Abstract
Carbon black, a particulate form of pure elemental carbon, is an industrial chemical with the high potential of occupational exposure. Although the relationship between exposure to particulate matters (PM) and cardiovascular diseases is well established, the cardiovascular risk of carbon black has not been characterized clearly. In this study, the cytotoxicity of carbon black to vascular smooth muscle and endothelial cells were examined to investigate the potential vascular toxicity of carbon black. Carbon black with distinct particle size, N330 (primary size, 28~36 nm) and N990 (250~350 nm) were treated to A-10, rat aortic smooth muscle cells and human umbilical vein endothelial cell line, ECV304, and cell viability was assessed by lactate dehydrogenase (LDH) leakage assay. Treatment of carbon black N990 resulted in the significant reduction of viability in A-10 cells at 100 μg/ml, the highest concentration tested, while N330 failed to cause cell death. Cytotoxicity to ECV304 cells was induced only by N330 at higher concentration, 200 μg/ml, suggesting that ECV304 cells were relatively resistant to carbon black. Treatment of 100 μg/ml N990 led to the elevation of reactive oxygen species (ROS) detected by dichlorodihydrofluorescein (DCF) in A-10 cells. Pretreatment of antioxidants, N-acetylcysteine (NAC) and sulforaphane restored decreased viability of N990-treated A-10 cells, and N-acetylcysteine, but not sulforaphane, attenuated N990-induced ROS generation in A-10 cells. Taken together, present study shows that carbon black is cytotoxic to vascular cells, and the generation of reactive oxygen contributes to the development of cytotoxicity. ROS scavenging antioxidant could be a potential strategy to attenuate the toxicity induced by carbon black exposure.
Keywords: Carbon black, Cytotoxicity, Antioxidants, Particulate matter
INTRODUCTION
Carbon black is a particulate form of pure elemental carbon with primary size, 10~500 nm, which is produced by partial combustion or pyrolysis of gaseous or liquid hydrocarbons under controlled conditions (Valberg et al., 2006). Carbon black may exhibit more or less different physicochemical properties depending on specific surface area, particle size and structure which are mainly determined by manufacturing processes. Based on annual tonnage, it is in the top 50 industrial chemicals, which is produced and consumed for diverse industrial purposes including reinforcement of rubber and coloring for dyestuffs (Baan, 2007). There is thus high risk of occupational exposure in the industry manufacturing tires and technical rubber articles, electrostatic discharge compounds, and toners and printing inks (Morfeld and McCunney, 2010).
Although extensive studies have been performed focused on respiratory and carcinogenic risk of carbon black exposure, there are only limited reports which provided implication for cardiovascular toxicity of carbon black (Valberg et al., 2006; Baan, 2007). Recently, a correlation between exposure to general particulate matter (PM) and the risk for cardiovascular diseases is well documented in epidemiologic studies, suggesting the positive association between increased level of ambient particles and cardiovascular morbidity and mortality (Simkhovich et al., 2008; Brook et al., 2010; Fang et al., 2010; Sun et al., 2010). Indeed, carbon black causes exacerbates atherosclerotic plaque formation in LDL receptor knockout animals fed with high cholesterol diet (Niwa et al., 2007), and exhibits cardioregulatory effects in animal experiments (Chang et al., 2007; Niwa et al., 2008). These experimental evidences suggest the cardiovascular toxicity of carbon black.
The most relevant mode of exposure to airborne carbon black is inhalation, because of its presence as a particulate form with low specific gravity which allows it fly in ambient. Generally, PM deposits in pulmonary system and hardly enters into circulatory system unless it is ultrafine or highly soluble in body fluid. In the case of carbon black, it has not been clear whether carbon black translocates into circulatory system, so exert its toxicity by direct interaction with target molecules. Recently, however, it was demonstrated that ultrafine particles are able to penetrate into the deep ling areas and pass through to reach the systemic circulation (Nemmar et al., 2002; Nemmar et al., 2004). Indeed, intratracheally instilled carbon black ultrafine particles was also shown to translocate into circulation system (Shimada et al., 2006), which can be facilitated by inflammation in lung (Inoue et al., 2009). These findings suggest that circulatory system is directly exposed to carbon black and vascular cells with blood serve as a primary target of absorbed carbon black.
