Abstract
We demonstrate that baicalein, a bioactive flavonoid originally isolated from Scutellaria baicalensis, inhibits LPS-induced expression of iNOS gene in RAW 264.7 cells. Treatment of peritoneal macrophages and RAW 264.7 cells with baicalein inhibited LPS-stimulated nitric oxide production in a dose-related manner. Immunohistochemical staining of iNOS and RT-PCR analysis showed that the decrease of NO was due to the inhibition of iNOS gene expression in RAW 264.7 cells. Immunostaining of p65, EMSA, and reporter gene assay showed that baicalein inhibited NF-κB nuclear translocation, DNA binding, and transcriptional activation, respectively. Collectively, these series of experiments indicate that baicalein inhibits iNOS gene expression by blocking NF-κB nuclear translocation. Due to the critical role that NO release plays in mediating inflammatory responses, the inhibitory effects of baicalein on iNOS suggest that baicalein may represent a useful anti-inflammatory agent.
Keywords: Baicalein, Macrophages, iNOS, NF-κB
INTRODUCTION
Baicalein extracted from Scutellaria baicalensis, a well known medicinal plant traditionally used in China as a herbal medicine. S. baicalensis has four major bioactive flavonoid compunds: baicain, baicalein, wogonin, and oroxylin- A which have anti-inflammation and anti-cancer effects (Lin and Shieh, 1996). Baicalein is also isolated from the Oroxylum indicum or Indain trumpetflower. These flavonoids have the multiple functions such as anti-cancer, anti-platelet, anti-ischemic, and anti-inflammatory activities (Hsieh et al., 2007). Some flavonoids are related to expression of genes that are involved in synthesis or activation of several pro-inflammatory mediators such as prostaglandins, reactive oxygen species, nitric oxide and so on. Interleukin (IL)- 1, a pro-inflammatory cytokine could induce nuclear factorkappa B (NF-κB), activator protein-1 (AP-1) and others to exert its inflammatory activity (Han et al., 2009). The induction of pro-inflammatory genes by LPS is mediated via the activation of inducible transcription factors (Lee et al., 2010). This procedures will demonstrate that baicalein inhibits iNOS gene expression through the inhibition of NF-κB and nuclear translocation. Excessive production of nitric oxide (NO) has been implicated in inflammation after stresses such as ischemia.
LPS-activated macrophage cell lines are routinely used to evaluate the anti-inflammatory activities of some extract from herb and so on. Activated macrophages by bacterial LPS induces local inflammations, antibody production, and so on because it secrets a numerous material of proinflammatory cytokines, chemokines, and inflammatory meidators such as cytokines, NO, and exhibit tumoricidal activity (Aldridge et al., 2008). One of them, NO, has been related to the mechanism of many disease processes such as septic shock, rheumatoid arthritis, cerebral malaria, and autoimmune diabetes. The most well-known pathway involved in LPS-induced proinflammatory responses is the mitogen-activated protein kinase (MAPK) pathway in macrophages, which is involved intracellular signaling cascade. The MAPK among extracellular membrane stimulates signal transduction, the cytoplasmic response, and the nuclear translocation and gene activation (Kang et al., 2010). The MAPK in macrophage activates transcription factors such as NF-κB or activator factor (Ap-1) (Whitmarsh and Davis, 1996). Especially NF-κB, a major transcription factor, is closely related to the expression of proinflammatory genes. The most abundant activated form of NF-κB is a heterodimer composed of a p50 subunit and a p65 subunit, which functions predominantly as a transcriptional activator.
Since the NO production is very important in the inflammatory response of macrophages, we investigated the effect of baicalein on the production of NO. To further investigate the mechanism by which baicalein inhibits the expression of iNOS gene, we assessed the effects of baicalein on the activation of NF-κB. Since NF-κB activation requires nuclear translocation of NF-κB component p65, we focused the effect of baicalein on the nuclear translocation of p65. The present studies demonstrate that baicalein inhibits iNOS gene expression through the inhibition of NF-κB nuclear translocation.
MATERIALS AND METHODS
Materials. Baicalein was purchased from CalBiochem (San Diego, CA, USA). LPS from Salmonella thyposa was purchased from Sigma (St. Louis, MO, USA). Reagents used for cell culture were purchased from Gibco BRL (Grand Island, NY, USA). Anti-iNOS and anti-p65 antibodies were purchased from Ustate Biotechnology (Lake Placid, NY, USA) and Santa Cruz Biotechnology (Santa Cruz, CA, USA), respectively.
