Abstract
Lipotoxicity involves pathological alterations to cells and tissues in response to elevated fat levels in blood. Furthermore, this process can disturb both cellular homeostasis and viability. In the current study, the authors show that neural progenitor cells (NPCs) are vulnerable to high levels of palmitic acid (PA) a saturated fatty acid. PA was found to cause cell death associated with elevated reactive oxygen species (ROS) levels, and to reduce NPCs proliferation. To evaluate the lipotoxicity of PA in adult NPCs in the hippocampus, male C57BL/6 mice were divided into two groups and maintained on either a normal diet (ND) or PA-rich high fat diet (HFD) for 2 weeks. Interestingly, short-term PA-rich HFD feeding reduced the survival of newly generated cells in the hippocampal dentate gyrus and hippocampal brain-derived neurotrophic factor levels. These findings suggest PA has a potent lipotoxicity in NPCs and that a PA-rich HFD disrupts hippocampal neurogenesis.
Keywords: High fat diet, Hippocampal neurogenesis, Neural progenitor cells, Brain-derived neurotrophic factor, Palmitic acid
INTRODUCTION
Lipotoxicity can be observed under altered energy balance conditions and limited fat storage, as exists in type 2 diabetes, in neurodegenerative diseases, such as, Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, and during heart failure (Ilieva et al., 2007; Chess and Stanley, 2008; Cnop, 2008; Fraser et al., 2010; Ruiperez et al., 2010). Furthermore, the plasma concentrations of free fatty acids (FFAs) are elevated in the obese and in those with metabolic disease, and these elevated levels can induce lipotoxicities, such as, cellular damage and the disruption of cellular homeostasis due to oxidative stress (Martinez de Morentin et al., 2010). In addition, elevated FA and nonesterified FA levels in plasma could impair insulin signaling and glucose response in pancreatic β-cells, and thus, aggravate the effects of obesity and metabolic disease (Cnop, 2008; Hansen et al., 2010). In particular, it has been reported that membrane phospholipids are degraded in traumatized and hypoxic-ischemic brains, and that this results in the release of FFAs, such as, palmitic acid (PA, C16:0), stearic acid (SA, C18:0), oleic acid (OA, C18:1), and docosahexaenoic acid (DHA, C22:6) (White et al., 2000). Of the FFAs, PA plays a critical role in the inhibition of the insulin signaling pathway and in the induction of ER stress in hypothalamic neurons (Mayer and Belsham, 2010). PA has been reported to induce an AD-like pathological pattern in primary cortical neurons by elevating oxidative stress and FFA metabolism in astrocytes (Patil et al., 2006; Patil et al., 2007), and PA-induced lipotoxicity has been reported to increase apoptotic cell death in PC12 cells. However, PA-induced lipotoxicity has not been previously studied in neural progenitor cells (NPCs).
NPCs have self-renewal and proliferative abilities and are capable of differentiating into neurons, astrocytes, and oligodendrocytes in the brain, and thus, NPCs are possibly of therapeutic utility for reconstruction in neurodegenerative disease (Einstein and Ben-Hur, 2008). Although NPCs exist in all brain regions during embryonic stage, it was known that NPCs in adult brain were limited only in the dentate gyrus of the hippocampus, and subventricular region of lateral ventricle. The continuation of neurogenesis into adulthood, particularly in the hippocampus, which is important for learning and memory, is of considerable importance, because it means that there is a continuous turnover of interneurons and granule cells and that newborn neurons replace dying cells and form functional synapses (van Praag et al., 2002). Adult hippocampal neurogenesis is influenced by various factors, such as aging, stress (Kuhn et al., 1996; Cameron and McKay, 1999; Warner-Schmidt and Duman, 2006; Walter et al., 2009), an enriched environment, physical exercise, and growth factors (Kempermann et al., 1997; van Praag et al., 1999; Olson et al., 2006). In particular, dietary restriction (DR) has shown been to enhance hippocampal neurogenesis by modulating brain-derived neurotrophic factor (BDNF) levels (Lee et al., 2000; Lee et al., 2002a; Lee et al., 2002b).
In this study, we examined whether PA and PA-rich HFD affects the cell proliferation in NPCs and adult hippocampal neurogenesis respectively. Our findings demonstrate that PA has lipotoxicity in NPCs, and that a PA-rich HFD harms adult hippocampal neurogenesis in young mice.
MATERIALS AND METHODS
Materials. PA, 3-[4, 5-dimethylthiazol-2-yl]-2, 5 diphenyl-tetrazolium bromide (MTT) and N-acetylcysteine (NAC) were obtained from Sigma (MO, USA). 5'-Bromo-2'-deoxyuridine (BrdU) was purchased from ACROS organics (New Jersey, USA). 2'-7'-Dichlorofluorescin diacetate (DCFDA), Hoechst 33342, and propidium iodide (PI) were supplied by Invitrogen (Oregon, USA).
Preparation of PA/BSA complex solution. PA was administered to NPCs by conjugating it with FFA-free bovine serum albumin (BSA; Roche, Germany). Briefly, PA was dissolved in ethanol and diluted in DMEM containing 2% (w/v) BSA, and incubated in a shaking water bath at 37℃ for 1 hr.
