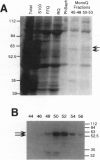
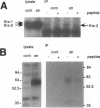
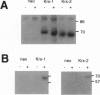
Abstract
The activation of protein kinases is a frequent response of cells to treatment with growth factors, chemicals, heat shock, or apoptosis-inducing agents. However, when several agents result in the activation of the same enzymes, it is unclear how specific biological responses are generated. We describe here two protein kinases that are activated by a subset of stress conditions or apoptotic agents but are not activated by commonly used mitogenic stimuli. Purification and cloning demonstrate that these protein kinases are members of a subfamily of kinases related to Ste20p, a serine/threonine kinase that functions early in a pheromone responsive signal transduction cascade in yeast. The specificity of Krs-1 and Krs-2 activation and their similarity to Ste20p suggest that they may function at an early step in phosphorylation events that are specific responses to some forms of chemical stress or extreme heat shock.
Full text
PDF

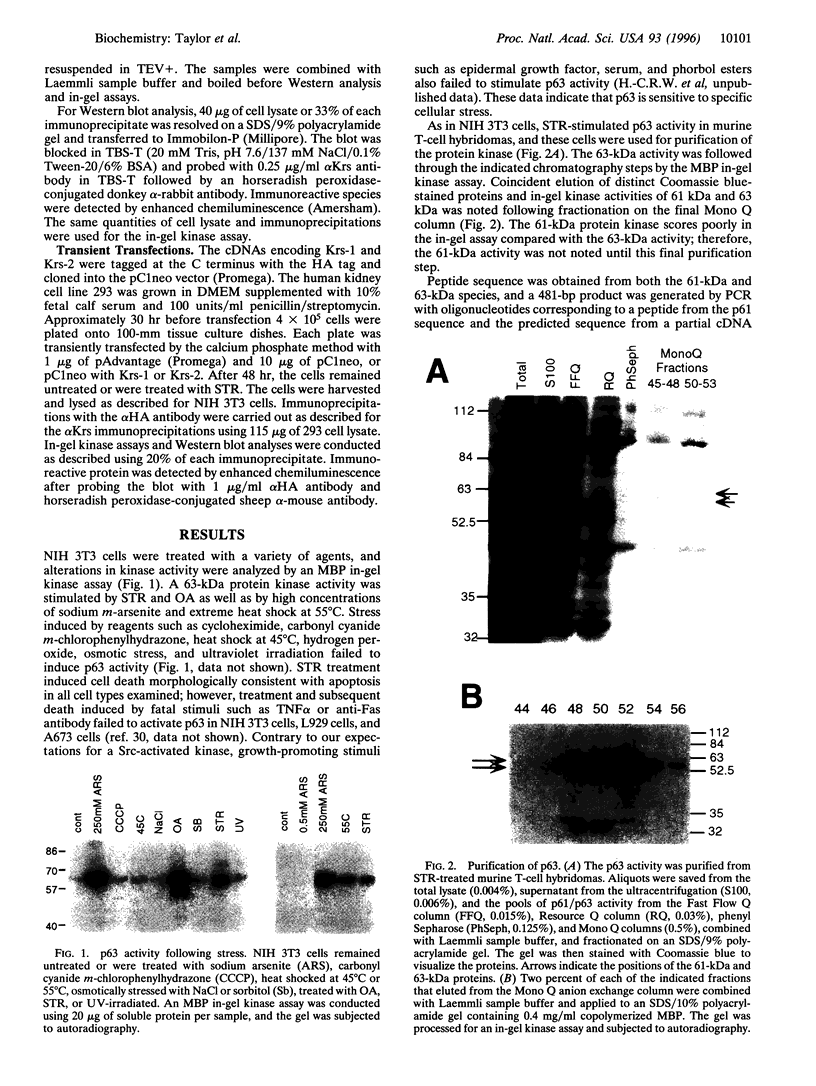


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N. G., Maller J. L., Tonks N. K., Sturgill T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990 Feb 15;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chenevert J., Corrado K., Bender A., Pringle J., Herskowitz I. A yeast gene (BEM1) necessary for cell polarization whose product contains two SH3 domains. Nature. 1992 Mar 5;356(6364):77–79. doi: 10.1038/356077a0. [DOI] [PubMed] [Google Scholar]
- Creasy C. L., Chernoff J. Cloning and characterization of a human protein kinase with homology to Ste20. J Biol Chem. 1995 Sep 15;270(37):21695–21700. doi: 10.1074/jbc.270.37.21695. [DOI] [PubMed] [Google Scholar]
- Creasy C. L., Chernoff J. Cloning and characterization of a member of the MST subfamily of Ste20-like kinases. Gene. 1995 Dec 29;167(1-2):303–306. doi: 10.1016/0378-1119(95)00653-2. [DOI] [PubMed] [Google Scholar]
- Cvrcková F., De Virgilio C., Manser E., Pringle J. R., Nasmyth K. Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev. 1995 Aug 1;9(15):1817–1830. doi: 10.1101/gad.9.15.1817. [DOI] [PubMed] [Google Scholar]
- Davis R. J. MAPKs: new JNK expands the group. Trends Biochem Sci. 1994 Nov;19(11):470–473. doi: 10.1016/0968-0004(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dérijard B., Hibi M., Wu I. H., Barrett T., Su B., Deng T., Karin M., Davis R. J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994 Mar 25;76(6):1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Dérijard B., Raingeaud J., Barrett T., Wu I. H., Han J., Ulevitch R. J., Davis R. J. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995 Feb 3;267(5198):682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- Friesen H., Lunz R., Doyle S., Segall J. Mutation of the SPS1-encoded protein kinase of Saccharomyces cerevisiae leads to defects in transcription and morphology during spore formation. Genes Dev. 1994 Sep 15;8(18):2162–2175. doi: 10.1101/gad.8.18.2162. [DOI] [PubMed] [Google Scholar]