In the present study we examined the effect of carbon black on viability of vascular cells such as smooth muscle and endothelial cells to evaluate the toxicity of carbon black to blood vessel. Additionally, N330 and N990, two distinct carbon black with different size were examined to explore the effect of size difference on cytotoxicity, considering the toxicity of PM is largely dependent on its size.
MATERIALS AND METHODS
Reagents. Carbon blacks, Corax® N330 and Thermax® floform N990 were purchased from Evonic Carbon Black Korea (Incheon, Korea) and Cancarb Ltd. (Medicine Hat, Alberta, Canada), respectively. Hydrogen peroxide and NADH were obtained from Sigma-Aldrich (St. Louis, MO, USA), and 2',7'-Dichlorodihydrofluorescein diacetate (DCF-DA) was from Molecular Probes (Eugene, OR, USA). All other chemicals used here were of the highest purity available and purchased from standard suppliers.
Cell culture. A-10, smooth muscle cells derived from embryonic rat thoracic aorta was purchased from American Type Culture Collection (Manassas, VA, USA), and human umbilical vein endothelial cell line, ECV304 was kindly provided by Dr. Keun-Wook Kang from Chosun University (Gwangju, Korea). All these cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were maintained at 37℃ and 5% CO2 in a humidified incubator, and were subcultured when they reached 80~ 90% confluence rate.
Dispersion of carbon black. For treatment, carbon black N330 or N990 were suspended in cell culture media, DMEM with 10% fetal bovine serum at the concentration required. For dispersion, sonication was applied to carbon black suspension at 100 W for 1 min prior to use.
Assessment of cytotoxicity. Cytotoxicity induced by carbon black was assessed by lactate dehydrogenase (LDH) leakage assay according to previous report (Lee et al., 2002). Briefly, cells were plated at a density of 1.2 × 105 cells/well in 24-well culture plates. After 24 hrs, cells were incubated with carbon black in the presence or absence of antioxidants for additional 24 hrs. Aliquots of culture medium were, then, taken and subjected to centrifugation. The resulting supernatant was used for the measurement of LDH activity. A 20 μl of either cell supernatant was added to 200 μl aliquot of working reagent containing 0.2 mM NADH and 2.5 mM sodium pyruvate in a well of 96-well plates. Decrease in absorbance at 340 nm was measured for 5 min with VersaMax microplate reader (Molecular Devices; Sunnyvale, CA, USA). Relative LDH activity was calculated from the slope of decrease in absorbance. LDH leakage was expressed as a percentage (%) of total LDH activity measured from total lysis with 1% Triton X-100. Menadione, a well known cytotoxicant, was used as a positive control.
Detection of reactive oxygen species (ROS). A-10 cells were treated with carbon black N990 in the presence or absence of antioxidants. Cells were washed once with phosphate buffered saline (PBS; 3.2 mM Na2HPO4, 0.5 mM KH2PO4, 1.3 mM KCl, 135 mM NaCl, pH 7.4), and incubated for additional 20 min with 2 μM DCF-DA dissolved in Krebs-Ringer solution (115.5 mM NaCl, 4.6 KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4 1.2, 2.5 mM CaCl2, 25.0 mM NaHCO3, 11.1 mM glucose, pH 7.4). Then, cells were immediately viewed by Eclipse TE2000-U inverted microscope (Nikon; Japan) at 480/535 nm of excitation/emission wavelength. Images of cells were analyzed with a Meta Imaging System (Molecular Devices; West Chester, PA, USA). For comparison of fluorescence intensity, all the images were obtained under the same optical condition and subtracted with background image.