Cell proliferation and viability. C17.2 NPCs were isolated from neonatal mouse cerebellum and immortalized (Snyder et al., 1992). This neural progenitor cell line can differentiate into three brain cell types, namely, neurons, astroglia, and oligodendrocytes (Snyder et al., 1992). C17.2 NPCs were generously provided by Dr. Cepko at Harvard University, USA. C17.2 NPCs were maintained in plastic culture flasks in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum, 5% horse serum, and 2 mM glutamine in a humidified 5% CO2/95% air atmosphere at 37℃. Cell viability and proliferation were measured using MTT assays. Briefly, for the cell proliferation assay, cells (1 × 104 cells/ml) were seeded in 96-well plates with DMEM containing 10% FBS, and for the cell viability assay, cells were seeded with DMEM containing 1% FBS. After 24 hr, cells were treated with different concentrations of PA (50, 100, 200, 400 μM) for 12, 24 and 48 hr. Media were then removed, cells were washed twice with PBS, 200 μl of 0.5 mg/ml MTT solution in PBS was added per well, and cells were incubated at 37℃ for 4 hr. The MTT solution was then removed and cells were lysed in solubilization solution (1 : 1 dimethyl sulfoxide:ethanol). Formazan dye product levels were quantified using an ELISA microplate reader at 560 nm. For anti-oxidant experiments, cells were pretreated with 1 mM of NAC for 1 hr before being treated with PA.
Nuclear staining with Hoechst 33342 and PI. Cell death measurements were carried out using a fluorometric method under a fluorescence microscope, as described previously (Wrede et al., 2002). The plasma membrane is permeable to Hoechst 33342 regardless of membrane damage, and emits a blue fluorescence after binding to the nucleus. However, PI is a polar nuclear stain and can only penetrate cells with damaged membranes. NPCs were seeded in 60- mm culture dishes, allowed to attach for 24 hr, and then incubated in the presence or absence of PA for 24 hr. Hoechst 33342 and PI were then added for 10 min at final concentrations of 200 μM and 400 μM, respectively. For the anti-oxidant experiments, cells were pretreated with NAC for 1 hr before being treated with PA treatment. Images were acquired using a Nikon ECLIPSE TE 2000-U microscope.
Oxidative stress measurements. Cells were seeded onto 96-well plates for 24 hr and then washed with PBS. Cells (1 × 104 cells/ml) in DMEM containing 1% FBS medium were then treated with DCFDA (80 μM) for 30 min in a final volume of 200 μl, washed twice with PBS, and then treated with PA at different concentrations. Changes in fluorescence intensity were measured after treatment with PA for 2, 10, 20, 30, 40, 50, and 60 min using a fluorescence plate reader (GloMax, Promega) with excitation and emission wavelengths of 485 and 530 nm, respectively.
Animals and Hippocampal neurogenesis. Young (5-week-old) male C57BL/6 mice were obtained from Daehan Biolink Co. Ltd. (Chungbuk, South Korea). Animals were maintained under temperature- and light-controlled conditions (20~23℃, 12 hr light/12 hr dark cycle) and provided with food and water ad libitum. The mice were divided randomly into two groups of 5 animals; the normal diet (ND; 10% fat by energy) group and the PA-rich HFD (HFD; 45% fat by energy) group. After an acclimatization period of 1 week, animals were fed either of the two diets for 2 weeks. The formulation of the PA-rich HFD diet was as described previously, and contained 30% PA on total fat (Yun et al., 2007). The survival of newly generated cells in the DG were assessed by injecting animals intraperitoneally (i.p) with BrdU (100 mg/kg body weight; ACROS Organics, USA) for 3 consecutive days prior to diet-feeding, and proliferation of newly generated cells was assessed by injecting BrdU at the same level for the last 3 consecutive days during dietfeeding. The institutional animal care committee of Pusan National University approved the experimental protocol.
Tissue processing. Mice were euthanatized and hippocampi were dissected for biochemical studies. Tissues were snap-frozen in liquid nitrogen and stored at −80℃ until required for analysis, when tissues were homogenized with homogenate buffer containing 10 mM Tris buffer (pH 8.0) with 1.5 mM MgCl2, 1 mM DTT, 0.1% NP-40, 2 μg/ml aprotinin, and 10 μg/ml pepstatin A. For histologic studies, mice were anesthetized and perfused intracardially with 4% paraformaldehyde (PFA) in 0.1 M PBS (pH 7.4). Brains were then removed, placed in the same fixation solution at 4℃ overnight, transferred to a 30% sucrose solution, and sectioned serially at 40 μm in the coronal plane using a freezing microtome (MICROM, Germany). Sections were collected in Dulbecco’s phosphate buffered saline (DPBS) solution containing 0.1% sodium azide and stored at 4℃.
