Abstract
Selective estrogen receptor modulators (SERMs) are synthetic molecules which bind to estrogen receptors (ER) and can modulate its transcriptional capabilities in different ways in diverse estrogen target tissues. Tamoxifen, the prototypical SERM, is extensively used for targeted therapy of ER positive breast cancers. Unfortunately, the use of tamoxifen is associated with acquired resistance and some undesirable side effects. This study investigated the availability of the conventional SERMs on the TAM-resistance breast cancer cells. SERMs showed more effectiveness in MCF-7 cells than tamoxifen resistant cells, except toremifene and ospemifene. Especially, toremifene was more efficacious in tamoxifen resistant cells than MCF-7. Ospemifene had similar cytotoxic activity on the two types of breast cancers. The other SERMs used in this experiment didn’t inhibit efficiently the proliferation of tamoxifen resistant cells. These results support the possibility to usage of toremifene on tamoxifen resistant cancer. The effectiveness by toremifene on tamoxifen resistant cells might be different pathways from the apoptosis and the autophagy. Further study should be needed to elucidate the underlying mechanism of effect of toremifene on tamoxifen resistant cancer.
Keywords: Selective estrogen receptor modulators (SERMs), Tamoxifen resistant breast cancer, Toremifene, Apoptosis, Autophagy
INTRODUCTION
Breast cancer is one of the common cancers over the world in women, approximately 180,000 new case and 40,000 per year in Unites States were reported. The incidence of this disease in several Asian countries has been dramatically increased. Over seventy per cent breast cancers have estrogen receptors, especially estrogen receptor alpha (ERα) and require the hormone to grow. Lowering the estrogen levels can slow the growth of the breast cancer. Breast cancers are treated with drugs that interfere with the estrogens not to bind to the estrogen receptors (ERs). Cells in other tissues in the body, such as bones and the uterus, also have ERs. But each ER has a slightly different structure depending on the kind of cell it is in. It means that breast cell estrogen receptors are different from bone cell estrogen receptors and both of those estrogen receptors are different from uterine estrogen receptors. The selective usage of drugs was made possible by the fact that the ERs of different target tissues vary in chemical structures (Levenson and Jordan, 1999; Osborne et al., 2000; Johnston, 2001). These drugs are called selective estrogen receptor modulators (SERMs). SERMs are synthetic molecules which bind to estrogen receptors ER-α and ER-β and can modulate its transcriptional factors in different ways in diverse estrogen target tissues (Jordan, 1988; Lerner and Jordan, 1990; Avioli, 1999).
Tamoxifen, the prototypical SERM, is extensively used for targeted therapy of ER positive breast cancers. For almost three decades, this drug has been as a first-line therapy in both early and advanced breast cancer. Unfortunately, a long term use of tamoxifen for the control of tumor growth is associated with acquired resistance and some undesirable side effects. Around 50% of advanced breast cancer does not have susceptibility to first-line therapy with tamoxifen. Also almost all patients with metastatic disease and approximately 40% of the patients that receive tamoxifen as a adjuvant therapy experienced tumor relapse and die from their disease (Normanno et al., 2005). The postulated mechanism of resistance and/or insensitivity to SERM therapy was related to followings; Loss of ER in the tumor, ER mutations, enhancement of coactivators and inhibition of corepressors and cross talk between the ER and the growth factor receptor pathways (Normanno et al., 2005; Adamo et al., 2007).
For these reasons, researchers are working on the development of SERMs without any of their harmful effects (Peng et al., 2009). But it is also true that there isn’t the appropriate data which compares the existing several SERMs’ potency for MCF-7 cells and TAM-R cells. It must be valuable to do a closer review of SERMs about the differences of their estrogen antagonistic properties on MCF-7 cells and TAM-R cells for the development of new SERMs. In this study we devised to investigate the estrogen antagonistic properties of several conventional SERMs on the two types of breast cancer cell lines, MCF-7 cell and TAM resistant cell.
MATERIALS AND METHOD
Materials and reagents. The chemicals and cell culture materials were obtained from following source: MCF-7 cell line was obtained from American Type Culture Collection (ATCC, Rochville, MD. U.S.A.). TAM resistance cell was offered by favorable professor Kang, Keon Wook of the department of pharmacy in Chosun University; Dulbecco’s Modified Eagle’s medium (DMEM), fetal bovine serum (FBS), antibiotics was from GIBCO (Gaithersburg, MD, U.S.A.), steroid-depleted fetal bovine serum from Hyclone (Logan, UT); Tamoxifen citrate, 4-hydroxytamoxifen, Toremifene and raloxifene hydrochloride was purchased from Sigma (St. Louis, MO). Ospemifene and idoxifene was gift from Dr. Shibutani S (State university of NewYork); methanethiosulfonate/ phenazine methosulfate solution (MTS) was from Promega Corp., (U.S.A.); dimethyl sulfoxide (DMSO), Acridine orange (AO), propidium iodode (PI) and RNase A were purchased from Sigma Chemical (St. Louis, MO, U.S.A). All chemicals were of the highest grade commercially available.