Statistical analysis. The means and standard errors (SE) of means were calculated for all experiment groups. The data were subjected to one way analysis of variance (ANOVA) followed by Dunn’s test to determine significant difference. Statistical analysis was performed using SigmaStat software (Version 3.5, Systat Software; San Jose, CA, USA). In all cases, a p value of < 0.05 was used to determine significance.
RESULTS
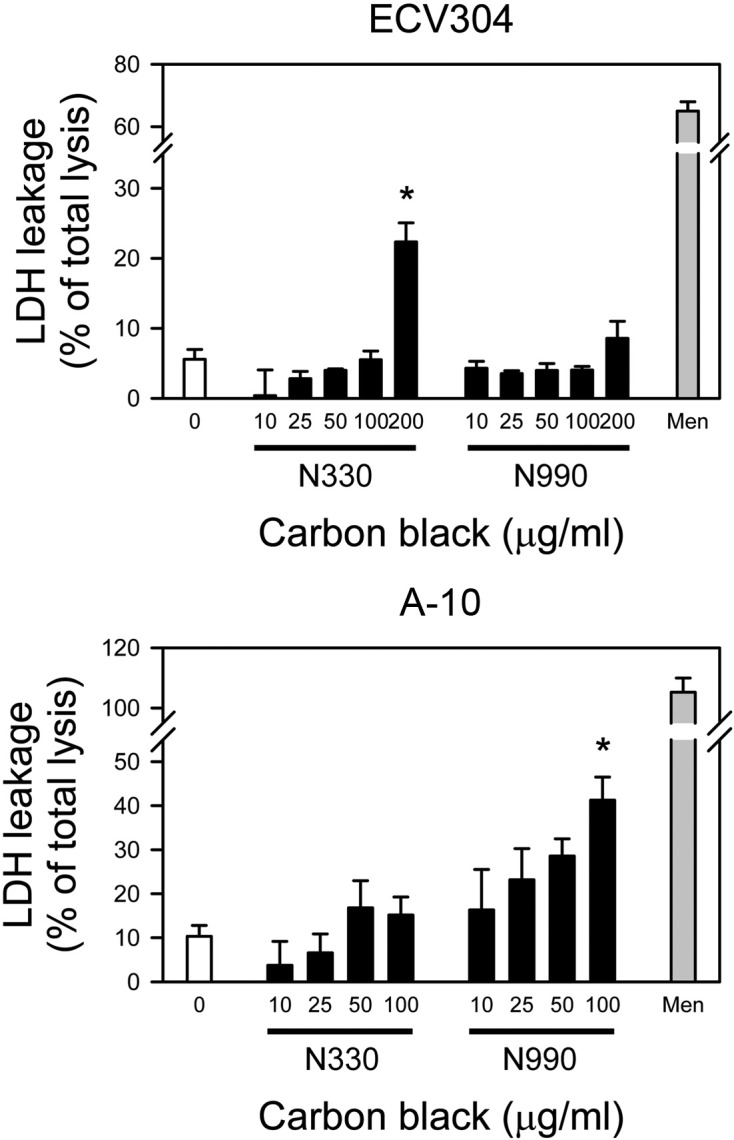
Cytotoxicity of carbon black to vascular smooth muscle and endothelial cells. To test the cytotoxicity of carbon black in vascular cells, carbon black N330 and N990 were treated to human umbilical vein endothelial ECV304 cells and smooth muscle cells derived from embryonic rat thoracic aorta, A-10 cells for 24 hrs, and cell viability was assessed by LDH leakage assay. Two different carbon black, N330 and N990 were employed to investigate the effect of size difference in cytotoxicity - primary particle sizes for N330 and N990 are 28~36 and 250~350 nm, respectively. As shown in Fig. 1, only N990, not N330, elicited statistically significant LDH leakage in A-10 cells at 100 μg/ml, the highest concentration tested (41.3 ± 5.2 versus 10.3 ± 3.9%; lower panel). In ECV304 cells, however, both N990 and N330 failed to induce cell death up to 100 μg/ml, which induced us to test higher concentration. Intriguingly, it was carbon black N330, not N990, that resulted in cytotoxicity to ECV304 cells, i.e., treatment of 200 μg/ml N330 resulted in LDH leakage in 22.3 ± 2.7% of cells (P < 0.05 versus untreated control cells), while N990 failed to exhibit toxicity (upper panel). Taken together, smooth muscle cells, A-10 was sensitive to the toxicity of carbon black, compared with ECV304 endothelial cells, and cytotoxicity of carbon black is dependent on cell type. In addition, toxicity of carbon black is also dependent on particle size, although it is hard to define the tendency.