BrdU immunostaining. BrdU immunohistochemistry was performed to quantify newly generated cells numbers, as described previously (Lee et al., 2002a). After sections staining with diaminobenzidine (DAB) solution for 5 min, images were acquired using a Nikon ECLIPSE TE 2000-U microscope (Nikon, Japan). Cells in every sixth section throughout the rostro-caudal region of the hippocampus were counted. The granular cell layer of the dentate gyrus was used for reference purposes. All cell counts were performed by a single blinded investigator (HRP).
Double-label immunostaining. BrdU immunostaining was performed in concert with immunostaining for several cell markers, that is, mature neuron marker, NeuN (mouse, Chemicon) or an immature neuron marker, DCX (rabbit, Cell Signaling Technology). To detect glial cell activation, sections were stained with an astrocyte marker, GFAP (mouse, Cell Signaling), and a microglia marker, Iba-1 (rabbit, Wako). The sections were then blocked with 3% normal goat serum (Gibco, Grand Island, USA), incubated with primary antibody at 4℃ overnight, washed with TBS, and incubated for 3 hr in the presence of secondary antibody labeled with Alexa Fluor-488 or 568. Images were acquired using FV10i FLUOVIEW confocal microscope (Olympus, Japan).
Nissl staining. Brian sections were mounted on slides, dried overnight, hydrated using an ethanol series, stained with cresyl violet, dehydrated in an ethanol series, and cleared with xylene. They were then mounted using permanent mounting medium (Fisher Scientific, Fair Lawn, NJ, USA) and coverslipped. Images were acquired using a Nikon ECLIPSE TE 2000-U microscope.
Analysis of BDNF levels. BDNF protein levels in hippocampus of ND or PA-rich HFD-fed mice were quantified using a commercially available kit (Promega Co., Madison, WI). Briefly, hippocampus homogenates were acidified and then neutralized. Ninety-six-well plates were then coated with monoclonal BDNF antibody, incubated in blocking and sample buffer, and washed in TBS/0.05% Tween 20 (TBST). Prepared samples were then added to three wells per plate, Plates were incubated in anti-Human BDNF polyclonal antibody for 2 hr. Wells were then washed with TBST and then incubated with anti-IgY HRP conjugate at RT for 1 hr. Finally, TMB One Solution (Promega Co., Madison, WI) was added. The reaction was stopped with 1 N HCl, and absorbance was measured at 450 nm using a plate reader.
Statistical analysis. Analysis of variance (ANOVA) with Fisher’s protected least significant difference (PLSD) procedure was used to determine the significances of differences between groups. The analysis was performed using Statview software®, and p values of < 0.05 were considered significant.
RESULTS
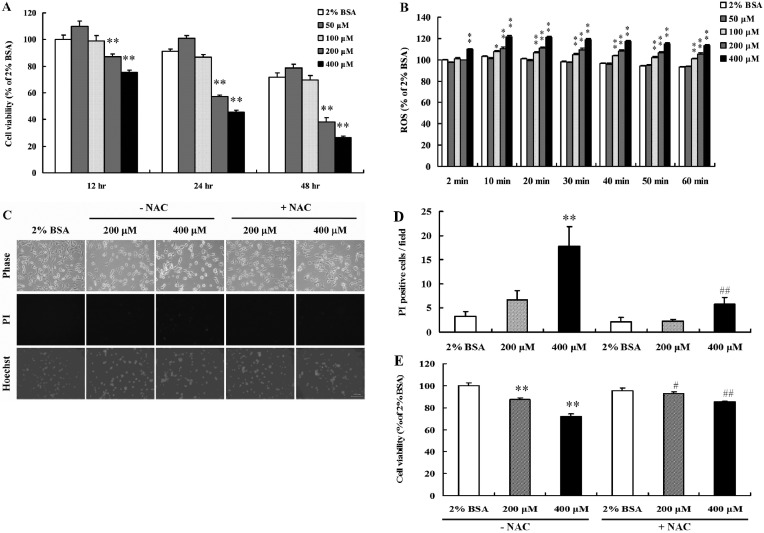
PA decreased neural progenitor cells viability and caused cell death by inducing oxidative stress. PA-induced lipotoxicity was evaluated in NPCs in low serum (1% FBS) containing medium. PA was conjugated with BSA because PA does not enter the intracellular space and BSA has high affinity for FFAs (Spector, 1975). BSA alone did not affect NPCs viability. At high concentrations (200 and 400 μM) PA significantly reduced NPCs viability (Fig. 1A). In addition, PA significantly elevated intracellular ROS levels significantly in a concentration-dependent manner (Fig. 1B), suggesting that PA-induced oxidative stress might be involved in lipotoxicity of PA. Thus, NPCs were pretreated with or without NAC (an antioxidant) for 1 hr, and then PA was administered at the indicated concentrations for 24 hr. Numbers of PI-stained cells were significantly increased by 200 and 400 μM PA (Fig. 1C and 1D). PA did not showed condensation of nuclei in Hoechst staining, thus we suggested that PA induced necrotic cell death in NPCs. However, NAC effectively blocked PA-mediated cell death and increased cell viability (Fig. 1C, 1D and 1E). These results suggest that PA-induced lipotoxicity is mediated by ROS generation and that it can be modulated by antioxidants.