Cell culture. The MCF-7 cells were cultured at 37℃ 5% CO2/95% air in Dulbecco’s Modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), 100 units/ ml penicillin, and 100 mg/ml streptomycin (Knowlden et al., 2003). Briefly, MCF-7 cells were washed with PBS, and the culture medium was changed to phenol-red-free DMEM containing 10% charcoal-stripped, steroid-depleted fetal bovine serum (Hyclone, Logan, UT) and 4-hydroxytamoxifen (0.1 μM). The cells were continuously exposed to this treatment regimen for 2 weeks and the concentration of 4- hydroxytamoxifen was gradually increased to 3 μM over a 9-month period. Initially, the cell growth rates were reduced. However, after exposure to the medium for 9 months, the rate of cell growth gradually increased, showing the establishment of a tamoxifen-resistant (TAM-R) cell line (Choi et al., 2007). To maintain the resistance of TAM-resistant cells, the cells continuously exposed to 4-hydroxy tamoxifen (3 μM).
Cell proliferation assay. The SERMs were dissolved in dimethyl sulfoxide (DMSO) to make 3 μM solutions. And the stocks of SERMs were kept in a deep freezer for the experiment. Cell proliferation assay was carried out using the Cell Titer 96 Aqueous One Solution Cell Proliferation Assay (Promega Corp., U.S.A.) as instructed by the manufacturer. Briefly, 6hr after seeding, Both breast cancer cells were replaced with fresh medium containing various concentrations of tamoxifen and its derivatives for 24 hr. Twenty microliters of methanethiosulfonate/phenazine methosulfate solution (MTS) was added to each well and incubated for 1~1.5 hr at 37℃. The absorbance was read at an optical density 490 nm using a precision microplate reader (Molecular Devices Corp., Sunnyvale, CA, USA) (Knowlden et al., 2003; Choi et al., 2007). The data of IC50 was processed by Graphpad Prism 5.01.
Cell cycle and sub-G1 group assays. MCF-7 and TAMR cells in 6-well plates (2 × 105 cells/well) were treated with tamoxifen, 4-hydroxytamoxifen, and toremifene and then incubated for 12 hr. Medium containing float cells was removed and trypsin was added to the cells in plates for 3 min, after which the cells were harvested by centrifugation at 1000 rpm for 5 min. Pellets were washed twice with cold PBS and then fixed by using 70% ethanol (in PBS) at 4℃ overnight. The cells were then washed twice with cold PBS and re-suspended in PBS containing 50 μg/ml PI, 1 μg/ml RNase in a dark room for 30 minutes at 37℃, the cells were then analyzed by FACScan flow cytometer (Becton Dickinson USA) by CellQuest software. Then the cell cycle and sub-G1 (apoptosis) groups were determined and analyzed (Lin et al., 2007).
Flowcytometric analysis of autophagy. MCF-7 and TAM-R cells(1×106 cells/well ) cells in 6-well plates were treated with SERMs including tamoxifen, 4-hydroxytamoxifen, and toremifene and then incubated for 12 hr. On fixed times, the cells were harvested by trypsin and rinsed with PBS two times by centrifugation at 1000 g. For measuring autophagy, the cell pellet was suspended with 10 μg/ml acridine orange solution at 37℃ for 15~20 min and then samples were analyzed by flowcytometry (Becton Dickinson FACScan, CA, U.S.A.) using the Cell Quest software (BD Biosciences, San Jose, CA, U.S.A.) to determine of autophagy as described Chen et al., 2009.
Statistical analysis. Data combined from at least three independent experiments were analyzed with Student’s test. All values were expressed as mean ± SD. A difference of at least p < 0.05 was considered statistically significant.
RESULTS
The availability of conventional SERMs on the TAMresistance breast cancer cells was investigated. To determine the effect on the TAM-resistance breast cancer cells, growth inhibition by SERMs was measured by cell proliferation assay.
