Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease, primarily affecting young females. Pregnancy in a woman with SLE remains a high risk situation with higher maternal and fetal mortality and morbidity. Although live births are achieved in majority of the pregnancies, active disease and major organ involvement can negatively affect the outcomes. Higher risk of fetal loss, pre-term birth, intra-uterine growth restriction and neonatal lupus syndromes are major fetal issues. Mothers are faced with disease flares, pre-eclampsia and other complications. Disease flares during SLE pregnancy pose the unique issue of recognition and differentiation between physiologic changes and disease state. Similarly pre-eclampsia and lupus nephritis may lead to diagnostic confusion. Treatment choices during pregnancy are limited to a few safe drugs, further restricting the options. Refractory pregnancy loss associated with anti-phospholipid antibodies and complete heart block associated with anti-Ro antibodies remain unresolved issues. A multidisciplinary approach, with close monitoring, is essential for optimal outcomes.
Keywords: Systemic lupus erythematosus, anti-phospholipid antibodies, pregnancy, fetal loss, pre-eclampsia, neonatal lupus syndromes
Introduction
Systemic lupus erythematosus (SLE) is an auto-immune disease with significant female predominance. The onset during reproductive years, coupled with improved survival, has led to increased numbers of pregnancies in SLE. The pregnancy outcomes have also significantly improved. The rate of pregnancy loss has decreased from 43% to 17% in recent years [1]. However, SLE patients have fewer children than their normal counterparts and SLE pregnancy still carries a high risk of complications [2-4]. A multidisciplinary approach, with close medical, obstetric and neonatal monitoring, is essential for optimal outcomes. This chapter will highlight major issues in SLE pregnancy and discuss the management strategies to minimize maternal and fetal risks.
Pregnancy planning in SLE
Active SLE at the time of conception is known to be the strongest predictor of adverse pregnancy outcomes [5]. Hence, ideally, all pregnancies in women with SLE should be planned during periods of disease control. Unplanned pregnancies during periods of disease activity highlight the often neglected need of effective contraceptive counseling of all young women with SLE [6]. Natural and barrier methods of contraception have a high failure rate and may not be sufficient in a patient with active disease. Safety of oral contraceptives has been documented in two large randomized controlled trials [7, 8]. However, patients with severely active disease were excluded from the studies. Patients with anti-phospholipid antibodies (aPL) are at high risk of thrombosis and should avoid estrogen containing contraceptives [9]. Certain drugs interfere with the oral contraceptive efficacy. This fact has recently been added to the FDA labeling of mycophenolate mofetil. Although effective, progesterone-only contraceptives have to be used judiciously. Long term use, especially of depot preparations, leads to negative effects on bone mineral density [10]. The intra-uterine contraceptive device remains a viable and safe option for many patients with SLE [8].
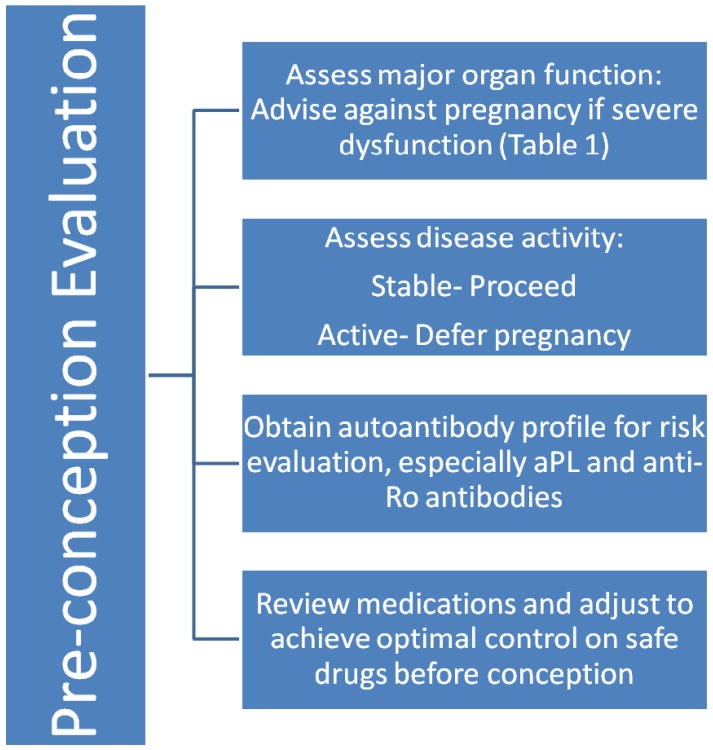
Pre-conception evaluation
Preconception assessment is an essential component of pregnancy planning in SLE. In a limited number of patients, pregnancy may pose an unacceptably high maternal risk, justifying an advice to defer or avoid pregnancy (Table 1). If there are no contra-indications, patient should undergo pre-conception counseling, maternal and fetal risk assessment, and medication review, before conception (Figure 1). A complete set of autoantibodies should be as certain specific maternal antibodies (aPL and anti-Ro antibodies) in mother poses unique fetal risks. Every effort should be made to ensure optimal disease control for at least 6 months prior to conception. Medications should be reviewed and adjusted to achieve good disease control on permitted medication. Thyroid function should be assessed as hypothyroidism in SLE is associated with poorer outcomes [11].
Table 1. Situations where pregnancy is not advisable in patient with SLE.
| Contra-indications to pregnancy: |
| Severe pulmonary hypertension (systolic pulmonary artery pressure > 50mm Hg) |
| Severe restrictive lung disease (Forced vital capacity < 1 L) |
| Advanced renal insufficiency (creatinine >2.8 mg/dL) |
| Advance heart failure |
| Previous severe preeclampsia or HELLP despite therapy |
| Pregnancy should be deferred: |
| Severe disease flare within last 6 months |
| Active lupus nephritis |
| Stroke within the previous 6 months |
Figure 1. Planning a pregnancy in the setting of SLE.
Pre-pregnancy Counseling
SLE pregnancies are considered to be high risk. All patients should be counseled about the possible issues including risk of disease flares, higher rates of pregnancy complications, sub-optimal obstetric outcomes, and the risk of neonatal lupus syndromes. The need for optimal disease control with safe medications during pregnancy should be explained.
Disease activity during pregnancy
One of the major issues is SLE pregnancy is the risk of disease exacerbation. Although it is generally agreed that pregnancy may lead to higher rates of disease flares, widely variable flare rates of between 25-65% have been reported [12-17]. Different organ systems may have variable response to pregnancy; musculoskeletal flares are less common while renal and hematologic flares are more common [18]. Majority of the flares in pregnancy are mild-to-moderate, with only small percentage of patients developing severe flares [16]. Active disease during the 6 months prior to conception, history of lupus nephritis and discontinuation of anti-malarial significantly increase the risk of flares during the pregnancy [14, 19-21].
Pregnancy Complications
Pregnancy in the setting of SLE is associated with a higher risk of complications, compared to normal women. A large national data base study of 16.7 million deliveries reported many fold increased risk of maternal death, preeclampsia, preterm labor, thrombosis, infection, and hematologic complications during SLE pregnancy [2]. However, these results have to be interpreted with caution. Non- pregnant SLE patients also have higher risk of medical complications and mortality rate. In addition, women with SLE in this study were older and had significantly higher rates of co-morbidities.