Fig. 1. PA decreased NPCs viability and induced oxidative stress. NPCs were seeded into 96-well plates (1 × 104 cells/ml), cultured for 24 hr, and treated with the indicated concentrations of PA for 12, 24, and 48 hr. (A) NPCs viabilities were determined using MTT assays. High concentrations of PA were found to have an inhibitory effect on NPCs viability. The values reported are means±SE (n=8). (B) Total intracellular ROS levels in NPCs exposed to several concentrations of PA were measured using the DCFDA method. PA increased ROS levels in a concentration-dependent manner. Values are reported as means ± SE (n = 8). *p < 0.05, **p < 0.01, compared with the 2% BSA-treated group (ANOVA with Fisher’s PLSD procedure). (C) NPCs pretreated with NAC for 1 hr were exposed to PA (200 or 400 μM) for 24 hr, and then 10 or 50 μM of Hoechst 33342 and PI were added for 10 min. Hoechst 33342- and PI-stained cells were stained bright blue and red, respectively. PA decreased cell numbers and induced necrotic cell death was detected by PI staining. Scale bar = 100 μm. (D) Quantitative analysis of the number of PI-stained NPCs. The values are reported as the means ± SE (n = 4 or 5). (E) NAC prevented the reduction in cell viability induced by PA. Cells were treated with several concentrations of PA in the presence or absence of NAC. Values are reported as the means ± SE (n = 8). **p < 0.01, compared with the 2% BSA-treated group in the absence of NAC, #p < 0.05, ##p < 0.01, compared with each PA concentration in the absence of NAC (ANOVA with Fisher’s PLSD procedure).
PA affected NPCs proliferation. The self-renewing and proliferative abilities of NPCs are important for maintaining the neural stem cell pool in the adult and in the developing brain. The effect of PA on NPCs proliferation was evaluated in normal serum (10% FBS) containing medium. In culture NPCs showed exponential growth. PA at 400 μM impaired NPCs proliferation at 24 hr, over 50 μM of PA significantly affected NPCs growth at 48 hr (Fig. 2A). This PA-mediated impairment of NPCs proliferation was confirmed by BrdU incorporation measurements (Fig. 2B). Taken together, these results show that PA impairs both NPCs viability and proliferation.
Fig. 2. PA reduced NPCs proliferation. (A) NPCs were seeded into 96-well plates (1 × 104 cells/ml) and cultured for 24 hr. The cells were then treated with the indicated concentrations of PA for 12, 24, or 48 hr. Proliferation was determined using MTT assays. High concentrations of PA were found to have an inhibitory effect on NPCs proliferation. (B) BrdU immunostained cells were counted under a microscope and quantified. Values are means ± SE (n = 4). **p < 0.01 compared with vehicle (ANOVA with Fisher’s PLSD procedure).
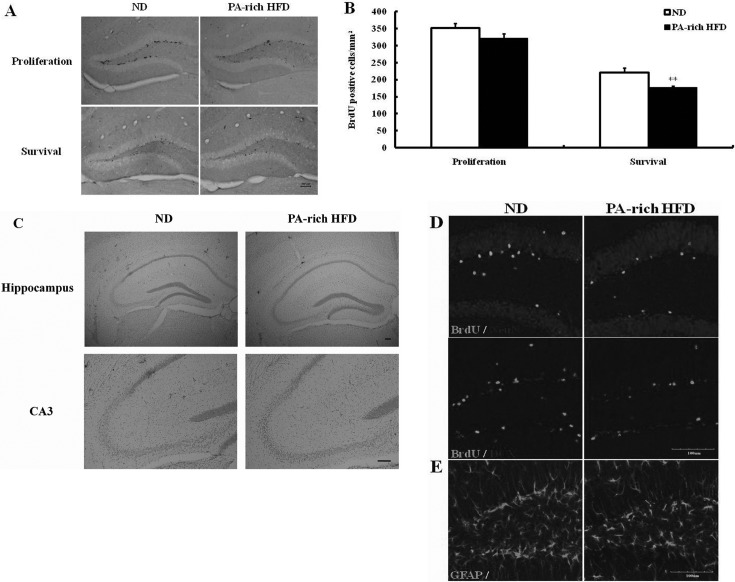
The PA-rich HFD decreased hippocampal neurogenesis but did not affect neuronal differentiation or glial activation. In order to determine whether PA-rich HFD affected adult NPCs in the hippocampus, six-week old C57BL/6 mice were fed either a ND or a PA-rich HFD for 2 weeks; body weight were monitored every two days. Body weight gradually increased in both groups and PA-rich HFD-fed mice significantly gained more body weight (data not shown). Six doses of BrdU were administered i.p. for the first or last 3 days of diet feeding to determine the survival and proliferation, respectively, of newly generated hippocampal NPCs. Dividing cells were visualized by BrdU immunohistochemistry. It was found that numbers of newly generated cells in the DG were unaffected by 2 weeks of PA-rich HFD. However, the survival of newly-generated cells after 2 weeks of PA-rich HFD were significantly diminished (Fig. 3A and B). Nissl staining revealed that a PA-rich HFD did not change neuronal density, hippocampal shape, or cause neuronal loss and damage, indicating that short-term PA-rich HFD was insufficient to cause histological and pathological changes in the hippocampus (Fig. 3C). To evaluate the neuronal differentiation of newly generated cells in the dentate gyrus, we performed double labeling using antibodies against the immature neuron marker, DCX, or the mature neuron marker, NeuN, in combination with BrdU. PA-rich HFD decreased the numbers of BrdU-positive cells in the dentate gyrus. The majority of BrdU-positive cells were located in the granule cell layer and colabeled with DCX or NeuN in the dentate gyrus of animals fed a ND or a PA-rich HFD. However, PA-rich HFD was not found to significantly affect the rate of neuronal differentiation (Fig. 3D). We also evaluated, by double labeling with a microglia marker (Iba-1) and an astrocyte marker (GFAP), whether PA-rich HFD induced glial activation, but no clear evidence of neuroinflammation was observed (Fig. 3E).
