Abstract
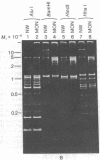
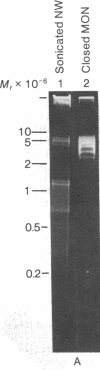
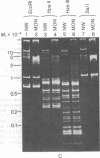
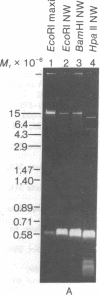
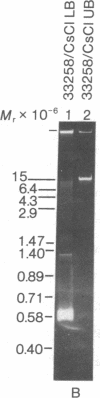
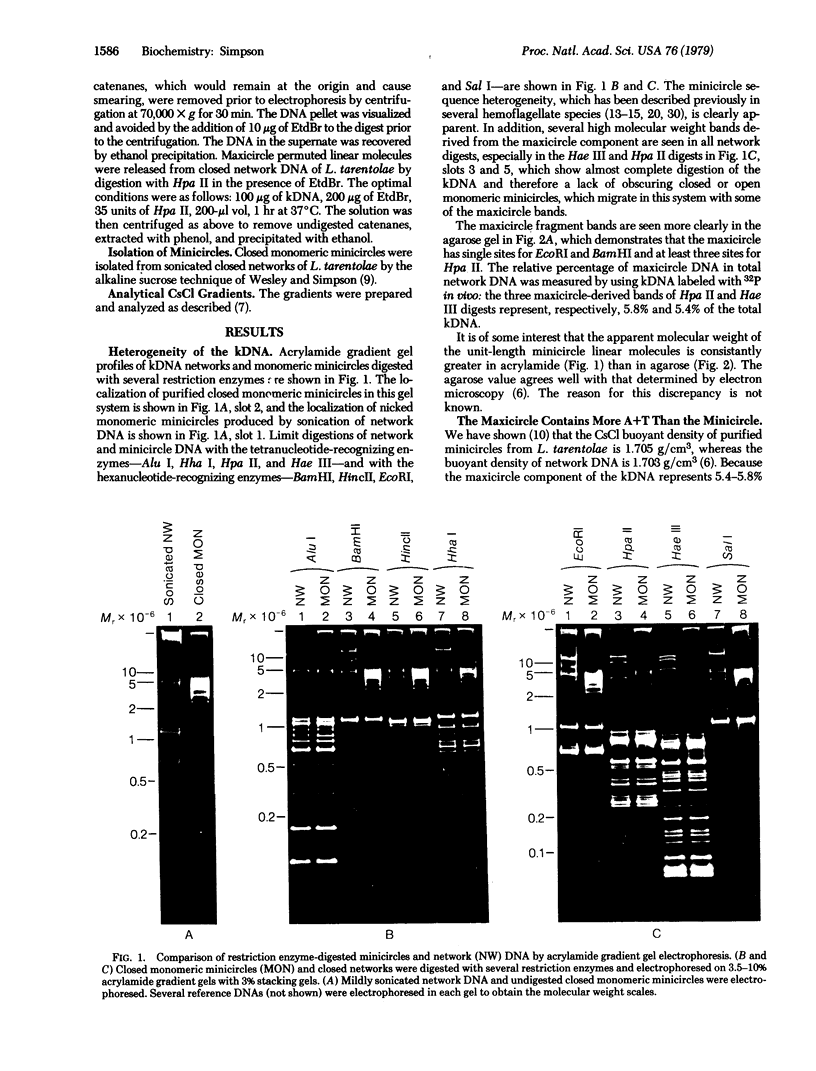
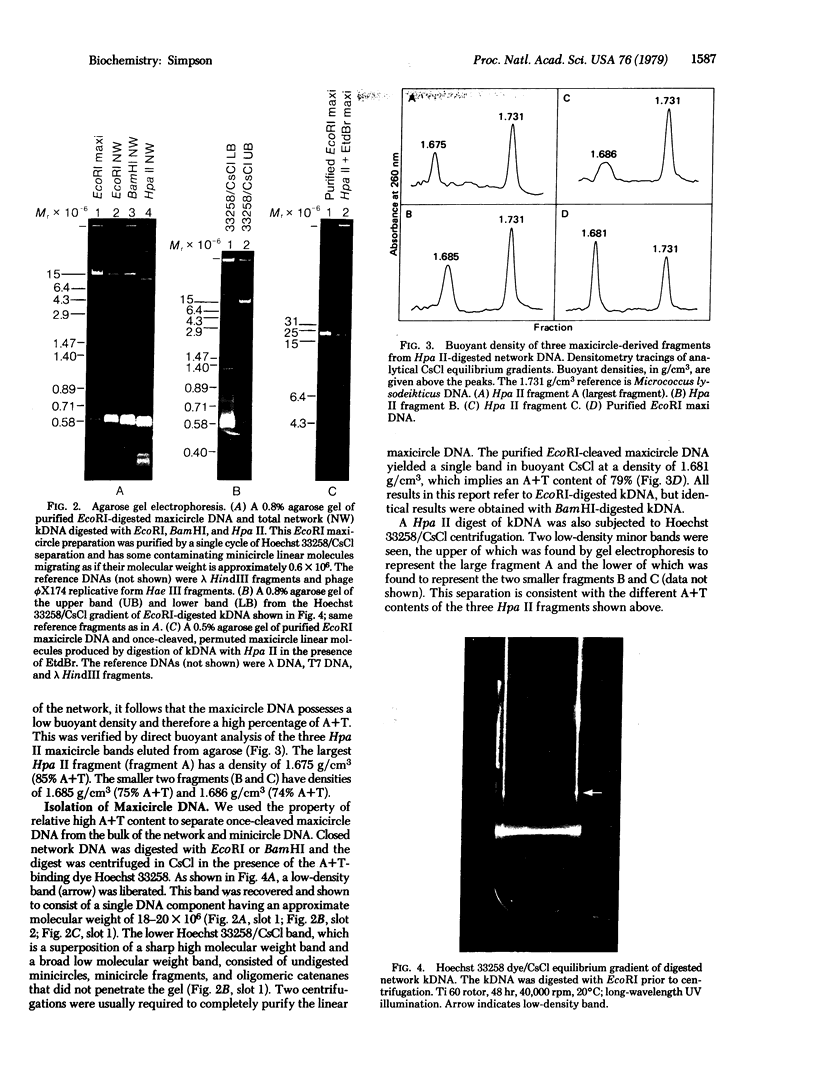
Maxicircle DNA has been isolated from the kinetoplast DNA of Leishmania tarentolae culture forms by bouyant separation in CsCl in the presence of the A + T-binding dye Hoechst 33258, after liberation from the kinetoplast DNA network by cleavage with the single-hit restriction endonuclease EcoRI. The purified linearized maxicircle DNA has a density in CsCl of 1.681 g/cm3 (79% A + T) and a molecular weight of approximately 18-20 x 10(6). The maxicircle molecule exhibited intramolecular base composition heterogeneity ranging from 85% A + T to 74% A + T.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borst P., Fase-Fowler F., Steinert M., Van Assel S. Maxi-circles in the kinetoplast DNA of Trypanosoma mega. Exp Cell Res. 1977 Nov;110(1):167–173. doi: 10.1016/0014-4827(77)90283-x. [DOI] [PubMed] [Google Scholar]
- Brodie S., Giron J., Latt S. A. Estimation of accessibility of DNA in chromatin from fluorescence measurements of electronic excitation energy transfer. Nature. 1975 Feb 6;253(5491):470–471. doi: 10.1038/253470a0. [DOI] [PubMed] [Google Scholar]
- Brunk C. F., Simpson L. Comparison of various ultraviolet sources for fluorescent detection of ethidium bromide-DNA complexes in polyacrylamide gels. Anal Biochem. 1977 Oct;82(2):455–462. doi: 10.1016/0003-2697(77)90183-x. [DOI] [PubMed] [Google Scholar]
- Cheng D., Simpson L. Isolation and characterization of kinetoplast DNA and RNA of Phytomonas davidi. Plasmid. 1978 Jun;1(3):297–315. doi: 10.1016/0147-619x(78)90047-1. [DOI] [PubMed] [Google Scholar]
- Fairlamb A. H., Weislogel P. O., Hoeijmakers J. H., Borst P. Isolation and characterization of kinetoplast DNA from bloodstream form of Trypanosoma brucei. J Cell Biol. 1978 Feb;76(2):293–309. doi: 10.1083/jcb.76.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennewald M., Prevatt W., Meyer R., Shapiro J. Isolation of inc P-2 plasmid DNA from Pseudomonas aeruginosa. Plasmid. 1978 Feb;1(2):164–173. doi: 10.1016/0147-619x(78)90036-7. [DOI] [PubMed] [Google Scholar]
