Abstract
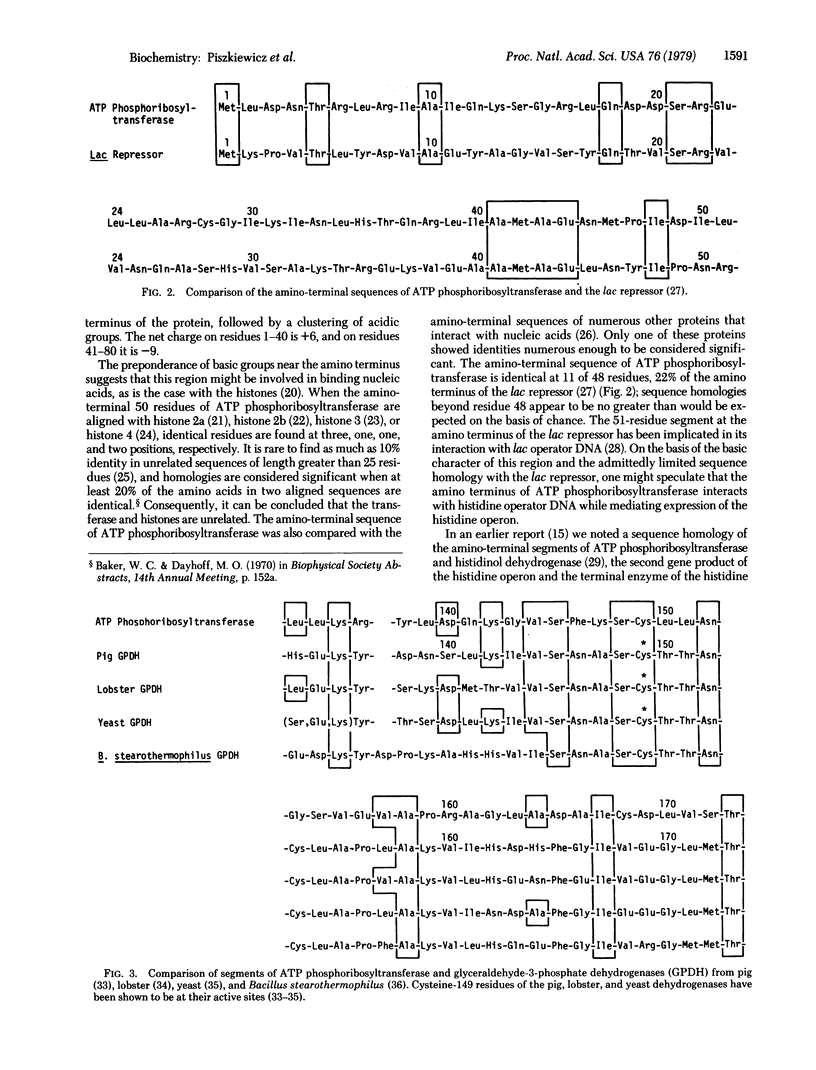
The amino acid sequence of ATP phosphoribosyltransferase [1-(5'-phosphoribosyl)-ATP:pyrophosphate phosphoribosyltransferase, EC 2.4.2.17] of Salmonella typhimurium has been determined. The amino acid sequence analysis was carried out with a combination of manual and automated methods. It was complemented by DNA sequence analysis (done in another laboratory) of the hisG gene, which codes for it. The subunit polypeptide chain contains 299 amino acid residues and has a molecular weight of 33,216. The amino-terminal segment of the protein is relatively basic in character and has limited sequence homologies with the lac repressor and histidinol dehydrogenase. In addition, the protein contains a 40-residue segment that has 13 residues identical with the sequence surrounding the active-site cysteine of glyceraldehyde-3-phosphate dehydrogenase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes W. M. DNA sequence from the histidine operon control region: seven histidine codons in a row. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4281–4285. doi: 10.1073/pnas.75.9.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyreuther K., Adler K., Geisler N., Klemm A. The amino-acid sequence of lac repressor. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3576–3580. doi: 10.1073/pnas.70.12.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker G., Harris J. I., Thierry J. C., Walker J. E., Wonacott A. J. Sequence and structure of D-glyceraldehyde 3-phosphate dehydrogenase from Bacillus stearothermophilus. Nature. 1977 Mar 24;266(5600):328–333. doi: 10.1038/266328a0. [DOI] [PubMed] [Google Scholar]
- Blasi F., Bruni C. B., Avitabile A., Deeley R. G., Goldberger R. F., Meyers M. M. Inhibition of transcription of the histidine operon in vitro by the first enzyme of the histidine pathway. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2692–2696. doi: 10.1073/pnas.70.9.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun D. H. Autoregulation of gene expression. Annu Rev Microbiol. 1975;29:275–299. doi: 10.1146/annurev.mi.29.100175.001423. [DOI] [PubMed] [Google Scholar]
- Davidson B. E., Sajgò M., Noller H. F., Harris J. I. Amino-acid sequence of glyceraldehyde 3-phosphate dehydrogenase from lobster muscle. Nature. 1967 Dec 23;216(5121):1181–1185. doi: 10.1038/2161181a0. [DOI] [PubMed] [Google Scholar]
- DeLange R. J., Fambrough D. M., Smith E. L., Bonner J. Calf and pea histone IV. II. The complete amino acid sequence of calf thymus histone IV; presence of epsilon-N-acetyllysine. J Biol Chem. 1969 Jan 25;244(2):319–334. [PubMed] [Google Scholar]