Fig. 3. PA-rich HFD decreased BrdU labeled cell counts in the hippocampal dentate gyrus. (A) Representative images showing BrdU-positive cells in the dentate gyrus. Scale bar = 100 μm. (B) Quantitative analysis of the number of BrdU-labeled cells and the areas of DG examined in the dentate gyri of mice fed a ND or a PA-rich HFD. The PA-rich HFD reduced numbers of BrdU-positive cells in the dentate gyrus. Values are means ± SE (n=5 mice/group). *p<0.05 compared with the ND group (ANOVA with Fisher’s PLSD procedure). (C) Prepared brain sections from experimental animals were stained with cresyl violet. Scale bar = 100 μm. (D) To determine the effect of PA-rich HFD on neuronal differentiation, immunohistochemistry was performed with primary antibodies against BrdU with NeuN or DCX. Scale bar = 100 μm. (E) In order to examine glial activation by the ND or the PA-rich HFD, double-labeling was performed with Iba-1 and GFAP. Scale bar = 100 μm.
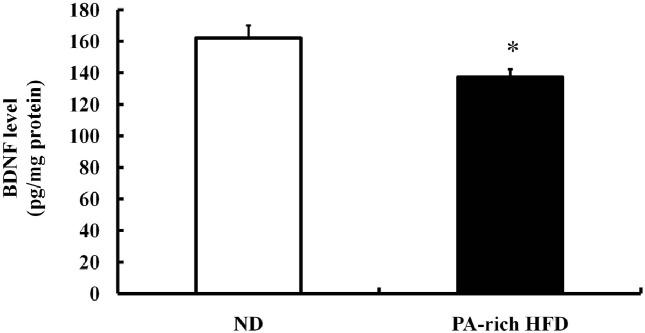
Hippocampal BDNF levels were reduced by PA-rich HFD. Adult hippocampal neurogenesis can be regulated by growth factors, such as, BDNF, VEGF, and IGF-1 (Lee et al., 2000; Anderson et al., 2002; Lee et al., 2002a; Cao et al., 2004). In the present study, we found that PA-rich HFD decreased levels of BDNF protein in the hippocampus (Fig. 4), which suggested a mechanism whereby PA-rich HFD impairs the survival of newly generated cells in the hippocampus.
Fig. 4. The PA-rich HFD reduced hippocampal BDNF levels. BDNF protein levels in hippocampal tissue homogenates from ND or PA-rich HFD mice were measured by quantitative ELISA. The PA-rich HFD reduced BDNF protein levels in the hippocampus. Values are means ± SE (n=5 mice/group). *p<0.05 compared with ND-fed group at each time point (ANOVA with Fisher’s PLSD procedure).
DISCUSSION
In the current study, we evaluated the lipotoxic potency of PA on the NPCs viability and proliferation, and on neurogenesis in the hippocampus. Our results indicate that PA reduces the viability of NPCs, and that its lipotoxicity is mediated by an elevation in ROS levels. Furthermore, a PA-rich HFD was found to impair hippocampal neurogenesis and to down-regulate hippocampal BDNF levels.