- Kleisen C. M., Borst P. Sequence heterogeneity of the mini-circles of kinetoplast DNA of Crithidia luciliae and evidence for the presence of a component more complex than mini-circle DNA in the kinetoplast network. Biochim Biophys Acta. 1975 Nov 4;407(4):473–478. doi: 10.1016/0005-2787(75)90301-9. [DOI] [PubMed] [Google Scholar]
- Kleisen C. M., Borst P., Weijers P. J. The structure of kinetoplast DNA. I. Properties of the intact multi-circular complex from Crithidia luciliae. Biochim Biophys Acta. 1975 May 1;390(2):155–167. [PubMed] [Google Scholar]
- Kleisen C. M., Weislogel P. O., Fonck K., Borst P. The structure of kinetoplast DNA. 2. Characterization of a novel component of high complexity present in the kinetoplast DNA network of Crithidia luciliae. Eur J Biochem. 1976 Apr 15;64(1):153–160. doi: 10.1111/j.1432-1033.1976.tb10283.x. [DOI] [PubMed] [Google Scholar]
- Kleisen M. C., Borst P., Weijers P. J. The structure of kinetoplast DNA. 1. The mini-circles of Crithidia lucilae are heterogeneous in base sequence. Eur J Biochem. 1976 Apr 15;64(1):141–151. doi: 10.1111/j.1432-1033.1976.tb10282.x. [DOI] [PubMed] [Google Scholar]
- Latt S. A. Microfluorometric detection of deoxyribonucleic acid replication in human metaphase chromosomes. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3395–3399. doi: 10.1073/pnas.70.12.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L. A simplified method for preparation of mouse satellite DNA. Anal Biochem. 1977 Apr;78(2):561–568. doi: 10.1016/0003-2697(77)90118-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Murray K., Murray N. E. Phage lambda receptor chromosomes for DNA fragments made with restriction endonuclease III of Haemophilus influenzae and restriction endonuclease I of Escherichia coli. J Mol Biol. 1975 Nov 5;98(3):551–564. doi: 10.1016/s0022-2836(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Parker R. C., Watson R. M., Vinograd J. Mapping of closed circular DNAs by cleavage with restriction endonucleases and calibration by agarose gel electrophoresis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):851–855. doi: 10.1073/pnas.74.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou G. F., Yot P. Heterogeneity of the kinetoplast DNA molecules of Trypanosoma cruzi. Biochemistry. 1977 May 31;16(11):2390–2396. doi: 10.1021/bi00630a013. [DOI] [PubMed] [Google Scholar]