The biggest issue is the 3-5 times higher risk of pre-eclampsia, complicating 16-30% of SLE pregnancies [22-24]. The predisposing factors for pre-eclampsia include advanced maternal age, previous personal or family history of preeclampsia, pre-existing hypertension or diabetes mellitus, and obesity [25]. In SLE, additional specific risk factors include active or history of lupus nephritis, presence of anti-phospholipid antibodies, declining complement levels, and thrombocytopenia [22-24, 26]. A genetic predisposition with heterozygous mutations in complement regulatory proteins was reported in the PROMISSE cohort, but needs further evaluation [27].
Obstetric Outcomes
The main obstetric issues in SLE pregnancy are higher rates of fetal loss, preterm birth, intra-uterine growth restriction (IUGR), and neonatal lupus syndromes. However, the rate of fetal loss has declined and live births rates of 80-90% have recently been reported [12, 14, 15]. Active disease and lupus nephritis increase the risk of fetal loss and other adverse outcomes [13, 20, 28, 29]. Proteinuria, hypertension, thrombocytopenia, and presence of anti-phospholipid antibodies are other negative predictors for fetal survival [13, 28, 30].
Pre-term births, and the morbidity associated with it, are the most frequent problems of SLE pregnancy. Variable rates have been reported but in the presence of the mentioned adverse prognostic factors, up to half of the pregnancies may end in premature delivery. Thyroid disease is also associated with higher risk of pre-term birth in SLE pregnancy [11]. About 10-30% of SLE pregnancies are complicated with fetal growth restriction and small for gestational age babies [14, 21].
Neonatal Lupus Syndromes
Neonatal Lupus Syndromes (NLS) is a form of passively acquired fetal autoimmunity from maternal antibodies, anti-Ro and anti-La antibodies. Majority of the manifestations, such as rash, hematologic and hepatic abnormalities, parallel the presence of maternal antibodies in the neonatal circulation. They tend to resolve with the clearance of the antibodies by six to eight months of life. In contrast, cardiac complications are a result of permanent damage to the fetal cardiac conduction system by maternal antibodies.
The cardiac manifestations of NLS include conduction defects, structural abnormalities, cardiomyopathy and congestive cardiac failure [31]. However, the most common issue is congenital heart block (CHB). CHB leads to high fetal mortality; rates of 15-30% have been reported. The majority of survivors require pacemakers, adding to the significant morbidity [32, 33]. CHB affects about 2% of children born to primigravid women with anti-Ro antibodies [34]. However, the risk rises to about 16-20% in subsequent pregnancies, after the birth of an affected child [35, 36]. Other suggested risk factors include higher levels of maternal antibodies, maternal hypothyroidism, and fetal genetic polymorphisms [36, 37].
Medication use during pregnancy
An essential component of pre-pregnancy counseling is discussion about the use of appropriate medications during the pregnancy. Unfortunately, concerns over presumed toxicity often lead to discontinuation of necessary therapy with resultant increase in disease activity, worsening the outcomes. The United States Food and Drug Administration (FDA) categories are often not helpful as they are mostly derived from animal data or are outdated. Although majority of SLE therapeutics are potentially harmful and contra-indicated, safe options exist and should be continued during the pregnancy (Table 2).
Table 2. Medications safe for use during SLE Pregnancy.
| Drugs | Comments | Recommendations |
|---|---|---|
| Non-steroidal anti-inflammatory drugs (NSAIDS) | First trimester use may be associated with higher risk of congenital malformations, fetal renal impairment and premature closure of ductus arteriosus with use in last trimester |
|
Corticosteroids
|
|
|
Antimalarials
|
Reduced risk of disease flares, CHB and NLS | Should be continued in all SLE pregnancies |
Immunosuppressants
|
Used in large number of transplant recipients. Recent report of late developmental delays in off springs with azathioprine |
|
Anti-Hypertensives
|
Concerns about growth retardation with labetalol and impaired utero-placental blood flow with hydralazine | Generally safe and preferred drugs for hypertension during pregnancy |
Non-steroidal anti-inflammatory drugs (NSAIDS) were considered safe during the first and second trimesters [38]. However, moderate associations between NSAID use in first trimester and specific birth defects were recently reported [39, 40]. There is also an increased risk of impaired fetal renal function with use after 20 weeks of gestation. Hence, caution needs to be exercised when using NSAIDs during early pregnancy. Continued use after the 32 week of gestation can increase the risk of premature closure of the ductus arteriosus by almost 15-fold, and should be avoided [41]. The data on the cyclooxygenase 2 inhibitors in pregnancy is very limited, and they are best avoided during pregnancy.
Steroid exposure should be limited to a minimum during the pregnancy. High doses during pregnancy are associated with an increased risk of diabetes, hypertension, pre-eclampsia and premature rupture of membranes [38]. However, in the case of disease flares, short courses of high doses and/or intravenous pulse methylprednisolone can be used. Patients on long term steroid therapy should also receive stress doses at the time of delivery. Use of fluorinated compounds, such as dexamethasone and betamethasone should be limited to a single course for fetal lung maturity, in cases of premature delivery. Repeated use has been associated with impaired neuro-psychological development of the child in later life, and should be avoided [42].
Hydroxychloroquine should be continued in all pregnant women with SLE. Multiple studies have proven the beneficial effects of hydroxychloroquine in SLE, including during pregnancy. Reduction in disease activity was noted with no harmful effects on the baby with use during pregnancy, while discontinuation led to an increase in disease flares [19, 43, 44]. The risk of CHB and neonatal lupus syndromes was also significantly reduced in at-risk pregnancies with sustained use of hydroxychloroquine [45, 46].
Azathioprine is one of the only few immunosuppressive agents that has documented safety during pregnancy [38]. The dose should be limited to maximum of 2mg/kg/day, to avoid risk of fetal cytopenias and immune suppression [38]. An association between maternal azathioprine therapy during pregnancy and late developmental delays in offspring was suggested by a recent study [47]. However, the confidence intervals were very wide and the study had serious limitations (small sample size, retrospective nature, lack of validated measures). Azathioprine can still be considered safe during pregnancy but it is prudent to counsel the women about the possible association. Other immunosuppressive drugs with no reported increase in fetal risk are the calcineurin inhibitors, tacrolimus and cyclosporine [48]. Leflunomide was considered to be teratogenic and traditional advice has been to discontinue for 2 years or perform a wash out procedure before conception. Recently, 2 cohort studies reported no increase in risk of malformations after inadvertent exposure during pregnancy [49, 50]. However, caution needs to be exercised and routine use of leflunomide during pregnancy is not recommended. Most other agents, such as cyclophosphamide, methotrexate, and mycophenolate, are contraindicated during pregnancy and should be discontinued at least 3 months before conception. Data on the biologics, such as rituximab or belimumab, during pregnancy are very limited, and they should be discontinued before conception.