Fig. 1. Cytotoxicity of carbon black N330 and N990 in ECV304 or A-10 cells. (A) ECV304 and (B) A-10 cells were incubated with various concentrations (0, 10, 25, 50, 100, or 200 μg/ml) of carbon black N330 or N990 for 24 hrs, and cell death were assessed by LDH activity released into the culture medium as described in the “Materials and Methods”. Data were expressed as mean ± SE of three independent experiments. Menadione (Men) at 100 μM was used as a positive control. *P < 0.05 versus untreated control cells.
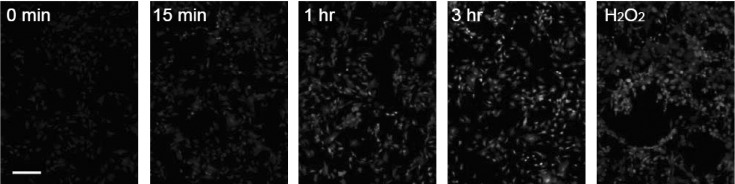
Generation of reactive oxygen species (ROS) by carbon black. Based on the previous studies that oxidative stress mediates diverse biological effect of carbon black, ROS generation was examined in the cells treated with carbon black in order to evaluate whether oxidative stress is involved in carbon black-induced cytotoxicity. Considering that A-10 was more vulnerable to carbon black than ECV304 and only N990 deteriorated viability, ROS was measured with fluorescence indicator, DCF in A-10 cells treated with carbon black N990. Analysis of fluorescence images revealed that treatment of N990 led to gradual generation of ROS over hours (Fig. 2). Comparable ROS was detected in parallel positive control, H2O2 treated cells.
Fig. 2. Detection of reactive oxygen species in A-10 cells treated with carbon black N990. A-10 cells were incubated with 100 μg/ml carbon black N990 for indicated durations (0, 15, 60, and 180 min). Then, cells were briefly washed with PBS, loaded with DCF-DA (2 μM), and were immediately viewed by fluorescence microscope. Hydrogen peroxide (H2O2, 200 μM), a positive control, was treated to cells for 30 min. Scale bar = 50 μm.