- DeLange R. J., Hooper J. A., Smith E. L. Complete amino-acid sequence of calf-thymus histone 3. Proc Natl Acad Sci U S A. 1972 Apr;69(4):882–884. doi: 10.1073/pnas.69.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nocera P. P., Avitabile A., Blasi F. In vitro transcription of the Escherichia coli histidine operon primed by dinucleotides. Effect of the first histidine biosynthetic enzyme. J Biol Chem. 1975 Nov 10;250(21):8376–8381. [PubMed] [Google Scholar]
- Di Nocera P. P., Blasi F., Di Lauro R., Frunzio R., Bruni C. B. Nucleotide sequence of the attenuator region of the histidine operon of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4276–4280. doi: 10.1073/pnas.75.9.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau G. R. Cleavage at glutamic acid with staphylococcal protease. Methods Enzymol. 1977;47:189–191. doi: 10.1016/0076-6879(77)47023-x. [DOI] [PubMed] [Google Scholar]
- Elgin S. C., Weintraub H. Chromosomal proteins and chromatin structure. Annu Rev Biochem. 1975;44:725–774. doi: 10.1146/annurev.bi.44.070175.003453. [DOI] [PubMed] [Google Scholar]
- Goldberger R. F. Autogenous regulation of gene expression. Science. 1974 Mar 1;183(4127):810–816. doi: 10.1126/science.183.4127.810. [DOI] [PubMed] [Google Scholar]
- Goldberger R. F., Kovach J. S. Regulation of histidine biosynthesis in Salmonella typhimurium. Curr Top Cell Regul. 1972;5:285–308. doi: 10.1016/b978-0-12-152805-8.50014-9. [DOI] [PubMed] [Google Scholar]
- Harris J. I., Perham R. N. Glyceraldehyde 3-phosphate dehydrogenase from pig muscle. Nature. 1968 Sep 7;219(5158):1025–1028. doi: 10.1038/2191025a0. [DOI] [PubMed] [Google Scholar]
- Hnilica L. S., Kappler H. A., Jordan J. J. Assymetry in the distribution of basic amino acid residues in the moderately lysine-rich histone F2b from calf thymus. Experientia. 1970 Apr 15;26(4):353–355. doi: 10.1007/BF01896882. [DOI] [PubMed] [Google Scholar]
- Hood J. M., Fowler A. V., Zabin I. On the evolution of beta-galactosidase. Proc Natl Acad Sci U S A. 1978 Jan;75(1):113–116. doi: 10.1073/pnas.75.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. M.T., Harris J. I. Glyceraldehyde 3-phosphate dehydrogenase: Amino acid sequence of enzyme from baker's yeast. FEBS Lett. 1972 May 1;22(2):185–189. doi: 10.1016/0014-5793(72)80040-1. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., Geisler N., Weber K. Amino-terminal fragments of Escherichia coli lac repressor bind to DNA. Nature. 1977 Oct 20;269(5630):668–672. doi: 10.1038/269668a0. [DOI] [PubMed] [Google Scholar]
- Kleeman J. E., Parsons S. M. Inhibition of histidyl-tRNA-adenosine triphosphate phosphoribosyltransferase complex formation by histidine and by guanosine tetraphosphate. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1535–1537. doi: 10.1073/pnas.74.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landon M., Piszkiewicz D., Smith E. L. Sequence of bovine liver glutamate dehydrogenase. II. Sequences of tryptic peptides. J Biol Chem. 1971 Apr 25;246(8):2374–2399. [PubMed] [Google Scholar]
