Abstract
In this research, the genotoxic effect of Picrorrhiza Rhizoma (PR) aqueous extract was evaluated using the mouse micronucleus test. PR extract was administered once a day for 2 continuous days by oral gavage to male ICR mice at doses of 2000, 1000 and 500 mg/kg. Cyclophosphamide was used as a known genotoxic agent in a positive control. The appearance of a micronucleus (MN) in polychromatic erythrocyte (PCE) is used as an index for genotoxic potential, and PCE ratio is used as an index of cytotoxicity. Although significant (p < 0.01) increase of the number of PCE with one or more nuclei (MNPCE) was detected in cyclophosphamide treated groups, no significant increases of MNPCE numbers were observed in all three different dosages of PR extracts treated mice with over 0.39 of the individual polychromatic erythrocyte ratio in all mice used in this study. The results obtained indicated that PR extract shows no genotoxicity effects up to 2000 mg/kg dosing levels.
Keywords: Picrorrhiza Rhizoma, Micronucleus test, Genotoxicity, Mice
INTRODUCTION
As increase of the concern in the functional food and well being in life, the demands and consumption of functional food originated form natural sources are increased (Lee et al., 2003). However, the toxicological aspects about these natural origin-functional foods has been neglected because of the reasons that they has been used as various purpose for long times. Therefore, it is considered that more detailed and systemic toxicological studies should be performed to control the abuse and potential toxicities even if they have been used as traditional folk medicine (Roh et al., 2009).
Bone marrow cytogenetics, micronucleus test is a useful short-term technique for elucidating the mechanism as well as to identify the substances for their clastogenic and anticlastogenic activity (Renner, 1990). In Korea Food and Drug Administration (KFDA) guideline (2009-116, 2009), the genotoxicity should be tested prior to develop a new drug even though they have natural origin. Most of natural herbal agents, genotoxicity has been performed using in vivo like micronucleus test (Kalantari et al., 2007; Roh et al., 2009).
A traditional Korean herbal medicine, Picrorrhiza Rhizoma (PR) is a dried root and stem of Picrorrhiza kurroa, and has been traditionally used as hepatoprotective agents such as jaundice (Lee and Ku, 2008a). Until now, the nitric oxide scavenging activity (Jagetia and Baliga, 2004), cardioprotective effect (Senthil Kumar et al., 2001), anti-cancer effect (Joy et al., 2000), anti-diabetic activity (Joy and Kuttan, 1999), anti-viral effect (Mehortra et al., 1990), immunostimulatory or immunomodulatory effects (Sharma et al., 1994), hypolipidemic and hepatoprotective effects (Lee et al., 2008), nephroprotective effects (Lee and Ku, 2008a), anti-inflammatory effects (Lee and Ku, 2008b; Park et al., 2008) of PR extracts have been evaluated with single mouse oral dose toxicity (Lee et al., 2006). However, there are no reports dealing the potential genotoxicity of PR extract upon our knowledge. The objective of the present study, therefore, was to obtain the genotoxic information about PR extracts, lyophilized water extract of Picrorrhiza kurroa,and further clarify their safety for clinical use.
MATERIALS AND METHODS
Animals and husbandry. Fifty male ICR mice (6-wk old upon receipt, SLC, Japan) were used after acclimatization for 10 days. The body weights of animals at receipt are ranged in 28~32 g. Animals were allocated five per polycarbonate cage in a temperature (20~25℃) and humidity (30~35%) controlled room. Light : dark cycle was 12 h : 12 h and feed (Samyang, Korea) and water were supplied free to access. Animals were marked by picric acid. This study was carried out with prior approval of the Animal Ethical Committee, The University of Daegu Haany University (Gyeongsan, Korea).