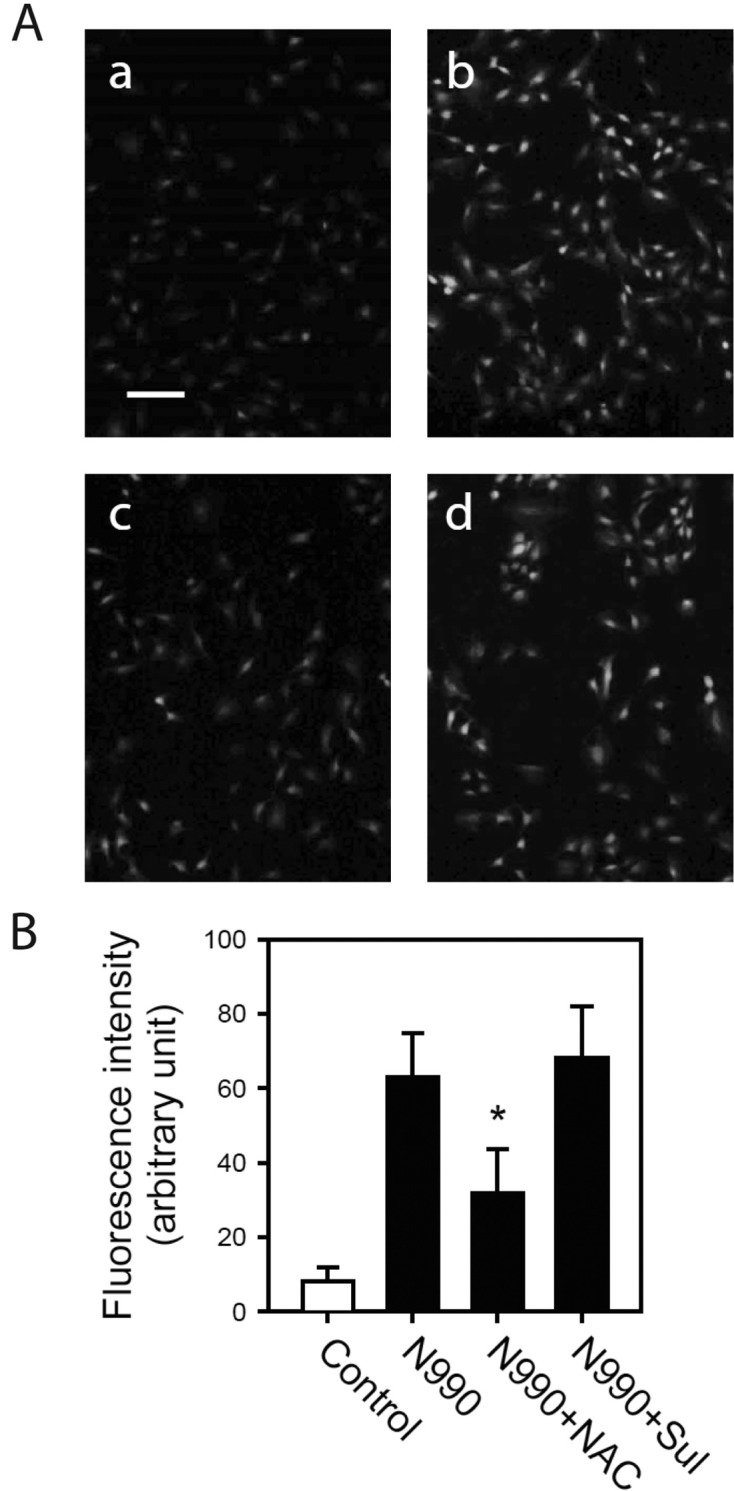
Effect of antioxidants on the toxicity of carbon black. To answer whether ROS generated by carbon black might contribute to the development of toxicity, we examined the effect of antioxidants on carbon black toxicity. N-acetylcysteine (NAC) and sulforaphane, acknowledged antioxidants, were employed for this study. Neither NAC nor sulforaphane induce cytotoxicity measured by LDH leakage assay up to 100 or 10 μM, respectively. However, pretreatment of NAC or sulforaphane significantly reduced LDH leakage nearly to basal level (Fig. 3). Such inhibition was observed at the concentration of 10~100 and 1~10 μM for NAC and sulforaphane, respectively, which are the commonly used concentration in most of the studies employed them as antioxidants (Ahmed et al., 2010; Keum, 2011). In the same experimental condition, pretreatment of NAC resulted in significant decrease in ROS generation, but such decrease was not observed in sulforaphane-pretreated cells (Fig. 4). These results suggested that carbon black N990 exert its cytotoxic effect at least in part by ROS generation.
Fig. 3. Protection of carbon black N990-induced cytotoxicity by antioxidants, N-acetylcysteine (NAC) and sulforaphane. A-10 cells were pretreated with (A) NAC (10 or 100 μM) or (B) sulforaphane (1 or 10 μM) for 1 hr and exposed to 100 μg/ml of carbon black N990 for an additional 24 hrs. Cytotoxicity was measured by LDH activity released into the medium. Data were obtained from three independent experiments and expressed as mean ± SE of total LDH activity measured from total lysis with 1% Triton X-100. #P < 0.05 versus untreated control cells; *P<0.05 versus carbon black N990-alone treated cells. Sul, sulforaphane.