Cell culture. Peritoneal macrophages and RAW 264.7 cells (murine macrophage line) were purchased from American Type Culture Collection (Bethesda, MD). Cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were then cultured in the presence of 5% CO2 at 37℃. Peritoneal cells were harvested by sterile peritoneal lavage with Hanks’ balanced salt solution, washed, resuspended in culture medium, and plate at 5 × 105 cells/ ml. Nonadherent cells were removed by pepeated washing after a 2-h incubation at 37℃.
Nitrite quantitation. NO2− accumulation was used as an indicator of NO production in the medium as previously described (Green et al., 1982). Cells were plated at 5 × 105 cells/ml in 96-well culture plates and treated with baicalein for 24 hr. The isolated supernatants were mixed with an equal volume of Griess reagent (1% sulfanilamide, 0.1% naphthylethylenediamine dihydrochloride, and 2% phosphoric acid) and incubated at room temperature for 10 min. Using NaNO2 to generate a standard curve, nitrite production was measured by an O.D. reading at 550 nm.
Western immunoblot analysis. Whole cell lysates (20 μg) were separated by 10% SDS-PAGE and then electrotransferred to nitrocellulose membranes (Amersham International, Buckinghamshire, UK). The membranes were preincubated for 1 hr at room temperature in Tris-buffered saline (TBS), pH 7.6 containing 0.05% Tween-20 and 3% bovine serum albumin. The nitrocellulose membranes were incubated with iNOS-specific antibodies. Immunoreactive bands were then detected by incubation with conjugates of anti-rabbit IgG with horseradish peroxidase and enhanced chemiluminescence reagents (Amersham).
RT-PCR. Total RNA was isolated using Tri Reagent (Molecular Researh Center, Cincinnati, OH, USA) as described previously (Chomczynski and Mackey, 1995). The forward and reverse primer sequences are: iNOS: 5'-CTG CAG CAC TTG GAT CAG GAA CCT G-3', 5'-GGG AGT AGC CTG TGT GCA CCT GGA A-3' and β-actin: 5'-TGG AAT CCT GTG GCA TCC ATG AAA C-3', 5'-TAA AAC GCA GCT CAG TAA CAG TCC G-3'. Equal amounts of RNA were reverse-transcribed into cDNA using oligo(dT)15 primers. PCR was performed with cDNA and each primer. Samples were heated to 94℃ for 5 min and cycled 30 times at 94℃ for 1 min, 55℃ for 1.5 min, and 72℃ for 1 min, after which an additional extension step at 72℃ for 5 min was included. PCR products were electrophoresed in 3% NuSieve 3 : 1 gels (FMC Bioproducts, Rockland, ME) followed by staining in ethidium bromide. The iNOS and β-actin primers produce amplified products at 311 bp and 349 bp, respectively.
Transient transfection of RAW 264.7 cells. Vector constructions were performed as previously described (Jeon et al., 1996). RAW 264.7 cells were transfected using the DEAE-dextran method (Xie et al., 1993b), diluted to 5 × 105 cells per 1 ml of complete media, plated on 24 well plates, and then incubated in the presence of 5% CO2 at 37℃ for 24 hr. The transfectants were treated with LPS and baicalein. Eighteen hours later the cells were lysed with lysis buffer (250 μl). The lysates were centrifuged (12,000 ×g for 10 min at 4℃), and the supernatant was assayed for the expression of CAT enzyme using CAT ELISA kit (Roche Molecular Biochemicals, Mannheim, Germany) according to the manufacturer’s instructions.