Test articles and formulation. Aqueous PR extracts (absorption rate 25.63%) were prepared by routine methods using rotary vacuum evaporator (Lab. Camp, Korea) and programmable freeze dryer (IlShin Lab., Korea) from PR, which were purchased from Cho-Heung Pharmaceutical Ind. Co. (Daegu, Korea) after confirm the morphology under microscopy. Powders of PR extracts are deep brown powder. PR extracts were stored in a refrigerator at −20℃ to protect from light and degeneration. The appearance of PR extracts in vehicle is clear deep brown solution in distilled water and it is well soluble upto 200 mg/ml concentration levels. The test article was orally administered at a dosage volume of 10 ml/kg, once a day for 2 days by oral gavage to mice; total 2000, 1000 and 500 mg/kg using distilled water as vehicle. Cyclophosphamide·H2O (CPA; Sigma, USA) was used as an identified genotoxic agents in a positive control group. CPA was dissolved in saline and once intraperitoneally administered at a volume of 10 ml/kg (70 mg/kg)
Grouping and dosing. The animals were allocated into five groups 10 mice each. The fixed highest dosage level of 2000 mg/kg oral dosing was chosen in accordance to the results of single oral dose toxicity test (Lee et al., 2006), in which no PR extract treatment-related toxicological evidences were detected upto 2000 mg/kg, the limited highest dosage in rodent recommended by KFDA guidelines (2009), and 500 and 250 mg/kg was selected using the common ratio 2. Control negative (taken vehicle) and control positive (CPA; 70 mg/kg-single treatment) were included by recommendation of KFDA guidelines (2009) and Organization for Economic Co-Operation and Development (OECD) guidelines (1997).
Observation of clinical signs. All abnormal clinical signs were recorded before and after dosing at least twice a day based on the functional observational battery test (Irwin, 1968; Dourish, 1987).
Body weight changes. Body weights were measured once a day.
Bone marrow preparation. All animals were sacrificed 24 h post administration using carbon dioxide, and bilateral femur was separated. Bone marrow preparations were made according to Schimid (1975). In brief, bone marrow cells were collected from aforementioned femur in 3 ml of inactivated fetal bovine serum (Gibco BRL, USA), centrifuged, and smeared on slides. Preparations were dried, and fixed by submerging in absolute methanol (for 10~20 min). Fixed slides were stained as follows;
May-Grunwald stain 3 min
May-Grunwald stain (1 : 1 diluted) 2 min
Giemsa stain (1 : 6 diluted) 10 min
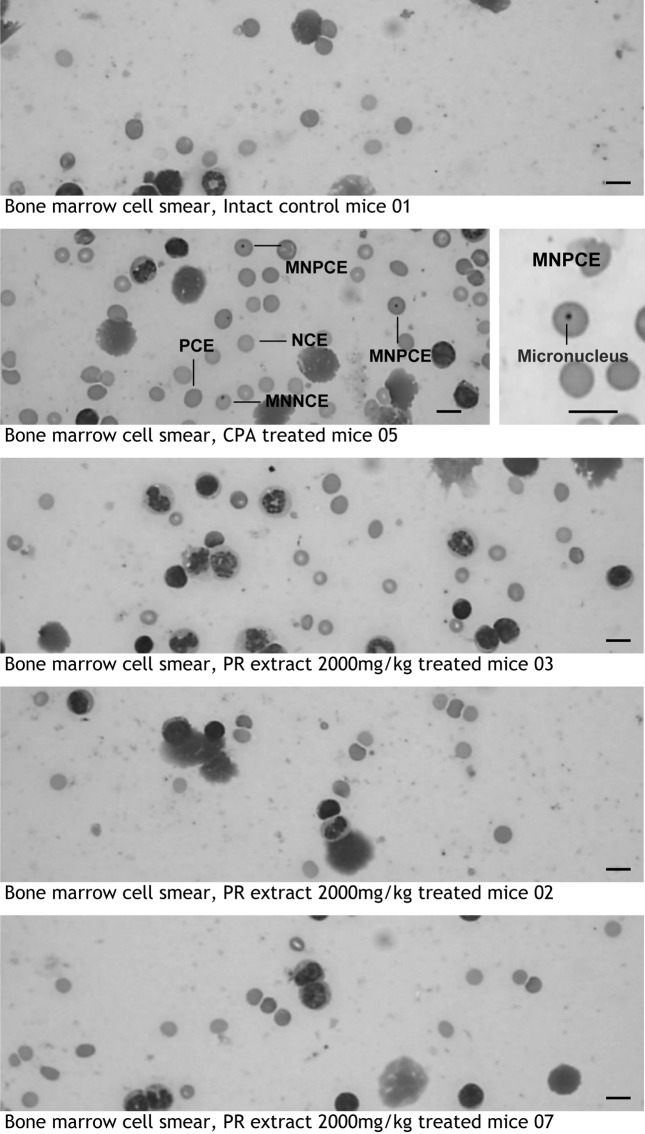
Observation and recoding of micronuclei. Slides were randomly coded and examined under × 1000 magnification by two different experts. Small round or oval shaped bodies, size of which ranging from 1/5 to 1/20 diameter of polychromatic erythrocytes (PCE), were counted as micronuclei (MN). Attention was given to discriminate micronuclei from artifacts (Fig 1). Results were expressed as the number of MNPCEs in 2000 PCEs. Mean number of MNPCE ± standard deviation was calculated for each treatment group. In addition, PCE ratio (PCE/(PCE + normochromatic erythrocytes (NCE)) ratio were also calculated by counting 1000 erythrocytes, for detecting the possibility of cytotoxicity (Heddle et al., 1984).