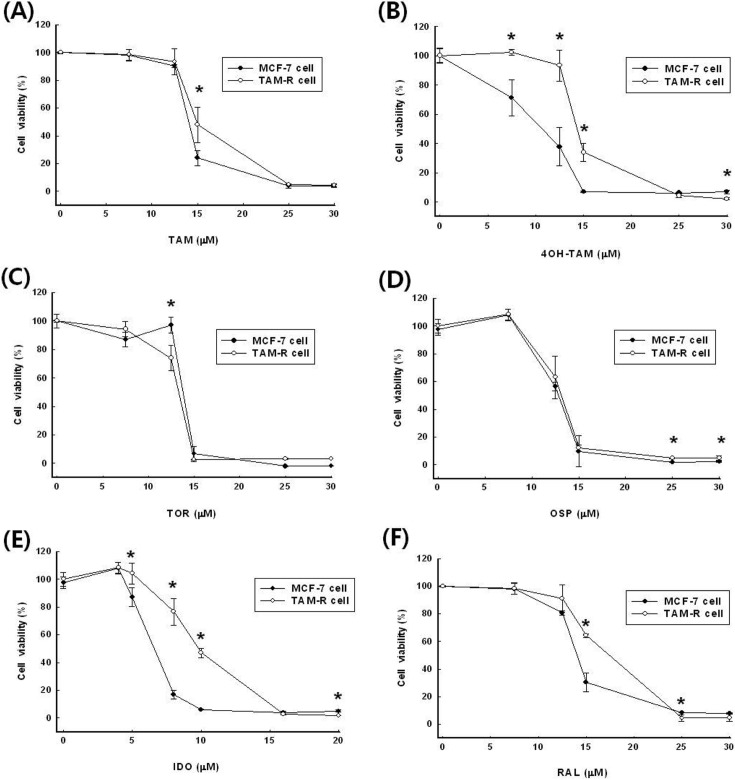
The inhibitory concentrations (IC50) against two types of breast cancers showed that TAM-R cells were need a higher concentration of SERMs than MCF-7 cells to meet IC50, except for TOR (Fig. 1, Table 1). The IC50 of tamoxifen (TAM) were 20.5 ± 4.0 μM on MCF-7 cells and 27.0 ± 1.9 μM on TAM-R cells. The antagonistic effect of TAM on MCF-7 cells was 131.5% higher than on TAM-R cells. Tamoxifen is mostly acting in vivo indirectly via hydroxylated metabolite, 4-hydroxy tamoxifen (4-OH TAM). 4-OH TAM showed more than two times powerful growth inhibition effect compared with that of tamoxifen itself in MCF-7 cells. The IC50 of 4-OH TAM was 11.3 ± 0.6 μM and 18.3 ± 1.1 μM on MCF-7 cells and TAM-R cells, respectively. TAM- R cells showed resistance more than 60% about 4- OH TAM compare with MCF-7.
Fig. 1. Effect of SERMs including (A) TAM, B) 4-OH TAM, (C) TOR, (D) OSP, (E) IDO and (F) RAL on the viability in MCF-7 and TAM-R cells. Cells were incubated with various concentrations of SERMs. Data are expressed as mean ± S.D. of three independent experiments (* p< 0.05 in comparison with control).
Table 1.
The inhibitory concentration of SERMs in MCF-7 and TAM-R cells
| SERMs | IC50 | P value summary | |
|---|---|---|---|
| MCF-7 | TAM-R | ||
| TAM | 20.5 ± 4.0 | 27.0 ± 1.9 | * |
| 4-OH TAM | 11.3 ± 0.6 | 18.3 ± 1.1 | *** |
| TOR | 18.9 ± 4.1 | 13.7 ± 1.2 | ns |
| OSP | 12.6 ± 0.3 | 12.7 ± 0.3 | ns |
| IDO | 6.5 ± 0.6 | 9.6 ± 0.5 | *** |
| RAL | 13.7 ± 0.3 | 15.7 ± 0.7 | ** |
Data are expressed as mean ± S.D. of three independent experiments. ns stands for not significance.
*, **, *** p< 0.05, 0.01, and 0.001 in comparison with MCF-7, respectively.
The IC50 of toremifene (TOR) was 18.9 ± 4.1 μM on MCF-7 cells and 13.7 ± 1.2 μM on TAM-R cells. Unlike other SERMs, TOR inhibited the proliferation of TAM-R cells more effectively than MCF-7 cells. This was a remarkable result when compared to other SERMs. The IC50 of ospemifene (OSP) was all about the same inhibitory concentration between two types of breast cancers.
The IC50 of idoxifene (IDO) was the most effective SERM used in the experiments, 6.5 ± 0.6 μM against MCF- 7 cells and 9.6 ± 0.5 μM against TAM-R cells. IDO showed the similar pattern of antagonic effects in both cells. The IC50 values of raloxifene (RAL) were 13.7 ± 0.3 μM and 15.7 ± 0.7 μM on MCF-7 cells and TAM-R cells, respectively.