Electrophoretic mobility shift assay (EMSA). Electrophoretic mobility shift assay (EMSA) was performed as previously described (Jeon et al., 1996). Nuclear extracts were prepared as previously described (Xie et al., 1993a). Treated and untreated RAW 264.7 cell line was lysed with hypotonic buffer (10 mM HEPES, 1.5 mM MgCl2, pH 7.5) and nuclei were pelleted by centrifugation at 3000 ×g for 5 min. Nuclear lysis was performed using a hypertonic buffer (30 mM HEPES, 1.5 mM MgCl2, 450 mM KCl, 0.3 mM EDTA, 10% glycerol, 1 mM DTT, 1 mM PMSF, 1 μg/ml of aprotinin, and 1 μg/ml of leupeptin). Following lysis, the samples were centrifuged at 14,500 ×g for 15 min, and supernatant was retained for use in the DNA binding assay. The double-stranded oligonucleotides were end-labeled with [γ-32P]-ATP. Nuclear extracts (5 μg) were incubated with poly (dI-dC) and the [32P]-labeled DNA probe in binding buffer (100 mM KCl, 30 mM HEPES, 1.5 mM MgCl2, 0.3 mM EDTA, 10% glycerol, 1 mM DTT, 1 mM PMSF, 1 μg/ml of aprotinin, and 1 μg/ml of leupeptin) for 10 min. DNA binding activity was separated from free probe using a 4% polyacrylamide gel in 0.5 × TBE buffer. Following electrophoresis, the gel was dried and subjected to autoradiography.
Statistical analysis. The mean ± SD was determined for each treatment group in a given experiment. When significant differences occurred, treatment groups were compared to the vehicle control using a Dunnett’s two-tailed t test (Dunnett, 1955).
RESULTS
Effect of baicalein on nitrite production in macrophages. We measured the accumulation of nitrite, the end product of NO, in the culture media using Griess reagent in order to investigate the effects of baicalein on NO production. Peritoneal macrophages and RAW 264.7 cells, mouse macrophage cell line, were treated with baicalein in the presence of LPS for 24 h. LPS (200 ng/ml) increased the production of nitrite ≥ 9- and 14-fold over basal levels in peritoneal macrophages and RAW 264.7 cells, respectively (Table 1). The induction in nitrite generation by LPS was inhibited by baicalein in a dose-dependent manner.
Table 1.
Inhibition of nitrite production in macrophages by baicalein
| Treatment | Nitrite (nmole/106 cells) | |
|---|---|---|
| Peritoneal cells | Control | 3.3 ± 1.2 |
| LPS (200 ng/ml) | 33.1 ± 2.3 | |
| LPS + BAI (5 μM) | 32.4 ± 1.8 | |
| LPS + BAI (10 μM) | 28.6 ± 4.3 | |
| LPS + BAI (25 μM) | 18.4 ± 3.9* | |
| LPS + BAI (50 μM) | 10.2 ± 2.3* | |
| RAW 264.7 | Control | 4.4 ± 3.5 |
| LPS (200 ng/ml) | 62.6 ± 3.4 | |
| LPS + BAI (5 μM) | 63.4 ± 2.7 | |
| LPS + BAI (10 μM) | 52.1 ± 2.1* | |
| LPS + BAI (25 μM) | 33.9 ± 6.7* | |
| LPS + BAI (50 μM) | 15.7 ± 3.3* | |
Each value shows the mean ± S.D. of triplicate determinations.
*, response that is significantly different from the control group as determined by Dunnett’s two-tailed t test at P < 0.05.
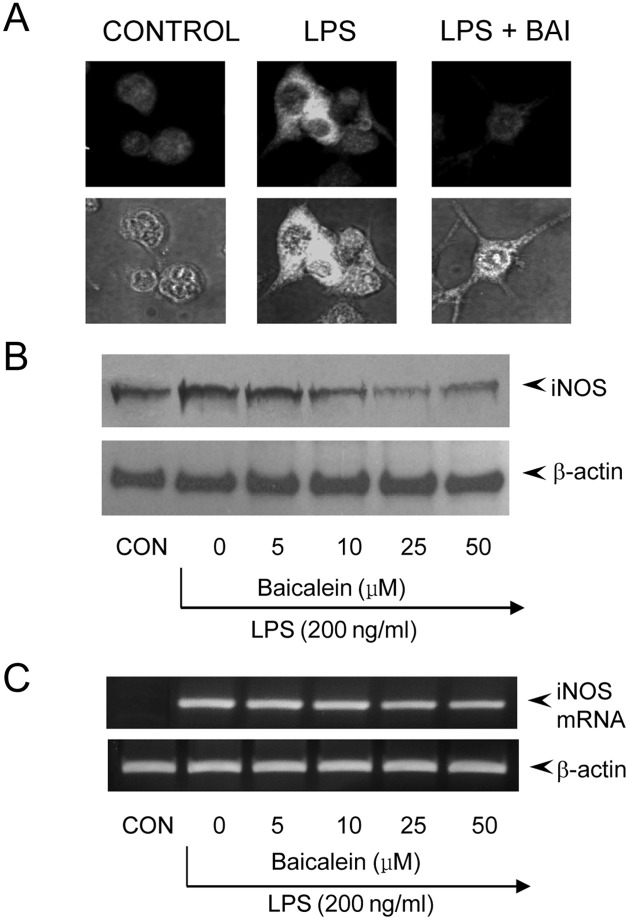