Fig. 1. Representative cytology of bone marrow cell smears. In prepared bone marrow cell smear, polychromatic erythrocyte (PCE), normochromatic erythrocyte (NCE), PCE with one or more nuclei (MNPCE) were counted based on the above morphology. NCE containing nucleus (MNNCE) was not calculated. Although, significant (p < 0.01) increase of MNPCEs was detected in CPA 70 mg/kg, no significant changes on MNPCE numbers were detected in all three different PR extract treated groups tested as compared with vehicle control. In addition, the PCE ratio was detected above 0.43 (in individual mice, over 0.39) in all tested groups including negative and positive control in this study. Scale bars = 10 μm.
Statistical analyses. Multiple comparison tests for different dose groups were conducted. Variance homogeneity was examined using the Levene test. If the Levene test indicated no significant deviations from variance homogeneity, the obtain data were analyzed by one way ANOVA test followed by the Scheffe test to determine which pairs of group comparison were significantly different. In case of significant deviations from variance homogeneity were observed at Levene test, a non-parametric comparison test, the Mann-Whitney U test was conducted to determine the specific pairs of group comparison. The result of statistical evaluation was regarded significantly when the P value was less than 0.05. In addition, the study was accepted when all of the PCE ratio are greater than 0.20 (Heddle et al., 1984). Statistical analyses were carried out using SPSS for Windows (Release 14.0K, SPSS Inc., USA).
RESULTS
Mortalities. No test article-treatment related unscheduled mortalities were detected in all tested doses during the observation periods.
Clinical signs. During the observation period, no abnormal clinical signs were observed from PR extract treatment.
Body weight changes. No meaningful changes on body weights were detected in CPA and all tested doses of PR extract treated groups as compared to that of control negative group (taken vehicle only) (Table 1).
Table 1.
Changes on the body weights
| Groups | Day after dosing | ||
|---|---|---|---|
| Day 01) | Day 1 | At a termination | |
| Intact control | 36.33 ± 1.30 | 41.07 ± 1.37 | 36.97 ± 1.30 |
| CPA control | 37.02 ± 1.41 | 41.30 ± 1.21 | 37.34 ± 1.53 |
| PR extract | |||
| 2000 mg/kg | 36.94 ± 1.84 | 40.97 ± 1.92 | 37.60 ± 1.57 |
| 1000 mg/kg | 36.09 ± 2.19 | 40.61 ± 3.12 | 37.14 ± 2.57 |
| 500 mg/kg | 36.78 ± 2.95 | 40.56 ± 2.59 | 37.51 ± 2.97 |
aValues are expressed as mean ± SD, g of ten mice.
1)Start day of test article administration.
All animals were overnight fasted at Day 0 and a termination, respectively.