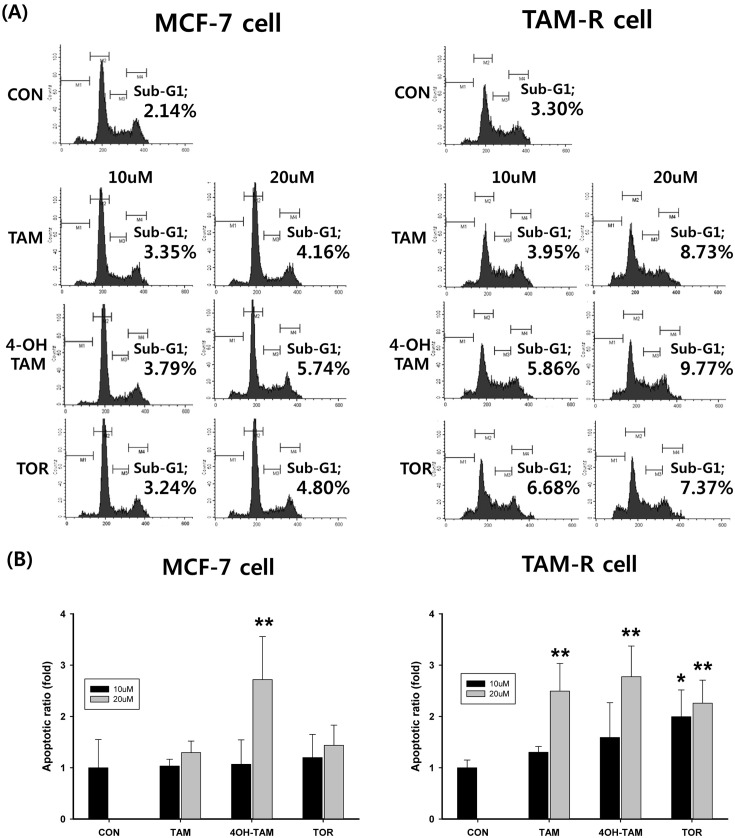
The effects of SERMs on the cell cycle distribution and sub-G1 phase from MCF-7 and TAM-R cells were measured to determine whether SERMs-decreased via apoptosis. Cells with DNA content were designated as being in the G0/G1, S or G2/M phase of the cell cycle. The number of cells in subG1 of the cell cycle was expressed as a percentage of the total number of cells examined. As shown in Fig. 2, the number of subG1 phase profiles for MCF-7 cells was only clearly increased after the treatment with 20 mM 4-OH TAM in MCF-7. The other SERM didn’t show any effect in this experimental condition. Apoptotic cell death in TAM-R cells was characterized by the treatment of SERM. Treatment of 10 μM TOR induced significantly subG1 group (apoptosis), but the other SERMs had no effect in concentration of 10 μM. Apoptosis occurred after treatment with all SERMs of 20 μM applied to TAM-R cells.
Fig. 2. Effects of SERMs-induced apoptosis in MCF-7 and TAM-R cells. MCF-7 cells and TAM-R cells were treated with SERMs including TAM, 4-OH TAM, and TOR and then incubated in an incubator for 24hr, re-suspended in 75% ethanol, and stained with 50 μg/ml PI containing 0.1% Triton X-100 and 0.02 mg/ml EDTA. The DNA content of cells was measured by flow cytometry and cell cycle profiles were analyzed using CellQuest software. (A) Flow cytometeric histogram of control group, TAM, 4-OH TAM, and TOR treat group. Sub- G1 area presented apoptosis. (B) Apoptotic ratio was calculated with the control group considered to be 1fold. Data are expressed as mean ± S.D. of three independent experiments (*p< 0.05, **p< 0.01, in comparison with control).
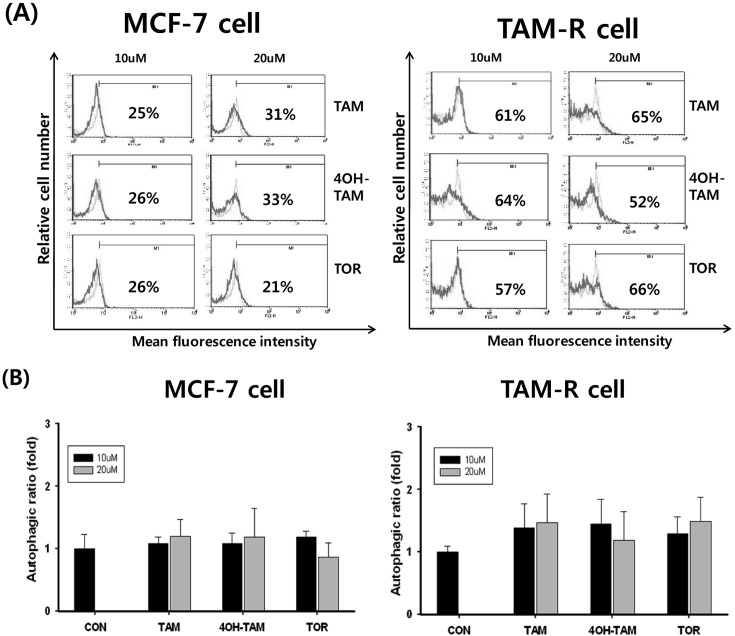
To determine the role of autophagy by SERMs, MCF-7 and TAM-R cells were treated with 4-OH TAM, TAM, and TOR and then incubated for 12 hr. Autophagy was analyzed after stained with acridine orange using flowcytometry. In this experimental condition, there was not found any evidence related autophagy by SERMs in both MCF-7 and TAM-R cells (Fig. 3).
Fig. 3. Effects of SERMs-induced autophagy in MCF-7 and TAM-R cells. MCF-7 and TAM-R cells were treated with SERMs including TAM, 4-OH TAM, and TOR and then incubated in an incubator for 24 hr. Cells were then harvested and stained with acridine orange. The AVOs (acidic vesicular organelles) of cells were measured by flow cytometry and cell cycle profiles were analyzed using CellQuest software. The percentage values represent M1, the percentage of cells with AVOs. (A) This shown isotype untreated (green) and treated (pink). (B) Autophagic ratio was calculated with the control group considered to be 1fold. Data are expressed as mean ± S.D. of three independent experiments.