We could assure that the decreased production of NO was caused by the blocking of iNOS production in immunohistochemical staining of iNOS (Fig. 1A). No effect on cell viability was observed at any treatment groups and always exceeded 90% as determined by trypan blue staining (data not shown). After RAW 264.7 cells were exposed to baicalein in the presence of LPS, the expression level of iNOS gene was monitored by Western immunoblot analysis and RT-PCR. As shown in Fig. 1B, iNOS protein production was inhibited by baicalein treatment in a dose-dependent manner. Consistent with this finding the transcription of iNOS mRNA was inhibited by baicalein (Fig. 1C). According to these findings, we knew that the decreased production of NO in macrophage was intervened by the blocking of iNOS gene expression, but control β-actin was constitutively expressed regardless of treating baicalein in macrophage. The above results suggested that baicalein effect on the gene expression of iNOS involved in inflammation.
Fig. 1. Blocking of iNOS gene expression by baicalein in LPSstimulated RAW 264.7 cells. (A) RAW 264.7 cells (5 × 105 cells/ml) were incubated with baicalein (100 ng/ml) in the presence of LPS (200 ng/ml) for 24 hr on cover slide in 12 well plates. Cells were subjected to immunohistochemical staining using an antibody specific for murine iNOS. Immunoreactivity of iNOS was localized along the margin of the cytoplasm of in control. (B) RAW 264.7 cells were treated with baicalein in the presence of LPS (200 ng/ml) for 24 hr. Cell lysates were then prepared and subjected to Western immunoblotting. (C) Cells were incubated with baicalein in the presence of LPS for 8 hr. Total RNA was isolated and analyzed for the magnitude of mRNA expression of iNOS using RT-PCR.
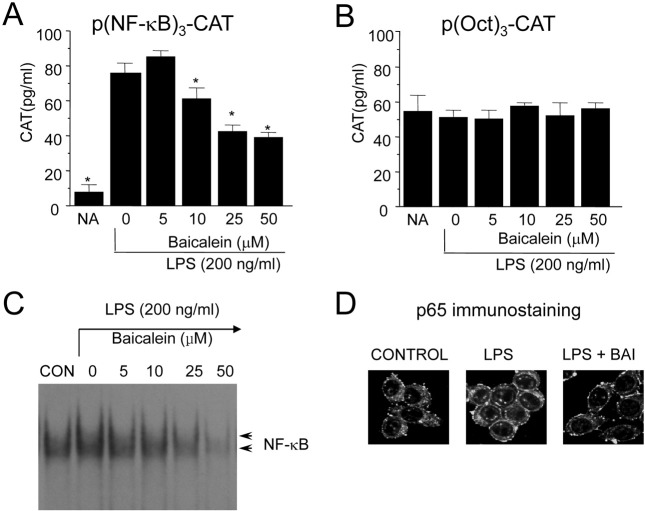
Inhibition of NF-κB in response to baicalein in LPS-stimulated RAW 264.7 cells. We examined the transcription factors whose binding sites were in the promoter of iNOS gene in order to investigate the molecular mechanism of baicalein-mediated inhibition of macrophages. The evaluation of baicalein on NF-κB was performed by using a transient transfection assay because the protein binding at the B binding site was necessary to confer inducibility by LPS of iNOS was well known (Xie et al., 1994). When RAW 264.7 cells were transiently treansfected with p(NF-κB)3-CAT, the CAT gene expressions were found to be blocked by baicalein in the presence of LPS (Fig. 3A). The basal levels of CAT expression in unstimulated RAW 264.7 cells were < 8 ± 3.1 pg/ml (mean ± standard deviation, two experiments). On LPS-stimulation, CAT expression by RAW 264.7 cells was increased by 10.3 times as much as basal level. Baicalein blocked the LPS-induced CAT expression by a dose-dependent manner. RAW 264.7 cells expressed very strong basal octamer-binding protein (Oct) activity, and its activity was influenced by neither LPS nor baicalein (Fig. 3B).