Changes on MNPCE numbers and PCE ratio. Significantly (p < 0.01) increase of number of MNPCEs among 2000 PCEs was detected in CPA 70 mg/kg a positive control group. However, no significant changes on MNPCE numbers were detected in all three different PR extract treated groups tested as compared with vehicle control. The PCE ratio in total 500 erythrocytes was detected above 0.43 (in individual mice, over 0.39) in all tested groups including negative and positive control (Fig. 1, Table 2).
Table 2.
Changes on MNPCE numbers and PCE/(PCE + NCE) ratio observed in mice
| Group | MNPCEs/ 2000 PCEs | PCE/(PCE + NCE) | |
|---|---|---|---|
| Ratio | Range | ||
| Intact control | 1.10 ± 0.99 | 0.43 ± 0.03 | 0.40~0.50 |
| CPA control | 64.00 ± 11.12* | 0.41 ± 0.02 | 0.39~0.43 |
| DHU001 2000 mg/kg | 0.90 ± 0.99 | 0.44 ± 0.02 | 0.40~0.48 |
| DHU001 1000 mg/kg | 0.80 ± 0.79 | 0.43 ± 0.02 | 0.40~0.49 |
| DHU001 500 mg/kg | 1.00 ± 0.82 | 0.45 ± 0.05 | 0.39~0.54 |
Values are expressed as mean ± SD of ten mice.
PCE, polychromatic erythrocyte, MN, micronuclei, NCE, normochromatic erythrocyte.
PCE+NCE=1000 erythrocytes.
* p < 0.01 compared with intact control by Mann-Whitney U test.
DISCUSSION
In the present study, the genotoxic effects of PR extracts were evaluated using the mouse micronucleus test. As the results obtained in the present study, PR extract shows no genotoxicity effect up to 2000 mg/kg dosing levels. The highest dosage used in the present study was selected as 2000 mg/kg oral dosing was chosen in accordance to the results of single oral dose toxicity test (Lee et al., 2006), in which no PR extract treatment-related toxicological evidences were detected upto 2000 mg/kg, the limited highest dosage in rodent recommended by KFDA guidelines (2009), and vehicle and positive control were added according to the recommendation of KFDA (2009) and OECD (1997) guidelines.
Micronucleus assays were first introduced in the early 1970's for the examination of genotoxic activity of chemical agents (Matter and Schmid, 1971; Heddle, 1973). The procedure is based on the observation that mitotic cells with chromatid breaks or incomplete exchanges or with malfunction of the spindle apparatus suffer from disturbances in anaphase distribution of their chromatin. After telophase, a sizable portion of this displaced chromatin is not included in the nuclei of the daughter cells but forms single or multiple micronuclei in the cell cytoplasm. The frequency of the appearance of micronuclei depends both upon the rate of chromosome breakage or loss and the rate of cell division (Von Ledebur and Schmid, 1973; Heddle et al., 1984). Although micronuclei can occur in almost all dividing cells, mouse bone marrow is usually the tissue used for the micronucleus test, and any agent which induces chromosomal aberrations can also produce micronuclei (Heddle et al., 1983, 1984).
Because of its simplicity and efficacy, the micronucleus test has become a popular and useful in vivo procedure for the detection of chemically-induced chromosome damage. The number of reports from micronucleus testing has increased dramatically in the scientific literature during the past decade (Ashby, 1985), and the value of this test for examining the mutagenicity and carcinogenicity of chemicals has been emphasized, particularly when it is used in combination with other cytogenetic assays (Heddle et al., 1984).
The PCE ratio was used as index of cytotoxicity and the study was accepted when all of the PCE ratio are greater than 0.20 (Heddle et al., 1984). The PCE ratio was detected as > 0.43 in all tested groups including negative and positive control in the present study. That is no problem from cytotoxicity of the tested articles used in this work.
CPA is a widely used anti-neoplasic drug, employed either alone or in combination with other products (Grochow, 1996). The parent drug is biologically inactive, however after biotransformation by microsomal enzymes a number of active metabolites capable of alkylating nucleic acids (Miyauchi et al., 1990), damage the chromosomes (through generation of free-radicals) and/or alkylating the DNA thereby producing mutagenicity (El-Bayoumy, 2001) were produced. In the present study, CPA used as a positive control, and it showed a significant increases of MNPCE ratios. This indicates that the experiment protocol and the results of the present study are acceptable, and no meaningful increases of MNPCE were reported up to 2000 mg/kg of PR extract.
