. [DOI] [PubMed] [Google Scholar]
- 59.Jett DA. Finding new cures for neurological disorders: a possible fringe benefit of biodefense research? Sci Transl Med. 2010;2(23):23ps12. doi: 10.1126/scitranslmed.3000752. [DOI] [PubMed] [Google Scholar]
- 60.Laggner C, Kokel D, Setola V, Tolia A, Lin H, Irwin JJ, et al. Chemical informatics and target identification in a zebrafish phenotypic screen. Nature chemical biology. 2012;8(2):144–6. doi: 10.1038/nchembio.732. [DOI] [PMC free article] [PubMed] [Google Scholar]