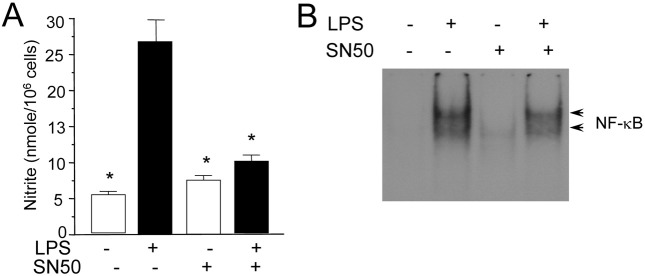
Fig. 3. Blocking of NO production by SN50, an inhibitor of NF-κB nuclear translocation. (A) RAW 264.7 cells were treated with SN50 (10 μM) in the presence or absence of LPS (200 ng/ml) for 24 hr. Nitrite generation was determined from the culture supernatant. (B), Cells were incubated with SN50 in the presence or absence of LPS for 2 hr. Nuclear extracts were then prepared and analyzed for the activity of NF-κB using EMSA.
Inhibition of NF-κB nuclear translocation by baicalein in LPS-stimulated RAW 264.7 cells. The effect of baicalein on the NF-κB whose binding motif is in the promoter of iNOS gene was evaluated using EMSA. LPS treatment of RAW 264.7 cells induced a marked increase in NF-κB binding to its cognate site. And the induction of NF-κB binding was inhibited by baicalein in a dose-related manner (Fig. 2C). The DNA binding of the NF-κB transcription factor is preceded by the nuclear translocation of NF-κB. To further investigate whether baicalein inhibits the nuclear translocation of p65, which is a component of NF-κB and has a transcriptional activation activity, we analyzed the activity using immunohistochemical staining. LPS-stimulated RAW 264.7 cells showed marked p65 staining in the nuclei, while unstimulated cells showed weaker nuclear NF-κB expression, but stronger staining in the cytoplasm. Baicalein treatment significantly inhibited LPS-induced nuclear translocation of p65 (Fig. 2D). These results indicate that baicalein decreases the nuclear translocation and DNA binding of NF-κB, which is important in the regulation of iNOS gene expression.
Fig. 2. Blocking of NF-κB activation by baicalein in LPS-stimulated RAW 264.7 cells. RAW 264.7 cells were transfected with p(NF-κB)3-CAT (A) or p(Oct)3-CAT (B) by DEAE dextran method. Twenty-four hours after transfection, cells were treated with the indicated concentrations of baicalein in the presence of LPS (200 ng/ml) for 18 hr. Cell extracts were then prepared and analyzed for the expression of CAT using CAT ELISA kit. (C). Cells (5 × 105 cells/ml) were incubated with baicalein (10, 50, 100, and 200 ng/ml) in the presence of LPS (200 ng/ml) for 2 hr. Nuclear extracts (5 μg/ml) were then isolated and analyzed for the activity of NF-κB. Reaction products were electrophoresed, and the gels were dried and autoradiographed. (D). Cells (5 × 105 cells/ ml) were incubated with baicalein (100 ng/ml) in the presence of LPS (200 ng/ml) for 2 hr on cover slide in 12 well plates. Cells were subjected to immunohistochemical staining using an antibody specific for murine p65.
We further confirmed the involvement of p65 nuclear translocation and NO production using NF-κB nuclear translocation inhibitor, SN50. SN50, cell-permeable inhibitor peptide, was reported to inhibit the nuclear translocation of NF-κB (Lin et al., 1995). Treatment of RAW 264.7 cells with SN50 blocked the LPS-induced nitrite generation (Fig. 3A) and NF-κB activation (Fig. 3B). Collectively these results demonstrate that NF-κB plays an important role in the activation of iNOS gene expression by LPS.