- Galcheva-Gargova Z., Dérijard B., Wu I. H., Davis R. J. An osmosensing signal transduction pathway in mammalian cells. Science. 1994 Aug 5;265(5173):806–808. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- Han J., Lee J. D., Bibbs L., Ulevitch R. J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994 Aug 5;265(5173):808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Harden N., Lee J., Loh H. Y., Ong Y. M., Tan I., Leung T., Manser E., Lim L. A Drosophila homolog of the Rac- and Cdc42-activated serine/threonine kinase PAK is a potential focal adhesion and focal complex protein that colocalizes with dynamic actin structures. Mol Cell Biol. 1996 May;16(5):1896–1908. doi: 10.1128/mcb.16.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995 Jan 27;80(2):187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- Hibi M., Lin A., Smeal T., Minden A., Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993 Nov;7(11):2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- Kameshita I., Fujisawa H. A sensitive method for detection of calmodulin-dependent protein kinase II activity in sodium dodecyl sulfate-polyacrylamide gel. Anal Biochem. 1989 Nov 15;183(1):139–143. doi: 10.1016/0003-2697(89)90181-4. [DOI] [PubMed] [Google Scholar]
- Katz P., Whalen G., Kehrl J. H. Differential expression of a novel protein kinase in human B lymphocytes. Preferential localization in the germinal center. J Biol Chem. 1994 Jun 17;269(24):16802–16809. [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis J. M., Banerjee P., Nikolakaki E., Dai T., Rubie E. A., Ahmad M. F., Avruch J., Woodgett J. R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994 May 12;369(6476):156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- L'Allemain G. Deciphering the MAP kinase pathway. Prog Growth Factor Res. 1994;5(3):291–334. doi: 10.1016/0955-2235(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane W. S., Galat A., Harding M. W., Schreiber S. L. Complete amino acid sequence of the FK506 and rapamycin binding protein, FKBP, isolated from calf thymus. J Protein Chem. 1991 Apr;10(2):151–160. doi: 10.1007/BF01024778. [DOI] [PubMed] [Google Scholar]
- Leberer E., Dignard D., Harcus D., Thomas D. Y., Whiteway M. The protein kinase homologue Ste20p is required to link the yeast pheromone response G-protein beta gamma subunits to downstream signalling components. EMBO J. 1992 Dec;11(13):4815–4824. doi: 10.1002/j.1460-2075.1992.tb05587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeuw T., Fourest-Lieuvin A., Wu C., Chenevert J., Clark K., Whiteway M., Thomas D. Y., Leberer E. Pheromone response in yeast: association of Bem1p with proteins of the MAP kinase cascade and actin. Science. 1995 Nov 17;270(5239):1210–1213. doi: 10.1126/science.270.5239.1210. [DOI] [PubMed] [Google Scholar]
- Lu X., Xie W., Reed D., Bradshaw W. S., Simmons D. L. Nonsteroidal antiinflammatory drugs cause apoptosis and induce cyclooxygenases in chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7961–7965. doi: 10.1073/pnas.92.17.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E., Leung T., Salihuddin H., Zhao Z. S., Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994 Jan 6;367(6458):40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995 Jan 27;80(2):179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Musacchio A., Gibson T., Rice P., Thompson J., Saraste M. The PH domain: a common piece in the structural patchwork of signalling proteins. Trends Biochem Sci. 1993 Sep;18(9):343–348. doi: 10.1016/0968-0004(93)90071-t. [DOI] [PubMed] [Google Scholar]