Dietary fatty acid levels may affect body weight regulation and cell/tissue function (Bueno et al., 2010; Hariri et al., 2010; Yu et al., 2010). Essential n-3 polyunsaturated fatty acids (PUFA) have been shown to have neuroprotective effects in Alzheimer’s disease and in a model of ischemia (Amtul et al., 2011; Zhang et al., 2010). In a recent study, it was found that the spatial learning and memory deficits induced by IL-1β in a murine model were reduced by dietary n-3 PUFA (Taepavarapruk and Song, 2010). However, previous studies have shown that FFAs can be produced during pathological conditions or conditions of energy imbalance, and that these negatively affect cells and organs (Martinez de Morentin et al., 2010). Hypoxic-ischemic injury causes tissue damage and membrane lipid degradation, which leads to the accumulation of FFAs, excessive oxidative stress and lipid peroxidation (White et al., 2000). Furthermore, the limited storage capacity of adipose tissues caused by an imbalance between energy intake and energy expenditure, induces the productions of toxic reactive lipid species that harm non-adipose tissues (Martinez de Morentin et al., 2010), and thus, the aberrant productions of FFAs could lead to secondary cells and tissue damage. In particular, PA is well known to be lipotoxic via elevated oxidative stress, apoptotic cell death, and ER stress (Ulloth et al., 2003; Yamato et al., 2007; Almaguel et al., 2009; Mayer and Belsham, 2010). However, its potency on proliferating neural stem cells has not been studied previously. In the present study, we found that a high concentration (200 and 400 μM) of PA reduced NPCs viability and induced necrosis, which concurs with a previous report that PA reduces cell viability and increases the apoptosis of PC12 cells and cortical neurons (Ulloth et al., 2003; Almaguel et al., 2009). In addition, FFA-induced ROS in neurons has been reported to impair mitochondrial functions and caused apoptotic cell death (Mattson, 1998; Almaguel et al., 2009), and lipotoxicity has been reported to induce oxidative stress and the subsequent activations of JNK and p38 (stress-mediated signal molecules) (Mayer and Belsham, 2010). Similarly, in the present study, elevated ROS levels in NPCs were observed after PA exposure, and the anti-oxidant NAC was found to block PA-mediated cytotoxicity effectively. These findings indicate that the lipotoxicity of PA is closely mediated by oxidative stress.
We also observed that PA reduced NPCs proliferation, suggesting the possibility that PA-induced lipotoxicity affects the NPCs growth. In order to evaluate lipotoxicity in adult hippocampal NPCs, we utilized a FFA containing diet model and examined hippocampal neurogenesis. In our previous study, we found that 7 weeks of PA-rich HFD feeding reduced the proliferation of newly generated cells in the hippocampus via BDNF down-regulation and increased lipid peroxidation (Park et al., 2010). However, in the present study, we applied short-term PA-rich HFD feeding to exclude the indirect effect of obesity on hippocampal neurogenesis, and although mice fed PA-rich HFD for 2 weeks had slightly greater body weights than ND mice, they could not be described as obese (data not shown). It has been reported that mice on a short-term PA-rich HFD do not develop obesity- induced symptoms or pathological changes (Araki et al., 2008; Comhair et al., 2011). In the present study, we found that the survival of newly generated NPCs after 2 weeks on the PA-rich HFD was significantly reduced, but NPCs proliferation in the dentate gyrus was not affected. These findings suggest that 2 weeks on a PA-rich HFD is not enough to alter NPCs proliferation in the hippocampus, but that this treatment does reduce the survival of newly generated cells. However, it is believed that short-term PA-rich HFD feeding did not affect the fate of newly generated cells because these cells differentiated into granule neurons in the dentate gyrus in both ND and PA-rich HFD mice.
The proliferation, differentiation, maturation, and integration of newly generated cells in the hippocampus are influenced by neurotrophic factors. In particular, BDNF promotes neural plasticity, neurogenesis, neuronal survival, and neurite outgrowth (Lee et al., 2000; Lee et al., 2002a; Lee et al., 2002b). Moreover, altered BDNF levels in BDNF mutant mice affect food intake and body weight, acting as an anorexigenic factor (Pelleymounter et al., 1995; Kernie et al., 2000; Rios et al., 2001). Taken together, BDNF regulates obesity and central energy balance. Furthermore, it has been suggested to play a critical role during neurogenesis, and reported a protective role during NPCs proliferation (Park et al., 2010). In the present study, we found that a PA-rich HFD decreased hippocampal BDNF levels without inducing neuronal damage or glial activation, which suggests that hippocampal neurogenesis might be markedly affected by a short duration PA-rich HFD.
Summarizing, PA was found to reduce NPCs viability and proliferation by elevating intracellular oxidative stress. Furthermore, short-term PA-rich HFD impairs hippocampal neurogenesis by reducing the survival of newly generated cells and BDNF levels in the hippocampus. Thus, we conclude that PA-induced lipotoxicity harmfully affects NPCs and adult hippocampal neurogenesis, and thus, could lead to learning and memory impairments and neurocognitive dysfunction.
Acknowledgments
This work was supported for two years by Pusan National University Research Grant (2009-2010).