- Riou G., Yot P. Etude de l'Adn kinétoplastique de Trypansoma cruzi à l'aide d'endonucléases de restriction. C R Acad Sci Hebd Seances Acad Sci D. 1975 Jun 16;280(23):2701–2704. [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Simpson A. M., Simpson L. Isolation and characterization of kinetoplast DNA networks and minicircles from Crithidia fasciculata. J Protozool. 1974 Nov;21(5):774–781. doi: 10.1111/j.1550-7408.1974.tb03751.x. [DOI] [PubMed] [Google Scholar]
- Simpson L., Berliner J. Isolation of the kinetoplast DNA of Leishmania tarentolae in the form of a network. J Protozool. 1974 May;21(2):382–393. doi: 10.1111/j.1550-7408.1974.tb03675.x. [DOI] [PubMed] [Google Scholar]
- Simpson L., Braly P. Synchronization of Leishmania tarentolae by hydroxyurea. J Protozool. 1970 Nov;17(4):511–517. doi: 10.1111/j.1550-7408.1970.tb04719.x. [DOI] [PubMed] [Google Scholar]
- Simpson L., Da Silva A. Isolation and characterization of kinetoplast DNA from Leishmania tarentolae. J Mol Biol. 1971 Mar 28;56(3):443–473. doi: 10.1016/0022-2836(71)90394-9. [DOI] [PubMed] [Google Scholar]
- Steinert M., Assel S. Large circular mitochondrial DNA in Crithidia luciliae. Exp Cell Res. 1975 Dec;96(2):406–409. doi: 10.1016/0014-4827(75)90274-8. [DOI] [PubMed] [Google Scholar]
- Weislogel P. O., Hoeijmakers J. H., Fairlamb A. H., Kleisen C. M., Borst P. Characterization of kinetoplast DNA networks from the insect trypanosome Crithidia luciliae. Biochim Biophys Acta. 1977 Sep 20;478(2):167–179. doi: 10.1016/0005-2787(77)90180-0. [DOI] [PubMed] [Google Scholar]
- Wesley R. D., Simpson L. Studies on kinetoplast DNA. 3. Kinetic complexity of kinetoplast and nuclear DNA from Leishmania tarentolae. Biochim Biophys Acta. 1973 Sep 7;319(3):267–276. doi: 10.1016/0005-2787(73)90165-2. [DOI] [PubMed] [Google Scholar]
- Wesley R. D., Simpson L. Studies on kinetoplast DNA. I. Isolation of kinetoplast DNA minicircles from Leishmania tarentolae. Biochim Biophys Acta. 1973 Sep 7;319(3):237–253. doi: 10.1016/0005-2787(73)90163-9. [DOI] [PubMed] [Google Scholar]
- Wesley R. D., Simpson L. Studies on kinetoplast DNA. II. Biophysical properties of minicircular DNA from Leishmania tarentolae. Biochim Biophys Acta. 1973 Sep 7;319(3):254–266. doi: 10.1016/0005-2787(73)90164-0. [DOI] [PubMed] [Google Scholar]