Based on the results, it is concluded that PR extract shows no genotoxicity and immunosuppress effects up to 2000 mg/kg dosing levels. In addition, it is also considered that there were no problems from cytotoxicity of PR extract because the polychromatic erythrocyte ratio was estimated as > 0.43 in all tested groups (in individual mice, over 0.39).
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A091049- 1012-0000400).
References
- 1.Ashby J. Is there a continuing role for the intraperitoneal injection route of exposure in short-term rodent genotoxicity assays? Mutat. Res. (1985);156:239–243. doi: 10.1016/0165-1218(85)90069-2. [DOI] [PubMed] [Google Scholar]
- 2.Dourish C.T., Greenshaw A.J., Dourish C.T. Effects of drugs on spontaneous motor activity in experimental psychopharmacology. Humana Press; Clifton: (1987). pp. 325–334. [Google Scholar]
- 3.El-Bayoumy K. The protective role of selenium on genetic damage and on cancer. Mutat. Res. (2001);475:123–139. doi: 10.1016/s0027-5107(01)00075-6. [DOI] [PubMed] [Google Scholar]
- 4.Grochow L.B., Perry M.C. Covalent-DNA binding drugs in The chemotherapy source book. Williams & Wilkins; Baltimore: (1996). pp. 293–316. [Google Scholar]
- 5.Heddle J.A. A rapid in vivo test for chromosome damage. Mutat. Res. (1973);18:187–190. doi: 10.1016/0027-5107(73)90035-3. [DOI] [PubMed] [Google Scholar]
- 6.Heddle J.A., Hite M., Kirkhart B., Mavournin K., MacGregor J.T., Newell G.W., Salamone M.F. The induction of micronuclei as a measure of genotoxicity: a report of the U.S. environmental protection agency gene-tox program. Mutat. Res. (1983);123:61–118. doi: 10.1016/0165-1110(83)90047-7. [DOI] [PubMed] [Google Scholar]
- 7.Heddle J.A., Stuart E., Salamone M.F., Kilbey B.J, Legator M., Nichols W., Ramel C. The bone marrow micronucleus test in Handbook of mutagenicity test procedures. Elsevier; Amsterdam: (1984). pp. 441–457. [Google Scholar]
- 8.Irwin S. Comprehensive observational assessment: Ia. A systemic, quantitative procedure for assessing the behavioral and physiological state of the mouse. Psychopharmacology. (1968);13:222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- 9.Jagetia G.C., Baliga M.S. The evaluation of nitric oxide scavenging activity of certain Indian medicinal plants in vitro: a preliminary study. J. Med. Food. (2004);7:343–348. doi: 10.1089/jmf.2004.7.343. [DOI] [PubMed] [Google Scholar]
- 10.Joy K.L., Kuttan R. Anti-diabetic activity of Picrorrhiza kurroa extract. J. Ethnopharmacol. (1999);67:143–148. doi: 10.1016/s0378-8741(98)00243-8. [DOI] [PubMed] [Google Scholar]
- 11.Joy K.L., Rajeshkumar N.V., Kuttan G., Kuttan R. Effect of Picrorrhiza kurroa extract on transplanted tumours and chemical carcinogenesis in mice. J. Ethnopharmacol. (2000);71:261–266. doi: 10.1016/s0378-8741(00)00168-9. [DOI] [PubMed] [Google Scholar]
- 12.Kalantari H., Larki A., Latifi S.M. The genotoxicity study of garlic and pasipy herbal drops by peripheral blood micronucleus test. Acta Physiol. Hung. (2007);94:261–266. doi: 10.1556/APhysiol.94.2007.3.10. [DOI] [PubMed] [Google Scholar]
- 13.Korea Food and Drug Administration. Notification No. 2009-116 Testing Guidelines for Safety Evaluation of Drugs. (2009) Aug 24;