References
- 1.Almaguel F.G., Liu J.W., Pacheco F.J., Casiano C.A., De Leon M. Activation and reversal of lipotoxicity in PC12 and rat cortical cells following exposure to palmitic acid. J. Neurosci. Res. (2009);87:1207–1218. doi: 10.1002/jnr.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amtul Z., Uhrig M., Rozmahel R.F., Beyreuther K. Structural basis for the differential effects of omega-3 and omega-6 fatty acids on Abeta production and amyloid plaques. J. Biol. Chem. (2010);286:6100–6107. doi: 10.1074/jbc.M110.183608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson M.F., Aberg M.A., Nilsson M., Eriksson P.S. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res. Dev. Brain Res. (2002);134:115–122. doi: 10.1016/s0165-3806(02)00277-8. [DOI] [PubMed] [Google Scholar]
- 4.Araki H., Nishihara T., Matsuda M., Fukuhara A., Kihara S., Funahashi T., Kataoka T.R., Kamada Y., Kiyohara T., Tamura S., Hayashi N., Shimomura I. Adiponectin plays a protective role in caerulein-induced acute pancreatitis in mice fed a high-fat diet. Gut. (2008);57:1431–1440. doi: 10.1136/gut.2007.135665. [DOI] [PubMed] [Google Scholar]
- 5.Bueno A.A., Oyama L.M., de Macedo Motoyama C.S., da Silva Biz C.R., Silveira V.L., Ribeiro E.B., Oller do Nascimento C.M. Long chain saturated fatty acids increase haptoglobin gene expression in C57BL/6J mice adipose tissue and 3T3-L1 cells. Eur. J. Nutr. (2010);49:235–241. doi: 10.1007/s00394-009-0069-z. [DOI] [PubMed] [Google Scholar]
- 6.Cameron H.A., McKay R.D. Restoring production of hippocampal neurons in old age. Nat. Neurosci. (1999);2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- 7.Cao L., Jiao X., Zuzga D.S., Liu Y., Fong D.M., Young D., During M.J. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat. Genet. (2004);36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 8.Chess D.J., Stanley W.C. Role of diet and fuel overabundance in the development and progression of heart failure. Cardiovasc. Res. (2008);79:269–278. doi: 10.1093/cvr/cvn074. [DOI] [PubMed] [Google Scholar]
- 9.Cnop M. Fatty acids and glucolipotoxicity in the pathogenesis of Type 2 diabetes. Biochem. Soc. Trans. (2008);36:348–352. doi: 10.1042/BST0360348. [DOI] [PubMed] [Google Scholar]
- 10.Comhair T.M., Garcia Caraballo S.C., Dejong C.H., Lamers W.H., Koehler S.E. Dietary cholesterol, female gender and n-3 fatty acid deficiency are more important factors in the development of non-alcoholic fatty liver disease than the saturation index of the fat. Nutr. Metab. (Lond) (2011);8:4. doi: 10.1186/1743-7075-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Einstein O., Ben-Hur T. The changing face of neural stem cell therapy in neurologic diseases. Arch. Neurol. (2008);65:452–456. doi: 10.1001/archneur.65.4.452. [DOI] [PubMed] [Google Scholar]
- 12.Fraser T., Tayler H., Love S. Fatty acid composition of frontal, temporal and parietal neocortex in the normal human brain and in Alzheimer's disease. Neurochem. Res. (2010);35:503–513. doi: 10.1007/s11064-009-0087-5. [DOI] [PubMed] [Google Scholar]
- 13.Hansen D., Dendale P., Beelen M., Jonkers R.A., Mullens A., Corluy L., Meeusen R., van Loon L.J. Plasma adipokine and inflammatory marker concentrations are altered in obese, as opposed to non-obese, type 2 diabetes patients. Eur. J. Appl. Physiol. (2010);109:397–404. doi: 10.1007/s00421-010-1362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hariri N., Gougeon R., Thibault L. A highly saturated fat-rich diet is more obesogenic than diets with lower saturated fat content. Nutr. Res. (2010);30:632–643. doi: 10.1016/j.nutres.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Ilieva E.V., Ayala V., Jove M., Dalfo E., Cacabelos D., Povedano M., Bellmunt M.J., Ferrer I., Pamplona R., Portero- Otin M. Oxidative and endoplasmic reticulum stress interplay in sporadic amyotrophic lateral sclerosis. Brain. (2007);130:3111–3123. doi: 10.1093/brain/awm190. [DOI] [PubMed] [Google Scholar]
- 16.Kempermann G., Kuhn H.G., Gage F.H. More hippocampal neurons in adult mice living in an enriched environment. Nature. (1997);386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 17.Kernie S.G., Liebl D.J., Parada L.F. BDNF regulates eating behavior and locomotor activity in mice. Embo J. (2000);19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn H.G., Dickinson-Anson H., Gage F.H. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. (1996);16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J., Duan W., Long J.M., Ingram D.K., Mattson M.P. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J. Mol. Neurosci. (2000);15:99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- 20.Lee J., Duan W., Mattson M.P. Evidence that brainderived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. (2002a);82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee J., Seroogy K.B., Mattson M.P. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J. Neurochem. (2002b);80:539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- 22.Martinez de Morentin P.B., Varela L., Ferno J., Nogueiras R., Dieguez C., Lopez M. Hypothalamic lipotoxicity and the metabolic syndrome. Biochim. Biophys. Acta. (2010);1801:350–361. doi: 10.1016/j.bbalip.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Mattson M.P. Modification of ion homeostasis by lipid peroxidation: roles in neuronal degeneration and adaptive plasticity. Trends Neurosci. (1998);21:53–57. doi: 10.1016/s0166-2236(97)01188-0. [DOI] [PubMed] [Google Scholar]