Most of the commonly used antihypertensive drugs have to be either avoided or used with extreme caution during pregnancy [51, 52]. Angiotensin-converting-enzyme (ACE) inhibitors and angiotensin II receptor blockers can cause specific malformations, the ACE-inhibitor fetopathy. In addition, neonatal arterial hypotension, renal failure, and death have been reported. Beta-adrenergic blockers have been associated with IUGR and fetal bradycardia. Diuretics can lead to maternal volume depletion and reduced uteroplacental perfusion. Hence, the safe armamentarium against hypertension during pregnancy is quite limited, including drugs such as hydralazine, methyl-dopa, nifedipine, and labetalol [51, 52].
Low-dose aspirin as an antiplatelet agent is safe during pregnancy but data on other antiplatelet agents are limited [53]. Heparin does not cross the placenta and is the anticoagulant of choice during pregnancy. Low-molecular weight heparin (LMWH) has similar efficacy and safety to unfractionated heparin (UFH). The ease of administration, higher antithrombotic to anticoagulant ratio, and predictable bioavailability has led to widespread use of LMWH instead of UFH [54]. Warfarin should be avoided during pregnancy, especially during the first trimester, due to the risk of warfarin embryopathy syndrome [55]. The data on direct factor Xa inhibitor, fondoparinux, are limited but reassuring. It does not cross placenta and may be a possible choice in women intolerant to heparin [54].
Calcium supplementation should be routinely provided to all pregnant women with SLE, especially those receiving corticosteroids and heparin. Insufficient vitamin D levels during pregnancy are associated with higher pregnancy morbidity including gestational diabetes, pre-eclampsia, and small for gestational age infants [56]. However, supplemental vitamin D during pregnancy did not consistently or significantly reduce the risk [57, 58]. Although guidelines differ, currently the safest approach is to supplement vitamin D during pregnancy in women at higher risk [59]. Bisphosphonates should be discontinued 6-12 months prior to pregnancy. Animal data showed higher maternal and fetal mortality and morbidity, albeit at levels many times higher than clinical doses. Limited human data did not show any serious adverse maternal or fetal effects but some reports of fetal hypocalcaemia and growth retardation have been noted [60].
Ante-natal management in SLE patients
Ante-natal management of pregnant patients will SLE requires close collaboration between rheumatologist and obstetrician. The monitoring should be more frequent and detailed than the usual standard of care. Each visit should include thorough physical examination, routine laboratory tests and specific investigations, tailored to the risk profile of the particular pregnancy (Table 3). Certain situations, such as disease flare or presence of specific antibodies, require specific strategies, as discussed below.
Table 3. Ante-natal monitoring in SLE pregnancy.
| Clinical review | Investigations | Specific Monitoring |
|---|---|---|
|
|
|
Pregnancy in the presence of anti-phospholipid antibodies
Presence of aPL during pregnancy is associated with significant risk of pregnancy morbidity and loss. Although aPL are present in about a quarter to half of patients with SLE, only a fraction of these patients develop antiphospholipid syndrome (APS), defined by the persistence of medium-to-high titre aPL (anticardiolipin, anti-β2 glycoprotein and/or the lupus anticoagulant) on at least two laboratory tests, 12 weeks apart, in the presence of at least one clinical criterion of thrombosis and/or pregnancy morbidity [61]. However, even asymptomatic women with aPL, not fulfilling the criteria, have higher rates of pregnancy loss. In addition, aPL increase the risk of pre-eclampsia, placental insufficiency, IUGR, and preterm delivery. Lupus anticoagulant is more specific in predicting the risk of adverse pregnancy outcomes, compared with other aPL [30].
The outcomes of pregnancies exposed to aPL have significantly improved and live birth rates of over 80% have been recently reported [62]. The management strategies differ, based on the risk profile of each pregnancy. Low dose aspirin alone is generally recommended for asymptomatic women with only persistently positive aPL and no prior event, despite limited evidence [63, 64]. The group with recurrent early losses or one or more late fetal loss, but no history of systemic thrombosis, is termed Obstetric APS. Aspirin, in combination with prophylactic doses of heparin, significantly reduces the risk of pregnancy loss in this group [65, 66]. Low-molecular weight heparin (LMWH) has similar efficacy to unfractionated heparin, but requires twice daily administration at all doses during pregnancy [67]. Low-molecular weight heparin must be transitioned to unfractionated heparin prior to delivery. Heparin treatment needs to be continued for 6 weeks post-partum [68]. The patients with prior systemic thrombosis should receive full therapeutic doses of heparin throughout pregnancy.
Some patients are refractory to the aspirin and heparin treatment and continue to have recurrent losses. The management of these patients requires individualized approach; all decisions have to be made in discussion with the woman and her partner. Addition of steroids has been reported to improve outcomes [69]. IVIg and plasmapheresis have been tried with benefit in case reports, but data are limited [70-72].
Pregnancy in the presence of anti-Ro antibodies
The biggest issue with exposure to the anti-Ro antibodies during pregnancy is the high risk of CHB. Most often CHB develops between 18–24 weeks of gestation. It is usually preceded by lesser degrees of conduction delays which may be reversed with early treatment [33]. However, conduction abnormalities can progress very rapidly and many times the first rhythm abnormality detected is CHB. Many tools have been developed for early detection of lesser degrees of heart block, including fetal doppler echocardiography, fetal kinetocardiogram and transabdominal fetal electrocardiogram [73-75].
Fetal doppler echocardiography remains the most commonly used modality. All exposed fetuses should be monitored weekly between 16–26 weeks of gestation, and bi-weekly thereafter [33]. Detection of an early conduction defect such as prolonged PR interval should be considered a danger signal. Although some early blocks are transient, the progression to CHB remains unpredictable. Prophylactic treatment should be discussed if the PR interval remains persistently prolonged. Maternal administration of fluorinated corticosteroids has shown fetal survival benefit in some studies. However, the results have not been consistent and the benefits have to be weighed against the higher risk of IUGR and preterm birth [76-78]. Treatment of established CHB remains even more unsatisfactory. Improved fetal outcomes were reported after trans-placental treatment with dexamethasone and beta-adrenergic stimulants in one study, but these findings were not replicated [76-78]. Hydroxychloroquine during pregnancy reduces the risk of cardiac NLS in at-risk fetuses [45, 79].
The recurrence risk of CHB in subsequent pregnancies after an affected pregnancy is many-fold higher. Hydroxychloroquine reduced this risk by 65% in one study [45]. Open label data showed beneficial effects of intravenous immunoglobulin (IVIG), but two large randomized controlled trials showed negative results [80-82]. The study design, dose of IVIG used and different composition of IVIG may have contributed to the negative outcomes [83]. In summary, currently there is no satisfactory treatment for established CHB.