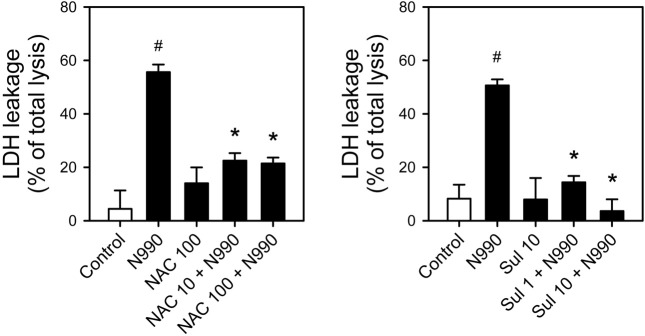
Fig. 4. Effect of NAC or sulforaphane on reactive oxygen species generation by carbon black N990. A-10 cells were pretreated with 100 μM NAC or 10 ìM sulforaphane for 1 hr and exposed to 100 μg/ml carbon black N990 for additional 3 hrs. After treatment, cells were washed with PBS and loaded with DCF-DA (2 μM). Then, images were immediately obtained by fluorescence microscope. (A) Images of DCF loaded cells. a: untreated control, b: N990-treated cells, c: cells treated with NAC and N990, d: cells treated with sulforaphane and N990. Scale bar = 30 μm. (B) Intensity of fluorescence in each pixel was measured and presented as mean ± SE. *P< 0.05 versus carbon black N990-alone treated cells. Sul, sulforaphane.
DISCUSSION
This current study investigated the toxicity of carbon black to vascular cells such as smooth muscle and endothelial cells in order to evaluate potential harmful effect on blood vessel. Carbon black N330 or N990 tested exhibited cytotoxicity at higher than 100 μM. Endothelial cells were more resistant to carbon black than smooth muscle cells. Relatively smaller particle, N330 could induce decrease in viability of endothelial cells, whereas only N990 exhibited cytotoxicity to smooth muscle cells. Carbon black treatment resulted in the elevation of ROS, and such increase as well as cytotoxicity was restored by pretreatment of antioxidants, NAC. Taken together, carbon black is toxic to vascular cells, especially to smooth muscle cells, and the induction of ROS generation is at least in part contributes to the development of cytotoxicity. These results suggest that antioxidant may serve as a potential strategy to attenuate or prevent vascular toxicity caused by carbon black, a particulate matter.
Carbon black is by nature a pure carbon chemical, hence has long been recognized to be, relatively, biochemically inert. This is the reason why toxicity studies have never been done so extensively, although carbon black is an industrial chemical of importance. However, as it gets widely known that physical characteristics as well as chemical properties play a critical role in the development of particulate matter (PM) toxicity, carbon black was revisited. Indeed, it has been reported recently that carbon black exposure is related with various toxicities including carcinogenicity, induction of immune response and cardiovasucular toxicity (Valberg et al., 2006). In addition to being an industrial chemical, carbon black has acquired the attention as a model compound for air pollutant study. Carbon black resembles to a certain extent carbonaceous core contained in urban dust and combustion particles such as diesel exhaust (Tankersley et al., 2004; Totlandsdal et al., 2010). Therefore, study of carbon black is of significance in the aspect of environmental toxicology as well as industrial hygiene.
The result of study reveals that the ROS production contributes to the cytotoxicity of carbon black, although underlying mechanism remains elusive. This result is well correlated with the previous studies reporting ROS generation by carbon black and its reduction by vitamin C or desferrioxamine, an iron chelator (Stone et al., 1998; Frikke-Schmidt et al., 2011). Target molecule responsible for ROS generation was not identified, however it is clear that the treatment of antioxidant is efficient way to attenuate the toxicity of carbon black. ROS scavengers or antioxidants may be applied for detoxification or prevention of toxicity caused by carbon black exposure.