- 24.Mayer C.M., Belsham D.D. Palmitate attenuates insulin signaling and induces endoplasmic reticulum stress and apoptosis in hypothalamic neurons: rescue of resistance and apoptosis through adenosine 5' monophosphate-activated protein kinase activation. Endocrinology. (2010);151:576–585. doi: 10.1210/en.2009-1122. [DOI] [PubMed] [Google Scholar]
- 25.Olson A.K., Eadie B.D., Ernst C., Christie B.R. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. (2006);16:250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- 26.Park H.R., Park M., Choi J., Park K.Y., Chung H.Y., Lee J. A high-fat diet impairs neurogenesis: involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci. Lett. (2010);482:235–239. doi: 10.1016/j.neulet.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 27.Patil S., Melrose J., Chan C. Involvement of astroglial ceramide in palmitic acid-induced Alzheimer-like changes in primary neurons. Eur. J. Neurosci. (2007);26:2131–2141. doi: 10.1111/j.1460-9568.2007.05797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patil S., Sheng L., Masserang A., Chan C. Palmitic acid-treated astrocytes induce BACE1 upregulation and accumulation of C-terminal fragment of APP in primary cortical neurons. Neurosci. Lett. (2006);406:55–59. doi: 10.1016/j.neulet.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Pelleymounter M.A., Cullen M.J., Wellman C.L. Characteristics of BDNF-induced weight loss. Exp. Neurol. (1995);131:229–238. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 30.Rios M., Fan G., Fekete C., Kelly J., Bates B., Kuehn R., Lechan R.M., Jaenisch R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. (2001);15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 31.Ruiperez V., Darios F., Davletov B. Alpha-synuclein, lipids and Parkinson’s disease. Prog. Lipid Res. (2010);49:420–428. doi: 10.1016/j.plipres.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Snyder E.Y., Deitcher D.L., Walsh C., Arnold-Aldea S., Hartwieg E.A., Cepko C.L. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. (1992);68:33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- 33.Spector A.A. Fatty acid binding to plasma albumin. J. Lipid Res. (1975);16:165–179. [PubMed] [Google Scholar]
- 34.Taepavarapruk P., Song C. Reductions of acetylcholine release and nerve growth factor expression are correlated with memory impairment induced by interleukin-1beta administrations: effects of omega-3 fatty acid EPA treatment. J. Neurochem. (2010);112:1054–1064. doi: 10.1111/j.1471-4159.2009.06524.x. [DOI] [PubMed] [Google Scholar]
- 35.Ulloth J.E., Casiano C.A., De Leon M. Palmitic and stearic fatty acids induce caspase-dependent and -independent cell death in nerve growth factor differentiated PC12 cells. J. Neurochem. (2003);84:655–668. doi: 10.1046/j.1471-4159.2003.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Praag H., Kempermann G., Gage F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. (1999);2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 37.van Praag H., Schinder A.F., Christie B.R., Toni N., Palmer T.D., Gage F.H. Functional neurogenesis in the adult hippocampus. Nature. (2002);415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walter J., Keiner S., Witte O.W., Redecker C. Agerelated effects on hippocampal precursor cell subpopulations and neurogenesis. Neurobiol. Aging. (2009) doi: 10.1016/j.neurobiolaging.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Warner-Schmidt J.L., Duman R.S. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. (2006);16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 40.White B.C., Sullivan J.M., DeGracia D.J., O’Neil B.J., Neumar R.W., Grossman L.I., Rafols J.A., Krause G.S. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J. Neurol. Sci. (2000);179:1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- 41.Wrede C.E., Dickson L.M., Lingohr M.K., Briaud I., Rhodes C.J. Protein kinase B/Akt prevents fatty acidinduced apoptosis in pancreatic beta-cells (INS-1). J. Biol. Chem. (2002);277:49676–49684. doi: 10.1074/jbc.M208756200. [DOI] [PubMed] [Google Scholar]
- 42.Yamato M., Shiba T., Yoshida M., Ide T., Seri N., Kudou W., Kinugawa S., Tsutsui H. Fatty acids increase the circulating levels of oxidative stress factors in mice with dietinduced obesity via redox changes of albumin. Febs J. (2007);274:3855–3863. doi: 10.1111/j.1742-4658.2007.05914.x. [DOI] [PubMed] [Google Scholar]
- 43.Yu H., Bi Y., Ma W., He L., Yuan L., Feng J., Xiao R. Long-term effects of high lipid and high energy diet on serum lipid, brain fatty acid composition, and memory and learning ability in mice. Int. J. Dev. Neurosci. (2010);28:271–276. doi: 10.1016/j.ijdevneu.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Yun J.W., Lee B.S., Kim C.W., Kim B.H. Comparison with 3 high-fat diet for studying obesity in C57BL/6 mouse. Lab. Anim. Res. (2007);23:245–250. [Google Scholar]
- 45.Zhang W., Hu X., Yang W., Gao Y., Chen J. Omega-3 polyunsaturated fatty acid supplementation confers long-term neuroprotection against neonatal hypoxic-ischemic brain injury through anti-inflammatory actions. Stroke. (2010);41:2341–2347. doi: 10.1161/STROKEAHA.110.586081. [DOI] [PMC free article] [PubMed] [Google Scholar]