Disease flare during pregnancy
An important management issue is the difficultly of recognizing disease flare in pregnant SLE patients. Many physiological changes of pregnancy may overlap with features of active disease, making differentiation difficult (Table 4). Some common laboratory tests also become less reliable: mild anemia and thrombocytopenia are common, erythrocyte sedimentation rate is raised, and up to 300mg/day proteinuria can occur during normal pregnancy. Complement levels rise by 10–50% during normal pregnancy and may appear to remain in the ‘normal’ range, despite disease activity. Thus, the trend of complement levels becomes more important than absolute values. Low and declining levels of complement during pregnancy have been associated with poor pregnancy outcomes [26, 84]. Anti-dsDNA antibodies may be helpful in evaluation of disease activity [84].
Table 4. Differentiation of SLE flare from physiological pregnancy changes.
| Characteristic | Pregnancy-related changes | SLE flare |
|---|---|---|
| Mucocutaneous | Facial flush Palmar erythema Postpartum hair loss |
Photosensitive rash Oral or nasal ulcers |
| Musculoskeletal | Arthralgias Myalgias |
Inflammatory arthritis |
| Hematologic | Mild anemia, Mild thrombocytopenia | Leucopenia, lymphopenia Immune hemolytic anemia Thrombocytopenia |
| Renal | Physiologic proteinuria <300mg/day | Active urinary sediment Proteinuria >300mg/day |
| Immunologic | Higher complement levels | Falling complement levels Rising anti DNA levels |
| Others | Fatigue Mild edema Mild resting dyspnea |
Fever Lymphadenopathy Pleuritis |
The SLE disease activity indices have similar limitations. They were derived in non-pregnant populations and physiologic pregnancy changes were not accounted in these indices. Pregnancy-specific disease activity scales, SLE Pregnancy Disease Activity Index (SLEPDAI), LAI in Pregnancy (LAI-P), and BILAG2004-Pregnancy index, have been developed with modifications to descriptors. However, they mostly remain as research tools. In real life, a combination of laboratory parameters coupled with the clinical judgment may the best tool to evaluate disease activity.
Treatment of flares during pregnancy is guided by the severity and organ involvement, similar to the non-pregnant state. However, the choice of agents is limited to safe drugs, as discussed above. Steroids in the lowest possible doses should be used, but short courses of high doses can be used for flares. NSAIDS can be used for mild symptoms in the first and second trimester. However, caution needs to be exercised in view of recent data on associations with malformations. Hydroxychloroquine should be continued throughout the pregnancy. Other safe immunosuppressants that can be used include azathioprine and calcineurin inhibitors. Although developmental delays in offsprings were recently reported with azathioprine, more studies are required to further evaluate this association. IVIG and plasmapheresis remain alternative options but the higher risk of thrombosis with IVIG and fluid overload have to be considered.
Pre-eclampsia during SLE pregnancy
Differentiation between pre-eclampsia and lupus nephritis flares during pregnancy may become difficult; both can present with increasing proteinuria, deteriorating renal function, hypertension, and thrombocytopenia. Certain features, if present, can help in distinguishing between the 2 conditions (Table 5). Multiple guidelines for diagnosis of pre-eclampsia have been proposed but are neither highly sensitive nor specific [85]. Abnormal uterine artery waveforms have been associated with a higher risk of pre-eclampsia and poor obstetric outcomes [86, 87]. However, the lack of standardization and limited data restricts the diagnostic value [88]. Multiple biomarkers, such as placental growth factor (PlGF), vascular endothelial growth factor (VEGF), soluble fms-like tyrosine kinase-1 (sFLT1), and soluble endoglin (sENG), have been evaluated as possible predictors and diagnostic tools. However, the sensitivities remain low and data in SLE are very limited [89, 90]. Prediction models incorporating clinical characteristics, uterine artery Doppler and biomarker levels have been developed but await prospective validation studies [91]. In certain situations, it may become extremely difficult to differentiate lupus nephritis and pre-eclampsia and they may also co-exist. Renal biopsy could help to differentiate but the higher risk of complications during pregnancy limits the use in advanced pregnancy. Sometimes, delivery of the baby may be the only definitive answer.
Table 5. Features differentiating Pre-eclampsia and Lupus nephritis.
| Clinical and Laboratory Features | Pre-eclampsia | Lupus nephritis |
|---|---|---|
| Hypertension | After 20 weeks of gestation | Any time during the pregnancy |
| Platelets | Low - normal | Low - normal |
| Complements | Normal - low | Low |
| Anti dsDNA | Absent or unchanged | Rising titers |
| Creatinine | Normal - raised | Normal to raised |
| Serum Uric Acid | Elevated (>5.5mg/dl) | Normal |
| 24 hour Urine Calcium | <195mg/dl | >195mg/dl |
| Urinary Sediment | Inactive | Active |
| Other Organs Involved | Occasionally CNS or HELLP | Evidence of active non-renal SLE |
| Response to steroids | No | Yes |
Summary
Pregnancy in women with SLE is a high risk condition. Despite considerable improvement in success rates, substantially high maternal and fetal morbidity and mortality still remain a cause for concern. Disease activity may worsen during the pregnancy and in turn may increase the risk of other maternal and fetal complications. Recognition and treatment of disease flares and pre-eclampsia, during SLE pregnancy, is fraught with difficulties including overlapping features, lack of specific diagnostic markers, and drug toxicities. Increased fetal loss, especially in the presence of aPL, pre-term births, IUGR, and neonatal syndromes including CHB are major unresolved issues. The key to success lies in the multidisciplinary care with close monitoring. Early detection of threats to maternal and fetal well-being, with judicious use of appropriate medications, is essential to achieve good outcomes.
Practice points
Pregnancy in the setting of SLE remains a high risk situation
Multidisciplinary care with close monitoring is essential for good outcomes
Active disease at conception is associated with adverse maternal and fetal outcomes
Pregnancy should be planned at times of disease quiescence with effective use of contraception
Preconception assessment should be done prior to the planned pregnancy
Specific monitoring and treatment protocols are required in high risk situations such as presence of specific antibodies (aPL and anti-Ro)
Disease flares, pre-eclampsia, fetal loss, prematurity, intra-uterine growth restriction and neonatal lupus syndromes (including CHB) remain the main issues
Safe treatment options exist and should be appropriately used for disease activity during pregnancy
Research agenda
Adverse obstetric outcomes including refractory pregnancy loss associated with aPL, and CHB associated with anti-Ro antibodies, remain a challenge.
The pathophysiologic mechanisms of these complications are now being understood such as the role of complement activation in aPL associated pregnancy loss. However, further studies are required for in depth understanding of these mechanisms.
There is an unmet need to develop targeted therapies, based on the underlying mechanisms, for pregnancy loss and CHB in at-risk pregnancies.
Late neuropsychiatric effects on children exposed to maternal anti-Ro antibodies and immunosuppressants in utero, need further evaluation.
The significance of thyroid abnormalities and benefits of thyroid replacement in pregnant women with SLE needs further evaluation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Aisha Lateef, Email: aisha_lateef@nuhs.edu.sg.
Michelle Petri, Email: mpetri@jhmi.edu.
