Abstract
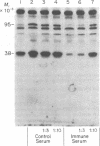
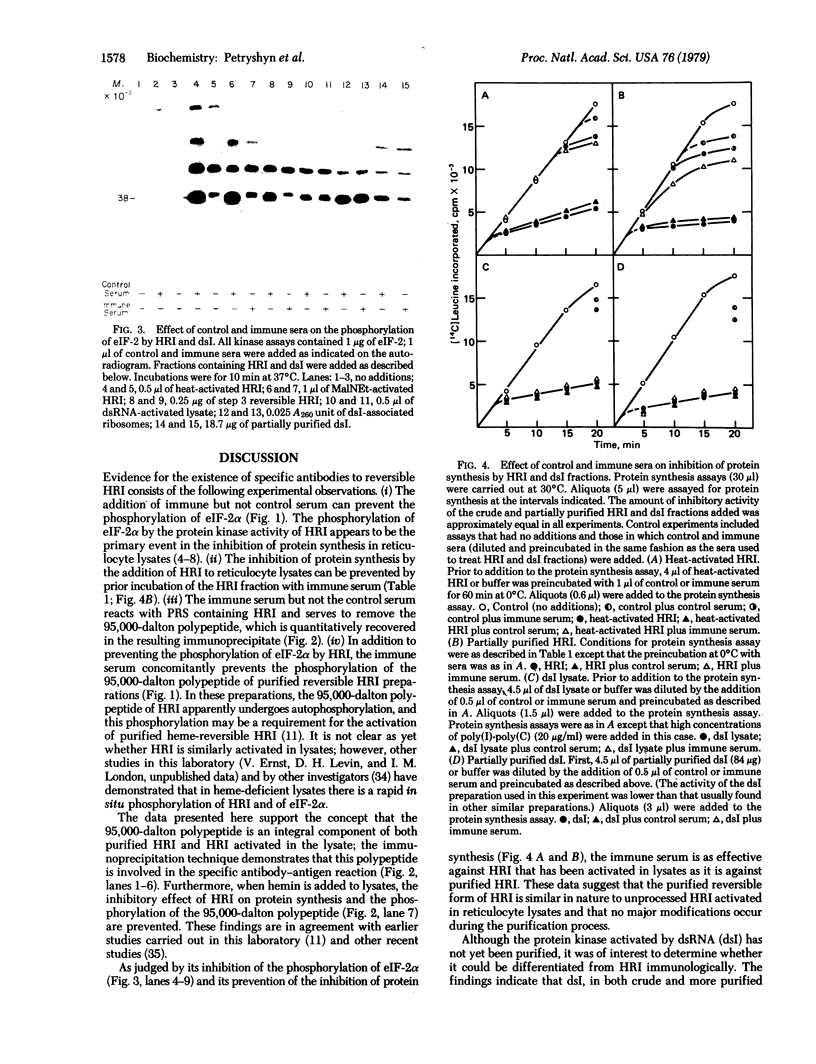
A specific immune serum to the heme-regulated inhibitor (HRI) has been prepared by immunizing chickens with highly purified reversible HRI prepared from rabbit reticulocyte lysates. Studies with this immune serum demonstrate that the behavior of purified reversible HRI is similar to that of the inhibitor activated in rabbit reticulocyte lysates: the immune serum (i) inhibits the phosphorylation of the small subunit (38,000 daltons) of the eukaryotic initiation factor eIF-2 by both crude and purified inhibitor preparations; (ii) prevents the concomitant inhibition of protein synthesis by both crude and purified inhibitor preparations; and (iii) prevents the autophosphorylation of the 95,000-dalton polypeptide in purified and crude HRI preparations. The protein kinase and inhibitory activities of crude and partially purified preparations of the double-stranded RNA-induced inhibitor of protein synthesis are not affected by the immune serum prepared to reversible HRI. These results indicate that the inhibitor induced by double-stranded RNA is antigenically distinct from the reversible HRI.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson S. D., Herbert E., Godchaux W. Factors affecting the rate of protein synthesis in lysate systems from reticulocytes. Arch Biochem Biophys. 1968 May;125(2):671–683. doi: 10.1016/0003-9861(68)90625-5. [DOI] [PubMed] [Google Scholar]
- Adamson S. D., Herbert E., Kemp S. F. Effects of hemin and other porphyrins on protein synthesis in a reticulocyte lysate cell-free system. J Mol Biol. 1969 Jun 14;42(2):247–258. doi: 10.1016/0022-2836(69)90041-2. [DOI] [PubMed] [Google Scholar]
- Anderson W. F., Bosch L., Cohn W. E., Lodish H., Merrick W. C., Weissbach H., Wittmann H. G., Wool I. G. International symposium on protein synthesis. Summary of Fogarty Center-NIH Workshop held in Bethesda, Maryland on 18-20 October, 1976. FEBS Lett. 1977 Apr 1;76(1):1–10. doi: 10.1016/0014-5793(77)80109-9. [DOI] [PubMed] [Google Scholar]
- BRUNS G. P., LONDON I. M. THE EFFECT OF HEMIN ON THE SYNTHESIS OF GLOBIN. Biochem Biophys Res Commun. 1965 Jan 18;18:236–242. doi: 10.1016/0006-291x(65)90746-1. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Henshaw E. C., Rahamimoff H., London I. M. Met-tRNAfMet binding to 40S ribosomal subunits: a site for the regulation of initiation of protein synthesis by hemin. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2946–2950. doi: 10.1073/pnas.71.8.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M. J., Safer B., Merrick W. C., Anderson W. F., London I. M. Inhibition of protein synthesis in rabbit reticulocyte lysates by double-stranded RNA and oxidized glutathione: indirect mode of action on polypeptide chain initiation. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1286–1290. doi: 10.1073/pnas.72.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnbrough C., Legon S., Hunt T., Jackson R. J. Initiation of protein synthesis: evidence for messenger RNA-independent binding of methionyl-transfer RNA to the 40 S ribosomal subunit. J Mol Biol. 1973 May 25;76(3):379–403. doi: 10.1016/0022-2836(73)90511-1. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E., Hunt T. Double-stranded poliovirus RNA inhibits initiation of protein synthesis by reticulocyte lysates. Proc Natl Acad Sci U S A. 1971 May;68(5):1075–1078. doi: 10.1073/pnas.68.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst V., Levin D. H., London I. M. Evidence that glucose 6-phosphate regulates protein synthesis initiation in reticulocyte lysates. J Biol Chem. 1978 Oct 25;253(20):7163–7172. [PubMed] [Google Scholar]