DISCUSSION
SERMs block ER activation and have affected on the therapy and survival in breast cancer patients. However the success of tamoxifen therapy is limited by intrinsic and acquired drug resistance. Resistance to tamoxifen as well as side effects, are a serious clinical therapeutic problem. For these reasons, researchers have been working on the development of SERMs without any of their unexpected effects (Peng et al., 2009). In this study, several conventional SERMs’ possibility for the treatment on tamoxifen resistant cancer patients was investigated.
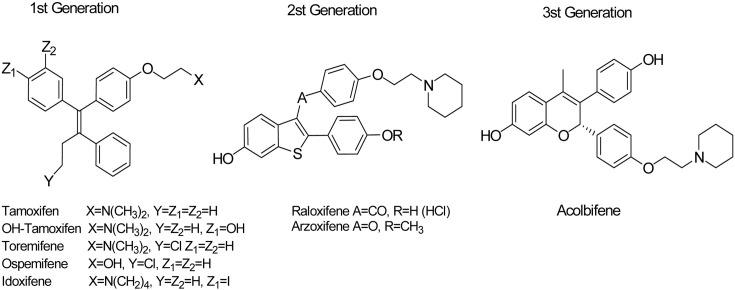
There are various types of conventional SERMs (Fig. 4), triphenylethylene and benzothiophene derivatives, based on their chemical structures. Each of them has its own unique responses to different types of body tissues. (Table 2) The SERMs with triphenylethylene structures are tamoxifen, droloxifene, toremifene, and idoxifene. Raloxifene is a SERM with a benzothiophene structure (Jirecek et al., 1999). Other types of SERMs have entered clinical development more recently, including benzothiophene derivatives (arzoxifene), benzopyrans (ormeloxifene, levormeloxifene, and EM-800), lasofoxifene, pipendoxifene, bazedoxifene, HMR-3339, and fulvestrant (Shelly et al., 2008).
Fig. 4. The structure of SERMs.
Table 2.
The target of SERMs
| Site | Pure estrogen | SERMs | Pure antiestrogen | |
|---|---|---|---|---|
| 1st generation | 2nd generation | |||
| Bone | Agonist | Agonist | Agonist | Antagonist |
| Cholesterol | Agonist | Agonist | Agonist | - |
| Uterus | Agonist | Partial agonist | Antagonist | Antagonist |
| Mammary | Agonist | Antagonist | Antagonist | Antagonist |
| Prototype | 17-estradiol | Tamoxifen | Raloxifene | ICI-164384 |
TAM is a prodrug that is metabolized to active metabolites 4-OH TAM and endoxifen by cytochrome P450 isoforms, CYP 2D6 and CYP 3A4 (Stearns et al., 2003). TAM acts as an estrogen antagonist in mammary gland and blocks estradiol-stimulated VEGF production in breast tumor cells. TAM binds to cytoplasm estrogen receptors in breast, anterior pituitary and prostate tissues. 4-OH TAM is a metabolite of the antiestrogen, TAM, in humans and other mammals. 4-OH TAM has a higher affinity than TAM and its other metabolites for binding to estrogen receptors, therefore it has greater potency of inhibiting cell multiplication in normal human breast cells (Malet et al., 1988) as well as in breast cancer cell lines in culture (Coezy et al., 1982).
TOR was made by the chlorination of TAM. It is metabolized by cytochrome P450 1A and 3A4 enzymes, resulting in the formation of N-desmethyl TOR, 4-OH TOR, and deamino-OH TOR (Berthou et al., 1994; Kim et al., 2003). TOR was known to inhibit chain reactions of lipoperoxidation and to eliminate free radicals in vitro. TOR also reduces the side effect of TAM which generates an intrinsic estrogenic effect and causes carcinoma on uterus (Labrie et al., 2003). Unlike TAM, TOR produced two orders of magnitude lower DNA adducts in rat liver and did not promote hepatocarcinoma in rats (Shibutani et al., 2001).
IDO is a TAM derivative with an iodine atom in the 4- position and a pyrrolidino ethoxy side chain replacing the diethylaminoethoxy group in the parent compound. The iodinated TAM placed on the 4-position enhances ER binding affinity and inhibits metabolic 4-hydroxylation. IDO has a lot of advantages than TAM for the treatment of breast cancer. IDO enhanced ER binding and antitumor effect of TAM. It improved antagonism of calmoduline dependent processes (McCague et al., 1989). OSP is a novel triphenylethylene compound and is metabolized into the major metabolite TOR (deamino-OH-TOR). OSP binds to estrogen receptors with more strong affinity than TAM. OSP has appeared to prevent estrogen depletion-induced bone loss in animal models, in addition to inhibiting the growth of human breast cancer MCF-7 cells and DMBA-induced mammary tumors. OSP shows a weak effect of preventing uterine cancer but has anti-osteoporosis effects. In addition, it is not associated with the liver toxicity seen with TAM. OSP also has revealed efficacy and safety in clinical trials in postmenopausal osteoporosis and urogenital atrophy (Rodriguez et al., 2004).