Different from NAC, sulforaphane failed to reduce ROS formation although it successfully protected cytotoxicity of carbon black (Fig. 3 and 4). Sulforaphane is a naturaloccurring isothiocyanate from cruciferous vegetables such as broccoli sprouts, which is widely used as an effective antioxidant and chemopreventive agent both in vivo and in vitro studies (Keum, 2011). It has been demonstrated that NF-E2-related factor 2 (Nrf2)-mediated induction of antioxidant enzymes is major mechanism by which sulforaphane protects cells from oxidative stress and carcinogens (Dinkova-Kostova et al., 2002). Although we did not evaluate in the present study whether sulforaphane activated Nrf2 pathway in A-10 cells, this cytoprotective mechanism could be conceivable by evidences showing that sulforaphane significantly abolished carbon black-induced cytotoxicity (Fig. 3) without direct radical scavenging effect (Fig. 4). Further study will be needed to investigate whether activation of Nrf2 signaling is effective in protection of carbon black toxicity.
Acknowledgments
This work was supported by Korea Occupational Safety and Health Agency (KOSHA) grants 2010-119-968 to M.Y.L.
References
- 1.Ahmed T., Tripathi A.K., Ahmed R.S., Banerjee B.D. Assessment of phosphamidon-induced apoptosis in human peripheral blood mononuclear cells: protective effects of N-acetylcysteine and curcumin. J. Biochem. Mol. Toxicol. (2010);24:286–292. doi: 10.1002/jbt.20337. [DOI] [PubMed] [Google Scholar]
- 2.Baan R.A. Carcinogenic hazards from inhaled carbon black, titanium dioxide, and talc not containing asbestos or asbestiform fibers: recent evaluations by an IARC Monographs Working Group. Inhal. Toxicol. (2007);19(Suppl 1):213–228. doi: 10.1080/08958370701497903. [DOI] [PubMed] [Google Scholar]
- 3.Brook R.D., Rajagopalan S., Pope C.A., 3rd, Brook J.R., Bhatnagar A., Diez-Roux A.V., Holguin F., Hong Y., Luepker R.V., Mittleman M.A., Peters A., Siscovick D., Smith S.C., Jr., Whitsel L., Kaufman J.D. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. (2010);121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 4.Chang C.C., Hwang J.S., Chan C.C., Cheng T.J. Interaction effects of ultrafine carbon black with iron and nickel on heart rate variability in spontaneously hypertensive rats. Environ. Health Perspect. (2007);115:1012–1017. doi: 10.1289/ehp.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinkova-Kostova A.T., Holtzclaw W.D., Cole R.N., Itoh K., Wakabayashi N., Katoh Y., Yamamoto M., Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. U.S.A. (2002);99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang S.C., Cassidy A., Christiani D.C. A systematic review of occupational exposure to particulate matter and cardiovascular disease. Int. J. Environ. Res. Public Health. (2010);7:1773–1806. doi: 10.3390/ijerph7041773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frikke-Schmidt H., Roursgaard M., Lykkesfeldt J., Loft S., Nojgaard J.K., Moller P. Effect of vitamin C and iron chelation on diesel exhaust particle and carbon black induced oxidative damage and cell adhesion molecule expression in human endothelial cells. Toxicol. Lett. (2011);203:181–189. doi: 10.1016/j.toxlet.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Inoue H., Shimada A., Kaewamatawong T., Naota M., Morita T., Ohta Y., Inoue K., Takano H. Ultrastructural changes of the air-blood barrier in mice after intratracheal instillation of lipopolysaccharide and ultrafine carbon black particles. Exp. Toxicol. Pathol. (2009);61:51–58. doi: 10.1016/j.etp.