References
- 1.Clark CA, Spitzer KA, Laskin CA. Decrease in pregnancy loss rates in patients with systemic lupus erythematosus over a 40-year period. J Rheumatol. 2005;32(9):1709–1712. [PubMed] [Google Scholar]
- 2.Clowse ME, Jamison M, Myers E, James AH. A national study of the complications of lupus in pregnancy. Am J Obstet Gynecol. 2008;199(2):127, e121–126. doi: 10.1016/j.ajog.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clowse ME, Chakravarty E, Costenbader KH, Chambers C, Michaud K. The effects of infertility, pregnancy loss, and patient concerns on family size of women with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2012 doi: 10.1002/acr.21593. [DOI] [PubMed] [Google Scholar]
- 4.Yasmeen S, Wilkins EE, Field NT, Sheikh RA, Gilbert WM. Pregnancy outcomes in women with systemic lupus erythematosus. J Matern Fetal Med. 2001;10(2):91–96. doi: 10.1080/714904302. [DOI] [PubMed] [Google Scholar]
- 5.Lateef A, Petri M. Management of pregnancy in systemic lupus erythematosus. Nat Rev Rheumatol. 2012;8(12):710–718. doi: 10.1038/nrrheum.2012.133. [DOI] [PubMed] [Google Scholar]
- 6.Yazdany J, Trupin L, Kaiser R, Schmajuk G, Gillis JZ, Chakravarty E, Schwarz EB. Contraceptive counseling and use among women with systemic lupus erythematosus: a gap in health care quality? Arthritis Care Res (Hoboken) 2011;63(3):358–365. doi: 10.1002/acr.20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, Lockshin M, Merrill JT, Belmont HM, Askanase AD, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353(24):2550–2558. doi: 10.1056/NEJMoa051135. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Guerrero J, Uribe AG, Jimenez-Santana L, Mestanza-Peralta M, Lara-Reyes P, Seuc AH, Cravioto MD. A trial of contraceptive methods in women with systemic lupus erythematosus. N Engl J Med. 2005;353(24):2539–2549. doi: 10.1056/NEJMoa050817. [DOI] [PubMed] [Google Scholar]
- 9.Lateef A, Petri M. Hormone replacement and contraceptive therapy in autoimmune diseases. J Autoimmun. 2012;38(2-3):J170–176. doi: 10.1016/j.jaut.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Shaarawy M, El-Mallah SY, Seoudi S, Hassan M, Mohsen IA. Effects of the long-term use of depot medroxyprogesterone acetate as hormonal contraceptive on bone mineral density and biochemical markers of bone remodeling. Contraception. 2006;74(4):297–302. doi: 10.1016/j.contraception.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Stagnaro-Green A, Akhter E, Yim C, Davies TF, Magder L, Petri M. Thyroid disease in pregnant women with systemic lupus erythematosus: increased preterm delivery. Lupus. 2011;20(7):690–699. doi: 10.1177/0961203310394894. [DOI] [PubMed] [Google Scholar]
- 12.Carvalheiras G, Vita P, Marta S, Trovao R, Farinha F, Braga J, Rocha G, Almeida I, Marinho A, Mendonca T, et al. Pregnancy and systemic lupus erythematosus: review of clinical features and outcome of 51 pregnancies at a single institution. Clin Rev Allergy Immunol. 2010;38(2-3):302–306. doi: 10.1007/s12016-009-8161-y. [DOI] [PubMed] [Google Scholar]
- 13.Cortes-Hernandez J, Ordi-Ros J, Paredes F, Casellas M, Castillo F, Vilardell-Tarres M. Clinical predictors of fetal and maternal outcome in systemic lupus erythematosus: a prospective study of 103 pregnancies. Rheumatology (Oxford) 2002;41(6):643–650. doi: 10.1093/rheumatology/41.6.643. [DOI] [PubMed] [Google Scholar]
- 14.Gladman DD, Tandon A, Ibanez D, Urowitz MB. The effect of lupus nephritis on pregnancy outcome and fetal and maternal complications. J Rheumatol. 2010;37(4):754–758. doi: 10.3899/jrheum.090872. [DOI] [PubMed] [Google Scholar]
- 15.Imbasciati E, Tincani A, Gregorini G, Doria A, Moroni G, Cabiddu G, Marcelli D. Pregnancy in women with pre-existing lupus nephritis: predictors of fetal and maternal outcome. Nephrol Dial Transplant. 2009;24(2):519–525. doi: 10.1093/ndt/gfn348. [DOI] [PubMed] [Google Scholar]
- 16.Petri M, Howard D, Repke J. Frequency of lupus flare in pregnancy. The Hopkins Lupus Pregnancy Center experience. Arthritis Rheum. 1991;34(12):1538–1545. doi: 10.1002/art.1780341210. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz-Irastorza G, Lima F, Alves J, Khamashta MA, Simpson J, Hughes GR, Buchanan NM. Increased rate of lupus flare during pregnancy and the puerperium: a prospective study of 78 pregnancies. Br J Rheumatol. 1996;35(2):133–138. doi: 10.1093/rheumatology/35.2.133. [DOI] [PubMed] [Google Scholar]
- 18.Petri M. The Hopkins Lupus Pregnancy Center: ten key issues in management. Rheum Dis Clin North Am. 2007;33(2):227–235. v. doi: 10.1016/j.rdc.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clowse ME, Magder L, Witter F, Petri M. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum. 2006;54(11):3640–3647. doi: 10.1002/art.22159. [DOI] [PubMed] [Google Scholar]
- 20.Clowse ME, Magder LS, Witter F, Petri M. The impact of increased lupus activity on obstetric outcomes. Arthritis Rheum. 2005;52(2):514–521. doi: 10.1002/art.20864. [DOI] [PubMed] [Google Scholar]
- 21.Saavedra MA, Cruz-Reyes C, Vera-Lastra O, Romero GT, Cruz-Cruz P, Arias-Flores R, Jara LJ. Impact of previous lupus nephritis on maternal and fetal outcomes during pregnancy. Clinical rheumatology. 2012;31(5):813–819. doi: 10.1007/s10067-012-1941-4. [DOI] [PubMed] [Google Scholar]
- 22.Bramham K, Hunt BJ, Bewley S, Germain S, Calatayud I, Khamashta MA, Nelson-Piercy C. Pregnancy outcomes in systemic lupus erythematosus with and without previous nephritis. J Rheumatol. 2011;38(9):1906–1913. doi: 10.3899/jrheum.100997. [DOI] [PubMed] [Google Scholar]
- 23.Chakravarty EF, Colon I, Langen ES, Nix DA, El-Sayed YY, Genovese MC, Druzin ML. Factors that predict prematurity and preeclampsia in pregnancies that are complicated by systemic lupus erythematosus. Am J Obstet Gynecol. 2005;192(6):1897–1904. doi: 10.1016/j.ajog.2005.02.063. [DOI] [PubMed] [Google Scholar]
- 24.Kwok LW, Tam LS, Zhu T, Leung YY, Li E. Predictors of maternal and fetal outcomes in pregnancies of patients with systemic lupus erythematosus. Lupus. 2011;20(8):829–836. doi: 10.1177/0961203310397967. [DOI] [PubMed] [Google Scholar]