Raloxifene (RAL), 2nd generation of SERM, produced both estrogen-agonistic effects on bone and lipid metabolism and estrogen-antagonistic effects on uterine endometrium and breast tissue (Morishima et al., 2008). The side effect was less than 1st generation SERMs. RAL caused fewer uterine cancers, which was a major adverse effect of TAM. The U.S. FDA announced approval of RAL for reducing the high risk of invasive breast cancer in postmenopausal women with osteoporosis (U.S. Food and Drug Administration (2007-09-14)). RAL has an antiestrogenic action to inhibit the growth of mammary or endometrial carcinoma in breast (Purdie and Beardsworth, 1999). Recently, RAL tried to be treated as 2nd line therapies to TAM resistance breast cancer patients (Normanno et al., 2005; Cummings et al., 1999). However, breast cancer cells often acquire resistance to SERMs used 2nd and 3rd line therapies (Miller et al., 2007).
The several SERMs used for the study were significantly inefficacious in TAM-R cells compared with MCF-7 cells except of TOR and OSP, although there isn’t a stark contrast between two types of cells. OSP had similar cytotoxic activity on the two types of breast cancers. Unlike the other SERMs, TOR had more anti-estrogenic effect on TAM-R cells than MCF-7 cells. These results support the possibility to usage of TOR on TAM resistance cancer patients. Also the researchers will be able to utilize the data of this study and design improved SERMs for TAM-resistant cancer.
The mechanism of effectiveness on TAM R cells by TOR was investigated two ways, apoptosis and autophagy. Cancer cells evade apoptotic signal, therefore distribution of the apoptotic pathway has important effects on the clinical outcome of chemotherapy (Pecorino, 2008). The effects of SERMs on the cell cycle distribution and sub-G1 phase from MCF-7 and TAM-R cells were measured to determine whether SERMs-decreased via apoptosis. Only the treatment with 20 μM 4-OH TAM leaded to apoptosis in MCF- 7. The other SERM didn’t show any effect in this experimental condition. Treatment of 10 μM TOR induced significantly subG1 group in TAM-R cells, but the other SERMs had no effect in same concentration. It was reported by Wärri et al. (1993) that TOR causes growth inhibition of estrogen-sensitive breast cancer cells by inducing some cells to undergo apoptosis and by inhibiting other cells from entering mitosis. Even though the treatment TOR in TAMR cells increased apoptosis, it’s not enough to explain the reason why TOR has better effect against TAM-R cells compared to MCF-7.
Autophagy is an intracellular self eating process involving lysosomal degradation of cytoplasmic materials (Chen et al., 2010). Targeting the prodeath and prosurvival functions of autophagy is as novel therapeutic strategies in cancer (Dalby et al., 2010). Autophagy plays different role depending on the cell type. In fully transformed cancer cells, it appears to function as a tumor suppressor as defective autophagy is associated with malignant transformation and carcinogenesis. In normal cells and in some cancer cells, it appears to function as a protective mechanism against cellular stress and yet the induction of autophagy is associated with cell death in some type of cancers. Recently, many researchers reported that the autophagy is a key mechanism of progression ER a-positive breast cancer cells to get antiestrogen resistance (Schoenlein et al., 2009; Gonzalez-Malerva et al., 2011; Samaddar et al., 2008). In our experimental condition, there were not found any evidence related autophagy by SERMs in both MCF-7 and TAM-R cells.
Unlike the other SERMs, TOR had more anti-estrogenic effect on TAM-R cells than MCF-7 cells. These results have valuable meaning as a preliminary experiment because TOR could be used the treatment for TAM resistant breast cancer patients. The action mechanism of TOR might be not related the apoptotic pathways and the autophagy. The effect of TOR in TAM-R cells was not extensively examined. To elucidate the main mechanisms, further study should be needed.
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2006- 331-E00431).