DISCUSSION
We demonstrate that baicalein treatment significantly attenuates LPS-induced NO production and iNOS transcription through the blocking of p65 nuclear translocation in the macrophage line RAW 264.7. Our study showed that NF-κB is positively regulated by LPS for iNOS gene expression, and baicalein treatment of RAW 264.7 cell significantly inhibited LPS-induced NF-κB activity. The NF-κB is a pleiotropic regulator of many genes involved in immune and inflammatory responses, including iNOS (Xie et al., 1994). The NF-κB family includes NF-κB1 (p50/p105), NF-κB2 (p52/p100), p65 (RelA), RelB, and c-Rel. Most members of this family (RelB being one exception) can homodimerize, as well as form heterodimers with each other. The most prevalent activated form of NF-κB is a heterodimer consisting of a p50 or p52 subunit and p65, which contains transactivation domains necessary for gene induction (Tak and Firestein, 2001). Activation of the NF-κB transcription family, by nuclear translocation of cytoplasmic complexes, plays a central role in inflammation through its ability to induce transcription of proinflammatory genes (Tak and Firestein, 2001). NF-κB exists mainly as a heterodimer consisting of subunits of the Rel Family p50 and p65, which are normally sequestered in the cystosol as an inactive complex due to binding with inhibitor proteins in unstimulated cells (Kim et al., 2010). EMSA studies showed strong induction by LPS of two separate kB binding complexes at 2 hr. Baicalein inhibited activation of both of these kB binding complexes. The inhibition of nuclear translocation of p65 by baicalein was further confirmed by Western immunoblot assay and the immunostaining of p65, respectively (Fig. 2C, Fig. 2D).
Several Chinese herbal medicines have anti-bacterial and viral properties and been used for treatment of chronic inflammation. Baicalein is flavonoid extracted from the root of Scutellaria baicalensis Georgi, which has been used as anti-inflammatory medicine in China for years. Baicalein, a ployphenolic flavonoid antioxidant, was known to have antiinflammatory, anticarcinogenic, and neuroprotective effects (Chen et al., 2004). NO which is synthesized from L-arginine by nitric oxide synthase (NOS) mediates a variety of functions including host defense, vascular homeostasis, neurotansmission, and vascular homeostasis. One of three isoforms of NOS, inducible NOS (iNOS) is the primary regulator of NO production and plays a role of the principal mediator of macrophage bactericidal and tumoricidal activities (Aldridge et al., 2008). Therefore, when there is excess or prolonged production of NO, it means that the possibility for inflammation associated tissue damage is present, implying an important role for targeted attenuation of iNOS mediated NO production (Guzik et al., 2003). Excessive production of nitric oxide in the tissue level mediated by activation of macrophage had been implicated in inflammation reaction, and baicalein inhibited the production of nitric oxide (Chen et al., 2004). Our results confirm that baicalein, as a flavonoid, could also strongly inhibit inflammatory reaction from activated macrophage because overproduction of NO predominantly via iNOS upregulation in the macrophage line RAW 264.7, contributes to numerous pathological processes, including inflammation. By our experiment the production of NO in macrophage is decreased by mediating in blocking of iNOS gene expression (Fig. 1).
In summary, these experiments demonstrate that baicalein, a bioactive flavonoid originally isolated from Scutellaria baicalensis, inhibits LPS-induced expression of iNOS gene in RAW 264.7 cells. Based on our findings, the most likely mechanism that can account for this biological effect involves the inhibition of p65 nuclear tranlocation. At least two significant points are brought out by these studies. First, these experiments further confirm the critical role of NF-κB in the regulation of iNOS. Second, due to the critical role that NO release plays in mediating inflammatory responses, the inhibitory effects of baicalein on iNOS suggest that baicalein may represent a useful anti-inflammatory agent.
Acknowledgments
This research was supported by research funds from Chosun University, 2007.