- Ernst V., Levin D. H., Ranu R. S., London I. M. Control of protein synthesis in reticulocyte lysates: effects of 3':5'-cyclic AMP, ATP, and GTP on inhibitions induced by hemedeficiency, double-stranded RNA, and a reticulocyte translationa inhibitor. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1112–1116. doi: 10.1073/pnas.73.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Hunt T., Jackson R. J. Analysis of phosphorylation of protein synthesis initiation factor eIF-2 by two-dimensional gel electrophoresis. Eur J Biochem. 1978 Sep 1;89(2):517–521. doi: 10.1111/j.1432-1033.1978.tb12556.x. [DOI] [PubMed] [Google Scholar]
- Gross M. Isolation and characterization of an antibody to a highly purified preparation of the hemin-controlled translational repressor from rabbit reticulocytes. Biochem Biophys Res Commun. 1974 Apr 8;57(3):611–619. doi: 10.1016/0006-291x(74)90590-7. [DOI] [PubMed] [Google Scholar]
- Gross M., Mendelewski J. Additional evidence that the hemin-controlled translational repressor from rabbit reticulocytes is a protein kinase. Biochem Biophys Res Commun. 1977 Jan 24;74(2):559–569. doi: 10.1016/0006-291x(77)90340-0. [DOI] [PubMed] [Google Scholar]
- Gross M., Mendelewski J. Control of protein synthesis by hemin. An association between the formation of the hemin-controlled translational repressor and the phosphorylation of a 100 000 molecular weight protein. Biochim Biophys Acta. 1978 Oct 24;520(3):650–663. doi: 10.1016/0005-2787(78)90150-8. [DOI] [PubMed] [Google Scholar]
- Gross M., Rabinovitz M. Control of globin synthesis by hemin: factors influencing formation of an inhibitor of globin chain initiation in reticulocyte lysates. Biochim Biophys Acta. 1972 Dec 6;287(2):340–352. doi: 10.1016/0005-2787(72)90383-8. [DOI] [PubMed] [Google Scholar]
- Gross M., Rabinovitz M. Partial purification of a translational repressor mediating hemin control of globin synthesis and implication of results on the site of inhibition. Biochem Biophys Res Commun. 1973 Feb 5;50(3):832–838. doi: 10.1016/0006-291x(73)91320-x. [DOI] [PubMed] [Google Scholar]
- Hunt T., Ehrenfeld E. Cytoplasm from poliovirus-infected HeLa cells inhibits cell-free haemoglobin synthesis. Nat New Biol. 1971 Mar 17;230(11):91–94. doi: 10.1038/newbio230091a0. [DOI] [PubMed] [Google Scholar]
- Hunt T., Vanderhoff G., London I. M. Control of globin synthesis: the role of heme. J Mol Biol. 1972 May 28;66(3):471–481. doi: 10.1016/0022-2836(72)90427-5. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. The use of hexose phosphates to support protein synthesis and generate [gamma-32P]ATP in reticulocyte lysates. FEBS Lett. 1978 Sep 15;93(2):235–238. doi: 10.1016/0014-5793(78)81111-9. [DOI] [PubMed] [Google Scholar]
- Kramer G., Cimadevilla J. M., Hardesty B. Specificity of the protein kinase activity associated with the hemin-controlled repressor of rabbit reticulocyte. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3078–3082. doi: 10.1073/pnas.73.9.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebleu B., Sen G. C., Shaila S., Cabrer B., Lengyel P. Interferon, double-stranded RNA, and protein phosphorylation. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3107–3111. doi: 10.1073/pnas.73.9.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon S., Jackson R. J., Hunt T. Control of protein synthesis in reticulocyte lysates by haemin. Nat New Biol. 1973 Jan 31;241(109):150–152. doi: 10.1038/newbio241150a0. [DOI] [PubMed] [Google Scholar]