References
- 1.Adamo V., Iorfida M., Montalto E., Festa V., Garipoli C., Scimone A., Zanghì M., Caristi N. Overview and new strategies in metastatic breast cancer (MBC) for treatment of tamoxifen-resistant patients. Ann. Oncol. (2007);18 Suppl 6:53–57. doi: 10.1093/annonc/mdm225. [DOI] [PubMed] [Google Scholar]
- 2.Avioli L.V. SERM drugs for the prevention of osteoporosis. Trends. Endocrinol. Metab. (1999);10:317–319. doi: 10.1016/S1043-2760(99)00176-9. [DOI] [PubMed] [Google Scholar]
- 3.Berthou F., Dreano Y., Belloc C., Kangas L., Gautier J.C., Beaune P. Involvement of cytochrome P450 3A enzyme family in the major metabolic pathways of toremifene in human liver microsomes. Biochem. Pharmacol. (1994);47:1883–1895. doi: 10.1016/0006-2952(94)90319-0. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y., Azad M.B., Gibson S.B. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. (2009);16:1040–1052. doi: 10.1038/cdd.2009.49. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y., Azad M.B., Gibson S.B. Methods for detecting autophagy and determining autophagy-induced cell death. Can. J. Physiol. Pharmacol. (2010);88:285–295. doi: 10.1139/Y10-010. [DOI] [PubMed] [Google Scholar]
- 6.Choi H.K., Yang J.W., Roh S.H., Han C.Y., Kang K.W. Induction of multidrug resistance associated protein 2 in tamoxifen-resistant breast cancer cells. Endocr. Relat. Cancer. (2007);14:293–303. doi: 10.1677/ERC-06-0016. [DOI] [PubMed] [Google Scholar]
- 7.Coezy E., Borgna J.L., Rochefort H. Tamoxifen and metabolites in MCF7 cells: correlation between binding to estrogen receptor and inhibition of cell growth. Cancer Res. (1982);42:317–323. [PubMed] [Google Scholar]
- 8.Cummings S.R., Eckert S., Krueger K.A., Grady D., Powles T.J., Cauley J.A., Norton L., Nickelsen T., Bjarnason N.H., Morrow M., Lippman M.E., Black D., Glusman J.E., Costa A., Jordan V.C. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple outcomes of raloxifene evaluation. JAMA. (1999);281:2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 9.Dalby K.N., Tekedereli I., Lopez-Berestein G., Ozpolat B. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy. (2010);6:322–329. doi: 10.4161/auto.6.3.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Malerva L., Park J., Zou L., Hu Y., Moradpour Z., Pearlberg J., Sawyer J., Stevens H., Harlow E., LaBaer J. High-throughput ectopic expression screen for tamoxifen resistance identifies an atypical kinase that blocks autophagy. Proc. Natl. Acad. Sci. U.S.A. (2011);108:2058–2063. doi: 10.1073/pnas.1018157108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jirecek S., Pavo I., Huber J.C. Effect of selective estrogen receptor modulators on estrogen-sensitive tissues. Gynakol Geburtshilfliche Rundsch. (1999);39:184–190. doi: 10.1159/000022308. [DOI] [PubMed] [Google Scholar]
- 12.Johnston S.R. Endocrine manipulation in advanced breast cancer: recent advances with SERM therapies. Clin. Cancer Res. (2001);7:4376s–4387s. [PubMed] [Google Scholar]
- 13.Jordan V.C. Chemosuppression of breast cancer with tamoxifen: laboratory evidence and future clinical investigations. Cancer Invest. (1988);6:589–595. doi: 10.3109/07357908809082124. [DOI] [PubMed] [Google Scholar]
- 14.Kim S.Y., Suzuki N., Santosh Laxmi Y.R., Rieger R., Shibutani S. Alpha-hydroxylation of tamoxifen and toremifene by human and rat cytochrome P450 3A subfamily enzymes. Chem. Res. Toxicol. (2003);16:1138–1144. doi: 10.1021/tx0300131. [DOI] [PubMed] [Google Scholar]
- 15.Knowlden J.M., Hutcheson I.R., Jones H.E., Madden T., Gee J.M., Harper M.E., Barrow D., Wakeling A.E., Nicholson R.I. Elevated levels of epidermal growth factor receptor/ c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. (2003);144:1032–1044. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- 16.Labrie F., El-Alfy M., Berger L., Labrie C., Martel C., Belanger A., Candas B., Pelletier G. The combination of a novel selective estrogen receptor modulator with an estrogen protects the mammary gland and uterus in a rodent model: the future of postmenopausal women’s health? Endocrinology. (2003);144:4700–4706. doi: 10.1210/en.2003-0269. [DOI] [PubMed] [Google Scholar]
- 17.Lerner L.J., Jordan V.C. Development of antiestrogens and their use in breast cancer: eighth Cain memorial award lecture. Cancer Res. (1990);50:4177–4189. [PubMed] [Google Scholar]
- 18.Levenson A.S., Jordan V.C. Selective oestrogen receptor modulation: molecular pharmacology for the millennium. Eur. J. Cancer. (1999);35:1974–1985. doi: 10.1016/S0959-8049(99)00297-X. [DOI] [PubMed] [Google Scholar]