References
- 1.Aldridge C., Razzak A., Babcock T.A., Helton W.S., Espat N.J. Lipopolysaccharide-stimulated RAW 264.7 macrophage inducible nitric oxide synthase and nitric oxide production is decreased by an omega-3 fatty acid lipid emulsion. J. Surg. Res. (2008);149:296–302. doi: 10.1016/j.jss.2007.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen C.J., Raung S.L., Liao S.L., Chen S.Y. Inhibition of inducible nitric oxide synthase expression by baicalein in endotoxin/cytokine-stimulated microglia. Biochem. Pharmacol. (2004);67:957–965. doi: 10.1016/j.bcp.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Chomczynski P., Mackey K. Substitution of chloroform by bromo-chloropropane in the single-step method of RNA isolation. Anal. Biochem. (1995);225:163–164. doi: 10.1006/abio.1995.1126. [DOI] [PubMed] [Google Scholar]
- 4.Dunnett C.W. A multiple comparison procedure for comparing several treatments with a control. J. Am. Statistics. Assoc. (1955);50:1096–1121. doi: 10.2307/2281208. [DOI] [Google Scholar]
- 5.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. (1982);126:131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- 6.Guzik T.J., Korbut R., Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. (2003);54:469–487. [PubMed] [Google Scholar]
- 7.Han E.H., Park J.H., Kang K.W., Jeong T.C., Kim H.S., Jeong H.G. Risk assessment of tetrabromobisphenol A on cyclooxygenase-2 expression via MAP kinase/NF-kappaB/ AP-1 signaling pathways in murine macrophages. J. Toxicol. Environ. Health A. (2009);72:1431–1438. doi: 10.1080/15287390903212873. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh C.J., Hall K., Ha T., Li C., Krishnaswamy G., Chi D.S. Baicalein inhibits IL-1beta- and TNF-alphainduced inflammatory cytokine production from human mast cells via regulation of the NF-kappaB pathway. Clin. Mol. Allergy. (2007);5:5. doi: 10.1186/1476-7961-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon Y.J., Yang K.H., Pulaski J.T., Kaminski N.E. Attenuation of inducible nitric oxide synthase gene expression by delta 9-tetrahydrocannabinol is mediated through the inhibition of nuclear factor- kappa B/Rel activation. Mol. Pharmacol. (1996);50:334–341. [PubMed] [Google Scholar]
- 10.Kang S.W., Choi J.S., Choi Y.J., Bae J.Y., Li J., Kim D.S., Kim J.L., Shin S.Y., Lee Y.J., Kwun I.S., Kang Y.H. Licorice isoliquiritigenin dampens angiogenic activity via inhibition of MAPK-responsive signaling pathways leading to induction of matrix metalloproteinases. J. Nutr. Biochem. (2010);21:55–65. doi: 10.1016/j.jnutbio.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Kim N.H., Son Y., Jeong S.O., Moon Hur J., Soo Bang H., Lee K.N., Kim E.C., Chung H.T., Pae H.O. Tetrahydroabietic acid, a reduced abietic acid, inhibits the production of inflammatory mediators in RAW264.7 macrophages activated with lipopolysaccharide. J. Clin. Biochem. Nutr. (2010);46:119–125. doi: 10.3164/jcbn.09-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y.J., Park S.Y., Kim S.G., Park da J., Kang J.S., Lee S.J., Yoon S., Kim Y.H., Bae Y.S., Choi Y.W. Identification of a novel compound that inhibits iNOS and COX-2 expression in LPS-stimulated macrophages from Schisandra chinensis. Biochem. Biophys. Res. Commu. (2010);391:1687–1692. doi: 10.1016/j.bbrc.2009.12.131. [DOI] [PubMed] [Google Scholar]
- 13.Lin C.C., Shieh D.E. The anti-inflammatory activity of Scutellaria rivularis extracts and its active components, baicalin, baicalein and wogonin. Am. J. Chin. Med. (1996);24:31–36. doi: 10.1142/S0192415X96000050. [DOI] [PubMed] [Google Scholar]
- 14.Lin Y.Z., Yao S.Y., Veach R.A., Torgerson T.R., Hawiger J. Inhibition of nuclear translocation of transcription factor NFkappa B by a synthetic peptide containing a cell membrane permeable motif and nuclear localization sequence. J. Biol. Chem. (1995);270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 15.Tak P.P., Firestein G.S. NF-kappaB: a key role in inflammatory diseases. J. Clin. Invest. (2001);107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitmarsh A.J., Davis R.J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol .Med. (1996);74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 17.Xie H., Chiles T.C., Rothstein T.L. Induction of CREB activity via the surface Ig receptor of B cells. J. Immunol. (1993a);151:880–889. [PubMed] [Google Scholar]
- 18.Xie Q.W., Kashiwabara Y., Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem. (1994);269:4705–4708. [PubMed] [Google Scholar]
- 19.Xie Q.W., Whisnant R., Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J. Exp. Med. (1993);177:1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]