- Levin D., London I. M. Regulation of protein synthesis: activation by double-stranded RNA of a protein kinase that phosphorylates eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1121–1125. doi: 10.1073/pnas.75.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D., Ranu R. S., Ernst V., London I. M. Regulation of protein synthesis in reticulocyte lysates: phosphorylation of methionyl-tRNAf binding factor by protein kinase activity of translational inhibitor isolated from hemedeficient lysates. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3112–3116. doi: 10.1073/pnas.73.9.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell C. R., Kamper C. S., Rabinovitz M. Hemin control of globin synthesis: an assay for the inhibitor formed in the absence of hemin and some characteristics of its formation. J Mol Biol. 1971 May 28;58(1):317–327. doi: 10.1016/0022-2836(71)90249-x. [DOI] [PubMed] [Google Scholar]
- Ranu R. S., Levin D. H., Delaunay J., Ernst V., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: characteristics of inhibition of protein synthesis by a translational inhibitor from heme-deficient lysates and its relationship to the initiation factor which binds Met-tRNAf. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2720–2724. doi: 10.1073/pnas.73.8.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: purification and initial characterization of the cyclic 3':5'-AMP independent protein kinase of the heme-regulated translational inhibitor. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4349–4353. doi: 10.1073/pnas.73.12.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. K., Hovanessian A., Brown R. E., Clemens M. J., Kerr I. M. Interferon-mediated protein kinase and low-molecular-weight inhibitor of protein synthesis. Nature. 1976 Dec 2;264(5585):477–480. doi: 10.1038/264477a0. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Taira H., Lengyel P. Interferon, double-stranded RNA, and protein phosphorylation. Characteristics of a double-stranded RNA-activated protein kinase system partially purified from interferon treated Ehrlich ascites tumor cells. J Biol Chem. 1978 Sep 10;253(17):5915–5921. [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Trachsel H., Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: purification and characterization of heme-reversible translational inhibitor. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3654–3658. doi: 10.1073/pnas.75.8.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein A., Federman P., Shulman L., Revel M. Specific phosphorylation in vitro of a protein associated with ribosomes of interferon-treated mouse L cells. FEBS Lett. 1976 Sep 15;68(1):119–124. doi: 10.1016/0014-5793(76)80418-8. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Kimchi A., Schmidt A., Revel M. Isolation of two interferon-induced translational inhibitors: a protein kinase and an oligo-isoadenylate synthetase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4734–4738. doi: 10.1073/pnas.75.10.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker W. V., Schulman H. M. Stimulation of globin-chain initiation by hemin in the reticulocyte cell-free system. Proc Natl Acad Sci U S A. 1968 Feb;59(2):582–589. doi: 10.1073/pnas.59.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]