- 19.Lin J.P., Yang J.S., Chang N.W., Chiu T.H., Su C.C., Lu K.W., Ho Y.T., Yeh C.C., Mei D., Lin H.J., Chung J.G. GADD153 mediates berberine-induced apoptosis in human cervical cancer Ca ski cells. Anticancer. Res. (2007);27:3379–3386. [PubMed] [Google Scholar]
- 20.Malet C., Gompel A., Spritzer P., Bricout N., Yaneva H., Mowszowicz I., Kuttenn F., Mauvais-Jarvis P. Tamoxifen and hydroxytamoxifen isomers versus estradiol effects on normal human breast cells in culture. Cancer Res. (1988);48:7193–7199. [PubMed] [Google Scholar]
- 21.McCague R., Leclercq G., Legros N., Goodman J., Blackburn G.M., Jarman M., Foster A.B. Derivatives of tamoxifen. Dependence of antiestrogenicity on the 4-substituent. J. Med. Chem. (1989);32:2527–2533. doi: 10.1021/jm00132a006. [DOI] [PubMed] [Google Scholar]
- 22.Miller W.R., Bartlett J.M., Canney P., Verrill M. Hormonal therapy for postmenopausal breast cancer: the science of sequencing. Breast. Cancer Res. Treat. (2007);103:149–160. doi: 10.1007/s10549-006-9369-7. [DOI] [PubMed] [Google Scholar]
- 23.Morishima S., Shibata M.A., Ohmichi M., Otsuki Y. Raloxifene, a selective estrogen receptor modulator, induces mitochondria-mediated apoptosis in human endometrial carcinoma cells. Med. Mol. Morphol. (2008);41:132–138. doi: 10.1007/s00795-008-0403-1. [DOI] [PubMed] [Google Scholar]
- 24.NCI-Naple Breast Cancer Group. Normanno N., Di Maio M., De Maio E., De Luca A., de Matteis A., Giordano A., Perrone F. Mechanisms of endocrine resistance and novel therapeutic strategies in breast cancer. Endocr. Relat. Cancer. (2005);12:721–747. doi: 10.1677/erc.1.00857. [DOI] [PubMed] [Google Scholar]
- 25.Osborne C.K., Zhao H., Fuqua S.A. Selective estrogen receptor modulators: structure, function, and clinical use. J. Clin. Oncol. (2000);18:3172–3186. doi: 10.1200/JCO.2000.18.17.3172. [DOI] [PubMed] [Google Scholar]
- 26.Pecorino L. Molecular biology and cancer. 2nd edition. Oxford University press; New York: (2008). pp. 137–160. [Google Scholar]
- 27.Peng J., Sengupta S., Jordan V.C. Potential of selective estrogen receptor modulators as treatments and preventives of breast cancer. Anticancer. Agents. Med. Chem. (2009);9:481–499. doi: 10.2174/187152009788451833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purdie D.W., Beardsworth S.A. The selective oestrogen receptor modulation: evolution and clinical applications. Br. J. Clin. Pharmacol. (1999);48:785–792. doi: 10.1046/j.1365-2125.1999.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez L., Carrillo M., Sorbera L.A., Zohar Y., Zany S. Effects of photoperiod on pituitary levels of three forms of GnRH and reproductive hormones in the male European sea bass (Dicentrarchus labrax, L.) during testicular differentiation and first testicular recrudescence. Gen. Comp. Endocrinol. (2004);136:37–48. doi: 10.1016/j.ygcen.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Samaddar J.S., Gaddy V.T., Duplantier J., Thandavan S.P., Shah M., Smith M.J., Browning D., Rawson J., Smith S.B., Barrett J.T., Schoenlein P.V. A role for macroautophagy in protection against 4-hydroxytamoxifen-induced cell death and the development of antiestrogen resistance. Mol. Cancer Ther. (2008);7:2977–2987. doi: 10.1158/1535-7163.MCT-08-0447. [DOI] [PubMed] [Google Scholar]
- 31.Schoenlein P.V., Periyasamy-Thandavan S., Samaddar J.S., Jackson W.H., Barrett J.T. Autophagy facilitates the progression of ERalpha-positive breast cancer cells to antiestrogen resistance. Autophagy. (2009);5:400–403. doi: 10.4161/auto.5.3.7784. [DOI] [PubMed] [Google Scholar]
- 32.Shelly W., Draper M.W., Krishnan V., Wong M., Jaffe R.B. Selective estrogen receptor modulators: an update on recent clinical findings. Obstet. Gynecol. Surv. (2008);63:163–181. doi: 10.1097/OGX.0b013e31816400d7. [DOI] [PubMed] [Google Scholar]
- 33.Shibutani S., Ravindernath A., Terashima I., Suzuki N., Laxmi Y.R., Kanno Y., Suzuki M., Apak T.I., Sheng J.J., Duffel M.W. Mechanism of lower genotoxicity of toremifene compared with tamoxifen. Cancer Res. (2001);61:3925–3931. [PubMed] [Google Scholar]
- 34.Stearns V., Johnson M.D., Rae J.M., Morocho A., Novielli A., Bhargava P., Hayes D.F., Desta Z., Flockhart D.A. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J. Natl. Cancer Inst. (2003);95:1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 35.Wärri A.M., Huovinen R.L., Laine A.M., Martikainen P.M., Härkönen P.L. Apoptosis in toremifene-induced growth inhibition of human breast cancer cells in vivo and in vitro. J. Natl. Cancer Inst. (1993);85:1412–1418. doi: 10.1093/jnci/85.17.1412. [DOI] [PubMed] [Google Scholar]