- 25.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Lateef A, Magder L, Petri M. Decrease in Complement (C3) Levels during Systemic Lupus Erythematosus Pregnancy is Associated with Higher Rates of Pre-eclampsia. Arthritis Rheum. 2010;62(10):S193. [Google Scholar]
- 27.Salmon JE, Heuser C, Triebwasser M, Liszewski MK, Kavanagh D, Roumenina L, Branch DW, Goodship T, Fremeaux-Bacchi V, Atkinson JP. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS Med. 2011;8(3):e1001013. doi: 10.1371/journal.pmed.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clowse ME, Magder LS, Witter F, Petri M. Early risk factors for pregnancy loss in lupus. Obstet Gynecol. 2006;107(2 Pt 1):293–299. doi: 10.1097/01.AOG.0000194205.95870.86. [DOI] [PubMed] [Google Scholar]
- 29.Wagner SJ, Craici I, Reed D, Norby S, Bailey K, Wiste HJ, Wood CM, Moder KG, Liang KP, Liang KV, et al. Maternal and foetal outcomes in pregnant patients with active lupus nephritis. Lupus. 2009;18(4):342–347. doi: 10.1177/0961203308097575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lockshin MD, Kim M, Laskin CA, Guerra M, Branch DW, Merrill J, Petri M, Porter F, Sammaritano L, Stephenson MD, et al. Lupus anticoagulant, but not anticardiolipin antibody, predicts adverse pregnancy outcome in patients with antiphospholipid antibodies. Arthritis Rheum. 2012 doi: 10.1002/art.34402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hornberger LK, Al Rajaa N. Spectrum of cardiac involvement in neonatal lupus. Scand J Immunol. 2010;72(3):189–197. doi: 10.1111/j.1365-3083.2010.02437.x. [DOI] [PubMed] [Google Scholar]
- 32.Hutter D, Silverman ED, Jaeggi ET. The benefits of transplacental treatment of isolated congenital complete heart block associated with maternal anti-Ro/SSA antibodies: a review. Scand J Immunol. 2010;72(3):235–241. doi: 10.1111/j.1365-3083.2010.02440.x. [DOI] [PubMed] [Google Scholar]
- 33.Buyon JP, Clancy RM, Friedman DM. Cardiac manifestations of neonatal lupus erythematosus: guidelines to management, integrating clues from the bench and bedside. Nat Clin Pract Rheumatol. 2009;5(3):139–148. doi: 10.1038/ncprheum1018. [DOI] [PubMed] [Google Scholar]
- 34.Brucato A, Doria A, Frassi M, Castellino G, Franceschini F, Faden D, Pisoni MP, Solerte L, Muscara M, Lojacono A, et al. Pregnancy outcome in 100 women with autoimmune diseases and anti-Ro/SSA antibodies: a prospective controlled study. Lupus. 2002;11(11):716–721. doi: 10.1191/0961203302lu252oa. [DOI] [PubMed] [Google Scholar]
- 35.Izmirly PM, Llanos C, Lee LA, Askanase A, Kim MY, Buyon JP. Cutaneous manifestations of neonatal lupus and risk of subsequent congenital heart block. Arthritis Rheum. 2010;62(4):1153–1157. doi: 10.1002/art.27333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Llanos C, Izmirly PM, Katholi M, Clancy RM, Friedman DM, Kim MY, Buyon JP. Recurrence rates of cardiac manifestations associated with neonatal lupus and maternal/fetal risk factors. Arthritis Rheum. 2009;60(10):3091–3097. doi: 10.1002/art.24768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaeggi E, Laskin C, Hamilton R, Kingdom J, Silverman E. The importance of the level of maternal anti-Ro/SSA antibodies as a prognostic marker of the development of cardiac neonatal lupus erythematosus a prospective study of 186 antibody-exposed fetuses and infants. J Am Coll Cardiol. 2010;55(24):2778–2784. doi: 10.1016/j.jacc.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 38.Ostensen M, Khamashta M, Lockshin M, Parke A, Brucato A, Carp H, Doria A, Rai R, Meroni P, Cetin I, et al. Anti-inflammatory and immunosuppressive drugs and reproduction. Arthritis Res Ther. 2006;8(3):209. doi: 10.1186/ar1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernandez RK, Werler MM, Romitti P, Sun L, Anderka M. Nonsteroidal antiinflammatory drug use among women and the risk of birth defects. Am J Obstet Gynecol. 2012;206(3):228, e221–228. doi: 10.1016/j.ajog.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams K, Bombardier C, van der Heijde DM. Safety of pain therapy during pregnancy and lactation in patients with inflammatory arthritis: a systematic literature review. J Rheumatol Suppl. 2012;90:59–61. doi: 10.3899/jrheum.120344. [DOI] [PubMed] [Google Scholar]
- 41.Koren G, Florescu A, Costei AM, Boskovic R, Moretti ME. Nonsteroidal antiinflammatory drugs during third trimester and the risk of premature closure of the ductus arteriosus: a meta-analysis. Ann Pharmacother. 2006;40(5):824–829. doi: 10.1345/aph.1G428. [DOI] [PubMed] [Google Scholar]
- 42.Wapner RJ, Sorokin Y, Mele L, Johnson F, Dudley DJ, Spong CY, Peaceman AM, Leveno KJ, Malone F, Caritis SN, et al. Long-term outcomes after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357(12):1190–1198. doi: 10.1056/NEJMoa071453. [DOI] [PubMed] [Google Scholar]
- 43.Levy RA, Vilela VS, Cataldo MJ, Ramos RC, Duarte JL, Tura BR, Albuquerque EM, Jesus NR. Hydroxychloroquine (HCQ) in lupus pregnancy: double-blind and placebo-controlled study. Lupus. 2001;10(6):401–404. doi: 10.1191/096120301678646137. [DOI] [PubMed] [Google Scholar]
- 44.Sperber K, Hom C, Chao CP, Shapiro D, Ash J. Systematic review of hydroxychloroquine use in pregnant patients with autoimmune diseases. Pediatr Rheumatol Online J. 2009;7:9. doi: 10.1186/1546-0096-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Izmirly PM, Costedoat-Chalumeau N, Pisoni CN, Khamashta MA, Kim MY, Saxena A, Friedman D, Llanos C, Piette JC, Buyon JP. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation. 2012;126(1):76–82. doi: 10.1161/CIRCULATIONAHA.111.089268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Izmirly PM, Kim MY, Llanos C, Le PU, Guerra MM, Askanase AD, Salmon JE, Buyon JP. Evaluation of the risk of anti-SSA/Ro-SSB/La antibody-associated cardiac manifestations of neonatal lupus in fetuses of mothers with systemic lupus erythematosus exposed to hydroxychloroquine. Ann Rheum Dis. 2010;69(10):1827–1830. doi: 10.1136/ard.2009.119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marder W, Ganser MA, Romero V, Hyzy MA, Gordon C, McCune WJ, Somers EC. In utero azathioprine exposure and increased utilization of special educational services in children born to mothers with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2013;65(5):759–766. doi: 10.1002/acr.21888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostensen M, Lockshin M, Doria A, Valesini G, Meroni P, Gordon C, Brucato A, Tincani A. Update on safety during pregnancy of biological agents and some immunosuppressive anti-rheumatic drugs. Rheumatology (Oxford) 2008;47(3):iii28–31. doi: 10.1093/rheumatology/ken168. [DOI] [PubMed] [Google Scholar]