- Meyers M., Blasi F., Bruni C. B., Deeley R. G., Kovach J. S., Levinthal M., Mullinix K. P., Vogel T., Goldberger R. F. Specific binding of the first enzyme for histidine biosynthesis to the DNA of histidine operon. Nucleic Acids Res. 1975 Nov;2(11):2021–2036. doi: 10.1093/nar/2.11.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons S. M., Koshland D. E., Jr A rapid isolation of phosphoribosyladenosine triphosphate synthetase and comparison to native enzyme. J Biol Chem. 1974 Jul 10;249(13):4104–4109. [PubMed] [Google Scholar]
- Parsons S. M., Lipsky M. A unique reactive residue in adenosine triphosphate phosphoribosyltransferase sensitive to five conformation and dissociation states. J Biol Chem. 1975 Jul 25;250(14):5660–5668. [PubMed] [Google Scholar]
- Parsons S. M., Lipsky M. Composition of the first enzyme of histidine biosynthesis isolated from wild-type and mutant operator strains of Salmonella typhimurium. J Bacteriol. 1975 Feb;121(2):485–490. doi: 10.1128/jb.121.2.485-490.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piszkiewicz D., Landon M., Smith E. L. Bovine glutamate dehydrogenase. Loss of allosteric inhibition by guanosine triphosphate and nitration of tyrosine-412. J Biol Chem. 1971 Mar 10;246(5):1324–1329. [PubMed] [Google Scholar]
- Piszkiewicz D., Rand-Meir T., Theodor I., Parsons S. M. The amino terminal sequence of ATP-phosphoribosyltransferase, the first gene product of the histidine operon. Biochem Biophys Res Commun. 1977 Sep 23;78(2):833–838. doi: 10.1016/0006-291x(77)90255-8. [DOI] [PubMed] [Google Scholar]
- Summers M. R., Smythers G. W., Oroszlan S. Thin-layer chromatography of sub-nanomole amounts of phenylthiohydantoin (PTH) amino acids on polyamide sheets. Anal Biochem. 1973 Jun;53(2):624–628. doi: 10.1016/0003-2697(73)90114-0. [DOI] [PubMed] [Google Scholar]
- Voll M. J., Appella E., Martin R. G. Purification and composition studies of phosphoribosyladenosine triphosphate:pyrophosphate phosphoribosyltransferase, the first enzyme of histidine biosynthesis. J Biol Chem. 1967 Apr 25;242(8):1760–1767. [PubMed] [Google Scholar]
- Yeoman L. C., Olson M. O., Sugano N., Jordan J. J., Taylor D. W., Starbuck W. C., Busch H. Amino acid sequence of the center of the arginine-lysine-rich histone from calf thymus. The total sequence. J Biol Chem. 1972 Oct 10;247(19):6018–6023. [PubMed] [Google Scholar]
- Yourno J. Similarity of cross-suppressible frameshifts in Salmonella typhimurium. J Mol Biol. 1971 Nov 28;62(1):223–231. doi: 10.1016/0022-2836(71)90141-0. [DOI] [PubMed] [Google Scholar]