- Nobes C. D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995 Apr 7;81(1):53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Ottilie S., Miller P. J., Johnson D. I., Creasy C. L., Sells M. A., Bagrodia S., Forsburg S. L., Chernoff J. Fission yeast pak1+ encodes a protein kinase that interacts with Cdc42p and is involved in the control of cell polarity and mating. EMBO J. 1995 Dec 1;14(23):5908–5919. doi: 10.1002/j.1460-2075.1995.tb00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pombo C. M., Kehrl J. H., Sánchez I., Katz P., Avruch J., Zon L. I., Woodgett J. R., Force T., Kyriakis J. M. Activation of the SAPK pathway by the human STE20 homologue germinal centre kinase. Nature. 1995 Oct 26;377(6551):750–754. doi: 10.1038/377750a0. [DOI] [PubMed] [Google Scholar]
- Raingeaud J., Whitmarsh A. J., Barrett T., Dérijard B., Davis R. J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996 Mar;16(3):1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer S. W., Davis R. W. A dominant truncation allele identifies a gene, STE20, that encodes a putative protein kinase necessary for mating in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):452–456. doi: 10.1073/pnas.90.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M., Zeng J., De Lemos-Chiarandini C., Rosenfeld M., Adesnik M., Sabatini D. D. In its active form, the GTP-binding protein rab8 interacts with a stress-activated protein kinase. Proc Natl Acad Sci U S A. 1996 May 14;93(10):5151–5155. doi: 10.1073/pnas.93.10.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992 Aug 7;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rouse J., Cohen P., Trigon S., Morange M., Alonso-Llamazares A., Zamanillo D., Hunt T., Nebreda A. R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994 Sep 23;78(6):1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Schultz S. J., Nigg E. A. Identification of 21 novel human protein kinases, including 3 members of a family related to the cell cycle regulator nimA of Aspergillus nidulans. Cell Growth Differ. 1993 Oct;4(10):821–830. [PubMed] [Google Scholar]
- Simon M. N., De Virgilio C., Souza B., Pringle J. R., Abo A., Reed S. I. Role for the Rho-family GTPase Cdc42 in yeast mating-pheromone signal pathway. Nature. 1995 Aug 24;376(6542):702–705. doi: 10.1038/376702a0. [DOI] [PubMed] [Google Scholar]
- Sánchez I., Hughes R. T., Mayer B. J., Yee K., Woodgett J. R., Avruch J., Kyriakis J. M., Zon L. I. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994 Dec 22;372(6508):794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- Wang H. C., Erikson R. L. Activation of protein serine/threonine kinases p42, p63, and p87 in Rous sarcoma virus-transformed cells: signal transduction/transformation-dependent MBP kinases. Mol Biol Cell. 1992 Dec;3(12):1329–1337. doi: 10.1091/mbc.3.12.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z. S., Leung T., Manser E., Lim L. Pheromone signalling in Saccharomyces cerevisiae requires the small GTP-binding protein Cdc42p and its activator CDC24. Mol Cell Biol. 1995 Oct;15(10):5246–5257. doi: 10.1128/mcb.15.10.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Geer P., Hunter T., Lindberg R. A. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu Rev Cell Biol. 1994;10:251–337. doi: 10.1146/annurev.cb.10.110194.001343. [DOI] [PubMed] [Google Scholar]