- 49.Cassina M, Johnson D, Robinson L, Braddock S, Xu R, Lopez-Jimenez J, Mirrasoul N, Salas E, Luo Y, Jones K, et al. Pregnancy outcome in women exposed to leflunomide before or during pregnancy. Arthritis Rheum. 2012 doi: 10.1002/art.34419. [DOI] [PubMed] [Google Scholar]
- 50.Chambers CD, Johnson DL, Robinson LK, Braddock SR, Xu R, Lopez-Jimenez J, Mirrasoul N, Salas E, Luo YJ, Jin S, et al. Birth outcomes in women who have taken leflunomide during pregnancy. Arthritis Rheum. 2010;62(5):1494–1503. doi: 10.1002/art.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.ACOG Practice Bulletin No. 125: Chronic hypertension in pregnancy. Obstet Gynecol. 2012;119(2 Pt 1):396–407. doi: 10.1097/AOG.0b013e318249ff06. [DOI] [PubMed] [Google Scholar]
- 52.Mustafa R, Ahmed S, Gupta A, Venuto RC. A comprehensive review of hypertension in pregnancy. J Pregnancy. 2012;2012:105918. doi: 10.1155/2012/105918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bell AD, Roussin A, Cartier R, Chan WS, Douketis JD, Gupta A, Kraw ME, Lindsay TF, Love MP, Pannu N, et al. The use of antiplatelet therapy in the outpatient setting: Canadian Cardiovascular Society guidelines. Can J Cardiol. 2011;27(A):S1–59. doi: 10.1016/j.cjca.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 54.Giannubilo SR, Tranquilli AL. Anticoagulant therapy during pregnancy for maternal and fetal acquired and inherited thrombophilia. Curr Med Chem. 2012;19(27):4562–4571. doi: 10.2174/092986712803306466. [DOI] [PubMed] [Google Scholar]
- 55.Abadi S, Einarson A, Koren G. Use of warfarin during pregnancy. Can Fam Physician. 2002;48:695–697. [PMC free article] [PubMed] [Google Scholar]
- 56.Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O'Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346:f1169. doi: 10.1136/bmj.f1169. [DOI] [PubMed] [Google Scholar]
- 57.De-Regil LM, Palacios C, Ansary A, Kulier R, Pena-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2012;(2):CD008873. doi: 10.1002/14651858.CD008873.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wagner CL, McNeil RB, Johnson DD, Hulsey TC, Ebeling M, Robinson C, Hamilton SA, Hollis BW. Health characteristics and outcomes of two randomized vitamin D supplementation trials during pregnancy: A combined analysis. J Steroid Biochem Mol Biol. 2013 doi: 10.1016/j.jsbmb.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steer PJ. Is vitamin D supplementation in pregnancy advisable? Lancet. 2013 doi: 10.1016/S0140-6736(13)60098-7. [DOI] [PubMed] [Google Scholar]
- 60.Stathopoulos IP, Liakou CG, Katsalira A, Trovas G, Lyritis GG, Papaioannou NA, Tournis S. The use of bisphosphonates in women prior to or during pregnancy and lactation. Hormones (Athens) 2011;10(4):280–291. doi: 10.14310/horm.2002.1319. [DOI] [PubMed] [Google Scholar]
- 61.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, PG DEG, Koike T, Meroni PL, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 62.Cervera R, Khamashta MA, Shoenfeld Y, Camps MT, Jacobsen S, Kiss E, Zeher MM, Tincani A, Kontopoulou-Griva I, Galeazzi M, et al. Morbidity and mortality in the antiphospholipid syndrome during a 5-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis. 2009;68(9):1428–1432. doi: 10.1136/ard.2008.093179. [DOI] [PubMed] [Google Scholar]
- 63.Del Ross T, Ruffatti A, Visentin MS, Tonello M, Calligaro A, Favaro M, Hoxha A, Punzi L. Treatment of 139 pregnancies in antiphospholipid-positive women not fulfilling criteria for antiphospholipid syndrome: a retrospective study. J Rheumatol. 2013;40(4):425–429. doi: 10.3899/jrheum.120576. [DOI] [PubMed] [Google Scholar]
- 64.Petri M, Qazi U. Management of antiphospholipid syndrome in pregnancy. Rheum Dis Clin North Am. 2006;32(3):591–607. doi: 10.1016/j.rdc.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 65.Empson M, Lassere M, Craig J, Scott J. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database Syst Rev. 2005;(2):CD002859. doi: 10.1002/14651858.CD002859.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mak A, Cheung MW, Cheak AA, Ho RC. Combination of heparin and aspirin is superior to aspirin alone in enhancing live births in patients with recurrent pregnancy loss and positive anti-phospholipid antibodies: a meta-analysis of randomized controlled trials and meta-regression. Rheumatology (Oxford) 2010;49(2):281–288. doi: 10.1093/rheumatology/kep373. [DOI] [PubMed] [Google Scholar]
- 67.Sephton V, Farquharson RG, Topping J, Quenby SM, Cowan C, Back DJ, Toh CH. A longitudinal study of maternal dose response to low molecular weight heparin in pregnancy. Obstet Gynecol. 2003;101(6):1307–1311. doi: 10.1016/s0029-7844(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 68.Bates SM, Greer IA, Pabinger I, Sofaer S, Hirsh J. Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (8th) 2008;133(6 Suppl):844S–886S. doi: 10.1378/chest.08-0761. [DOI] [PubMed] [Google Scholar]
- 69.Bramham K, Thomas M, Nelson-Piercy C, Khamashta M, Hunt BJ. First-trimester low-dose prednisolone in refractory antiphospholipid antibody-related pregnancy loss. Blood. 2011;117(25):6948–6951. doi: 10.1182/blood-2011-02-339234. [DOI] [PubMed] [Google Scholar]
- 70.Branch DW, Peaceman AM, Druzin M, Silver RK, El-Sayed Y, Silver RM, Esplin MS, Spinnato J, Harger J. A multicenter, placebo-controlled pilot study of intravenous immune globulin treatment of antiphospholipid syndrome during pregnancy. The Pregnancy Loss Study Group. Am J Obstet Gynecol. 2000;182:122–127. doi: 10.1016/s0002-9378(00)70500-x. [DOI] [PubMed] [Google Scholar]
- 71.Diejomaoh MF, Al-Azemi MM, Bandar A, Egbase PE, Jirous J, Al-Othman S, Bukhadour N, Al-Sweih N. A favorable outcome of pregnancies in women with primary and secondary recurrent pregnancy loss associated with antiphospholipid syndrome. Arch Gynecol Obstet. 2002;266(2):61–66. doi: 10.1007/s004040100179. [DOI] [PubMed] [Google Scholar]