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Keum Y.S. Regulation of the Keap1/Nrf2 system by chemopreventive sulforaphane: implications of posttranslational modifications. Ann. N. Y. Acad. Sci. (2011);1229:184–189. doi: 10.1111/j.1749-6632.2011.06092.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee M.Y., Bae O.N., Chung S.M., Kang K.T., Lee J.Y., Chung J.H. Enhancement of platelet aggregation and thrombus formation by arsenic in drinking water: a contributing factor to cardiovascular disease. Toxicol. Appl. Pharmacol. (2002);179:83–88. doi: 10.1006/taap.2001.9356. [DOI] [PubMed] [Google Scholar]
- 11.Morfeld P., McCunney R.J. Bayesian bias adjustments of the lung cancer SMR in a cohort of German carbon black production workers. J. Occup. Med. Toxicol. (2010);5:23. doi: 10.1186/1745-6673-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nemmar A., Hoet P.H., Vanquickenborne B., Dinsdale D., Thomeer M., Hoylaerts M.F., Vanbilloen H., Mortelmans L., Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. (2002);105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- 13.Nemmar A., Hoylaerts M.F., Hoet P.H., Nemery B. Possible mechanisms of the cardiovascular effects of inhaled particles: systemic translocation and prothrombotic effects. Toxicol. Lett. (2004);149:243–253. doi: 10.1016/j.toxlet.2003.12.061. [DOI] [PubMed] [Google Scholar]
- 14.Niwa Y., Hiura Y., Murayama T., Yokode M., Iwai N. Nano-sized carbon black exposure exacerbates atherosclerosis in LDL-receptor knockout mice. Circ. J. (2007);71:1157–1161. doi: 10.1253/circj.71.1157. [DOI] [PubMed] [Google Scholar]
- 15.Niwa Y., Hiura Y., Sawamura H., Iwai N. Inhalation exposure to carbon black induces inflammatory response in rats. Circ. J. (2008);72:144–149. doi: 10.1253/circj.72.144. [DOI] [PubMed] [Google Scholar]
- 16.Shimada A., Kawamura N., Okajima M., Kaewamatawong T., Inoue H., Morita T. Translocation pathway of the intratracheally instilled ultrafine particles from the lung into the blood circulation in the mouse. Toxicol. Pathol. (2006);34:949–957. doi: 10.1080/01926230601080502. [DOI] [PubMed] [Google Scholar]
- 17.Simkhovich B.Z., Kleinman M.T., Kloner R.A. Air pollution and cardiovascular injury epidemiology, toxicology, and mechanisms. J. Am. Coll. Cardiol. (2008);52:719–726. doi: 10.1016/j.jacc.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Stone V., Shaw J., Brown D.M., Macnee W., Faux S.P., Donaldson K. The role of oxidative stress in the prolonged inhibitory effect of ultrafine carbon black on epithelial cell function. Toxicol. In Vitro. (1998);12:649–659. doi: 10.1016/S0887-2333(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 19.Sun Q., Hong X., Wold L.E. Cardiovascular effects of ambient particulate air pollution exposure. Circulation. (2010);121:2755–2765. doi: 10.1161/CIRCULATIONAHA.109.893461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tankersley C.G., Campen M., Bierman A., Flanders S.E., Broman K.W., Rabold R. Particle effects on heart-rate regulation in senescent mice. Inhal. Toxicol. (2004);16:381–390. doi: 10.1080/08958370490439551. [DOI] [PubMed] [Google Scholar]
- 21.Totlandsdal A.I., Refsnes M., Lag M. Mechanisms involved in ultrafine carbon black-induced release of IL-6 from primary rat epithelial lung cells. Toxicol. In Vitrov . (2010);24:10–20. doi: 10.1016/j.tiv.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Valberg P.A., Long C.M., Sax S.N. Integrating studies on carcinogenic risk of carbon black: epidemiology, animal exposures, and mechanism of action. J. Occup. Environ. Med. (2006);48:1291–1307. doi: 10.1097/01.jom.0000215342.52699.2a. [DOI] [PubMed] [Google Scholar]