- 14.Lee H.S., Ku S.K. Effect of Picrorrhiza rhizoma extracts on early diabetic nephropathy in streptozotocininduced diabetic rats. J. Med. Food. (2008a);11:294–301. doi: 10.1089/jmf.2007.578. [DOI] [PubMed] [Google Scholar]
- 15.Lee H.S., Lee I.K., Ku S.K. Single oral dose toxicity study of Water Extracts of Picrorrhiza Rhizoma in ICR mice. J. Toxicol. Pub. Health. (2006);22:117–126. [Google Scholar]
- 16.Lee H.S., Ku S.K. Effects of Picrorrhiza Rhizoma on acute inflammation in mice. Biomolecules. &. Therapeutics. (2008b);16:137–140. [Google Scholar]
- 17.Lee H.S., Woo S.J., Ku S.K. Hypolipemic and hepatoprotective effects of picrorrhiza rhizome in high fat diet supplied mice. A prevention study. Biomolecules. &. Therapeutics. (2008);16:46–53. [Google Scholar]
- 18.Lee J.E., Kim H.J., Lee C.H., Lee K.C., Choi E.K., Chai H.Y., Yun Y.W., Kim D.J., Nam S.Y., Lee B.J., Ahn B.W. Four-week repeated-dose toxicity study on Pinellia extract. Korean J. Lab. Anim. Sci. (2003);19:127–141. [Google Scholar]
- 19.Matter B., Schmid W. Trenimon-induced chromosomal damage in bone-marrow cells of six mammalian species, evaluated by the micronucleus test. Mutat. Res. (1971);12:417–425. doi: 10.1016/0027-5107(71)90092-3. [DOI] [PubMed] [Google Scholar]
- 20.Mehrotra R., Rawat S., Kulshreshtha D.K., Patnaik G.K., Dhawan B.N. In vitro studies on the effect of certain natural products against hepatitis B virus. Indian J. Med. Res. (1990);92:133–138. [PubMed] [Google Scholar]
- 21.Miyauchi A., Hiramine C., Tanaka S., Hojo K. Differential effects of a single dose of cyclophosphamide on T cell subsets of the thymus and spleen in mice: flow cytofluorometry analysis. Tohoku J. Exp. Med. (1990);162:147–167. doi: 10.1620/tjem.162.147. [DOI] [PubMed] [Google Scholar]
- 22.Organization for Economic Co-Operation and Development. Guideline for the Testing of Chemicals TG No. 474. Mammalian Erythrocyte Micronucleus Test. (1997) Jul 21;
- 23.Park J.H., Lee S.N., Ku S.K. Effects of Picrorrhiza Rhizoma on dinitroflurobenzene-induced contact dermatitis (Type I allergy). Biomolecules. &. Therapeutics. (2008);16:237–242. [Google Scholar]
- 24.Renner H.W. In vivo effects of single or combined dietary antimutagens on mutagen-induced chromosomal aberrations. Mutat. Res. (1990);244:185–188. doi: 10.1016/0165-7992(90)90070-z. [DOI] [PubMed] [Google Scholar]
- 25.Roh S.S., Lee H.S., Ku S.K. Micronucleus test of DHU001, a polyherbal formula, in bone marrow cells of male ICR mice. Toxicol. Res. (2009);25:225–230. doi: 10.5487/TR.2009.25.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schimid W. The Micronucleus Test. Mutat. Res. (1975);31:9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- 27.Senthil Kumar S.H., Anandan R., Devaki T., Santhosh Kumar M. Cardioprotective effects of Picrorrhiza kurroa against isoproterenol-induced myocardial stress in rats. Fitoterapia. (2001);72:402–405. doi: 10.1016/s0367-326x(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 28.Sharma M.L., Rao C.S., Duda P.L. Immunostimulatory activity of Picrorhiza kurroa leaf extract. J. Ethnopharmacol. (1994);41:185–192. doi: 10.1016/0378-8741(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 29.Von Ledebur M., Schmid W. The micronucleus test - methodological aspects. Mutat. Res. (1973);19:109–117. doi: 10.1016/0027-5107(73)90118-8. [DOI] [PubMed] [Google Scholar]