- 72.El-Haieg DO, Zanati MF, El-Foual FM. Plasmapheresis and pregnancy outcome in patients with antiphospholipid syndrome. Int J Gynaecol Obstet. 2007;99(3):236–241. doi: 10.1016/j.ijgo.2007.05.045. [DOI] [PubMed] [Google Scholar]
- 73.Friedman DM, Kim MY, Copel JA, Davis C, Phoon CK, Glickstein JS, Buyon JP. Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation. 2008;117(4):485–493. doi: 10.1161/CIRCULATIONAHA.107.707661. [DOI] [PubMed] [Google Scholar]
- 74.Rein AJ, Mevorach D, Perles Z, Gavri S, Nadjari M, Nir A, Elchalal U. Early diagnosis and treatment of atrioventricular block in the fetus exposed to maternal anti-SSA/Ro-SSB/La antibodies: a prospective, observational, fetal kinetocardiogram-based study. Circulation. 2009;119(14):1867–1872. doi: 10.1161/CIRCULATIONAHA.108.773143. [DOI] [PubMed] [Google Scholar]
- 75.Sonesson SE. Diagnosing foetal atrioventricular heart blocks. Scand J Immunol. 2010;72(3):205–212. doi: 10.1111/j.1365-3083.2010.02434.x. [DOI] [PubMed] [Google Scholar]
- 76.Breur JM, Visser GH, Kruize AA, Stoutenbeek P, Meijboom EJ. Treatment of fetal heart block with maternal steroid therapy: case report and review of the literature. Ultrasound Obstet Gynecol. 2004;24(4):467–472. doi: 10.1002/uog.1713. [DOI] [PubMed] [Google Scholar]
- 77.Friedman DM, Kim MY, Copel JA, Llanos C, Davis C, Buyon JP. Prospective evaluation of fetuses with autoimmune-associated congenital heart block followed in the PR Interval and Dexamethasone Evaluation (PRIDE) Study. Am J Cardiol. 2009;103(8):1102–1106. doi: 10.1016/j.amjcard.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jaeggi ET, Fouron JC, Silverman ED, Ryan G, Smallhorn J, Hornberger LK. Transplacental fetal treatment improves the outcome of prenatally diagnosed complete atrioventricular block without structural heart disease. Circulation. 2004;110(12):1542–1548. doi: 10.1161/01.CIR.0000142046.58632.3A. [DOI] [PubMed] [Google Scholar]
- 79.Tunks RD, Clowse ME, Miller SG, Brancazio LR, Barker PC. Maternal autoantibody levels in congenital heart block and potential prophylaxis with antiinflammatory agents. Am J Obstet Gynecol. 2013;208(1):64, e61–67. doi: 10.1016/j.ajog.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 80.Friedman DM, Llanos C, Izmirly PM, Brock B, Byron J, Copel J, Cummiskey K, Dooley MA, Foley J, Graves C, et al. Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block: Results of a multicenter, prospective, open-label clinical trial. Arthritis Rheum. 2010;62(4):1138–1146. doi: 10.1002/art.27308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaaja R, Julkunen H. Prevention of recurrence of congenital heart block with intravenous immunoglobulin and corticosteroid therapy: comment on the editorial by Buyon et al. Arthritis Rheum. 2003;48(1):280–281. doi: 10.1002/art.10716. author reply 281-282. [DOI] [PubMed] [Google Scholar]
- 82.Pisoni CN, Brucato A, Ruffatti A, Espinosa G, Cervera R, Belmonte-Serrano M, Sanchez-Roman J, Garcia-Hernandez FG, Tincani A, Bertero MT, et al. Failure of intravenous immunoglobulin to prevent congenital heart block: Findings of a multicenter, prospective, observational study. Arthritis Rheum. 2010;62(4):1147–1152. doi: 10.1002/art.27350. [DOI] [PubMed] [Google Scholar]
- 83.Routsias JG, Kyriakidis NC, Friedman DM, Llanos C, Clancy R, Moutsopoulos HM, Buyon J, Tzioufas AG. Association of the idiotype:antiidiotype antibody ratio with the efficacy of intravenous immunoglobulin treatment for the prevention of recurrent autoimmune-associated congenital heart block. Arthritis Rheum. 2011;63(9):2783–2789. doi: 10.1002/art.30464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clowse ME, Magder LS, Petri M. The clinical utility of measuring complement and anti-dsDNA antibodies during pregnancy in patients with systemic lupus erythematosus. J Rheumatol. 2011;38(6):1012–1016. doi: 10.3899/jrheum.100746. [DOI] [PubMed] [Google Scholar]
- 85.Verghese L, Alam S, Beski S, Thuraisingham R, Barnes I, MacCallum P. Antenatal screening for pre-eclampsia: evaluation of the NICE and pre-eclampsia community guidelines. J Obstet Gynaecol. 2012;32(2):128–131. doi: 10.3109/01443615.2011.635224. [DOI] [PubMed] [Google Scholar]
- 86.Cnossen JS, Morris RK, ter Riet G, Mol BW, van der Post JA, Coomarasamy A, Zwinderman AH, Robson SC, Bindels PJ, Kleijnen J, et al. Use of uterine artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis. CMAJ. 2008;178(6):701–711. doi: 10.1503/cmaj.070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Le Thi Huong D, Wechsler B, Vauthier-Brouzes D, Duhaut P, Costedoat N, Andreu MR, Lefebvre G, Piette JC. The second trimester Doppler ultrasound examination is the best predictor of late pregnancy outcome in systemic lupus erythematosus and/or the antiphospholipid syndrome. Rheumatology (Oxford) 2006;45(3):332–338. doi: 10.1093/rheumatology/kei159. [DOI] [PubMed] [Google Scholar]
- 88.Sciscione AC, Hayes EJ. Uterine artery Doppler flow studies in obstetric practice. Am J Obstet Gynecol. 2009;201(2):121–126. doi: 10.1016/j.ajog.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 89.Qazi U, Lam C, Karumanchi SA, Petri M. Soluble Fms-like tyrosine kinase associated with preeclampsia in pregnancy in systemic lupus erythematosus. J Rheumatol. 2008;35(4):631–634. [PubMed] [Google Scholar]
- 90.Kleinrouweler C, Wiegerinck M, Ris-Stalpers C, Bossuyt P, van der Post J, von Dadelszen P, Mol B, Pajkrt E. Accuracy of circulating placental growth factor, vascular endothelial growth factor, soluble fms-like tyrosine kinase 1 and soluble endoglin in the prediction of pre-eclampsia: a systematic review and meta-analysis. BJOG. 2012;119(7):778–787. doi: 10.1111/j.1471-0528.2012.03311.x. [DOI] [PubMed] [Google Scholar]
- 91.Kuc S, Wortelboer EJ, van Rijn BB, Franx A, Visser GH, Schielen PC. Evaluation of 7 serum biomarkers and uterine artery Doppler ultrasound for first-trimester prediction of preeclampsia: a systematic review. Obstet Gynecol Surv. 2011;66(4):225–239. doi: 10.1097/OGX.0b013e3182227027. [DOI] [PubMed] [Google Scholar]