Abstract
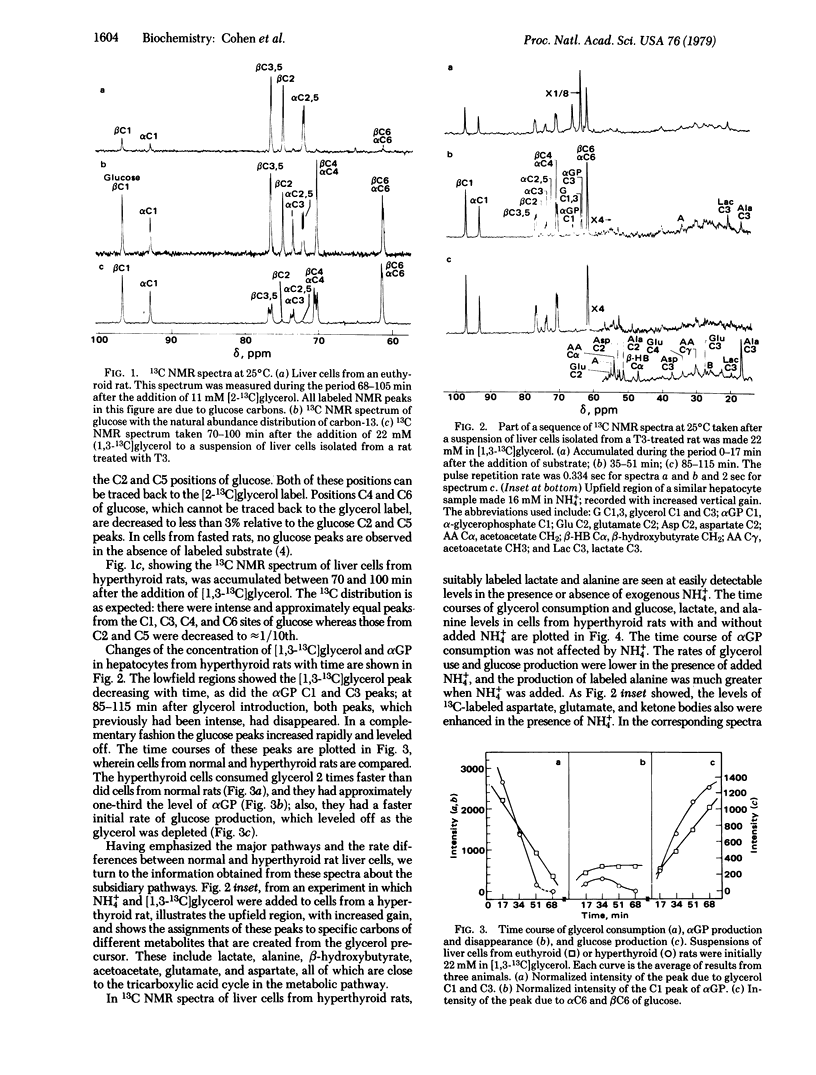
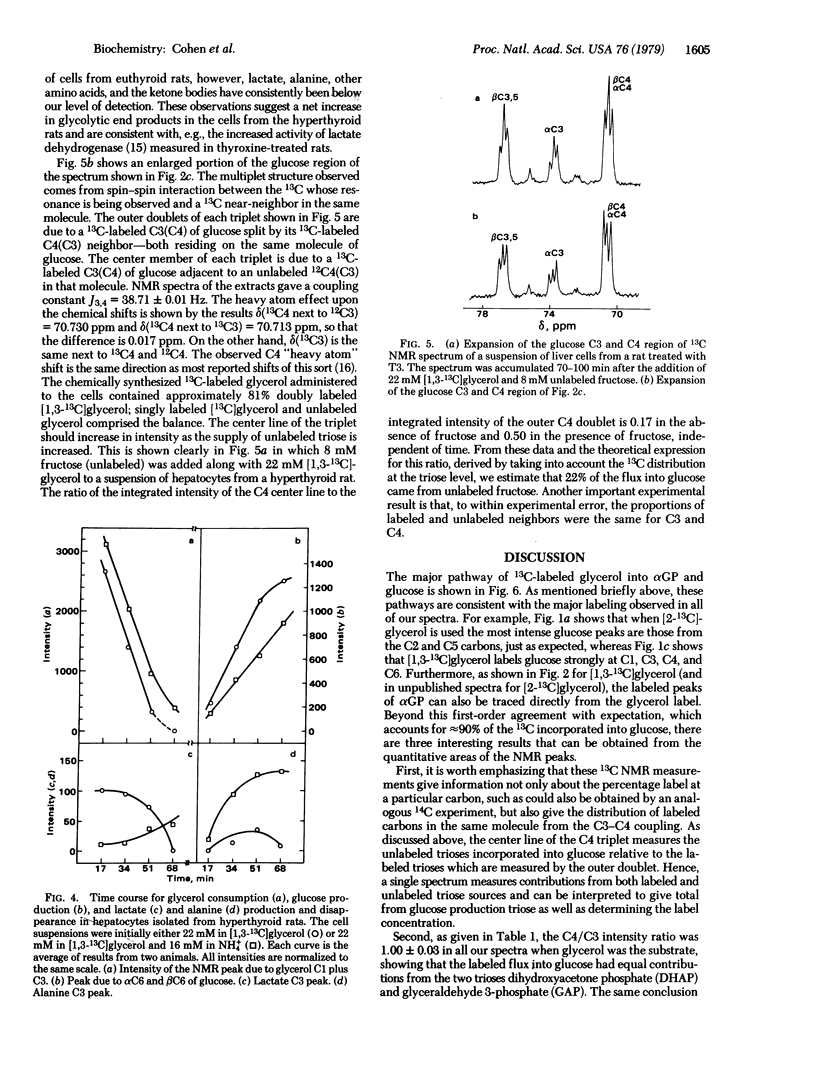
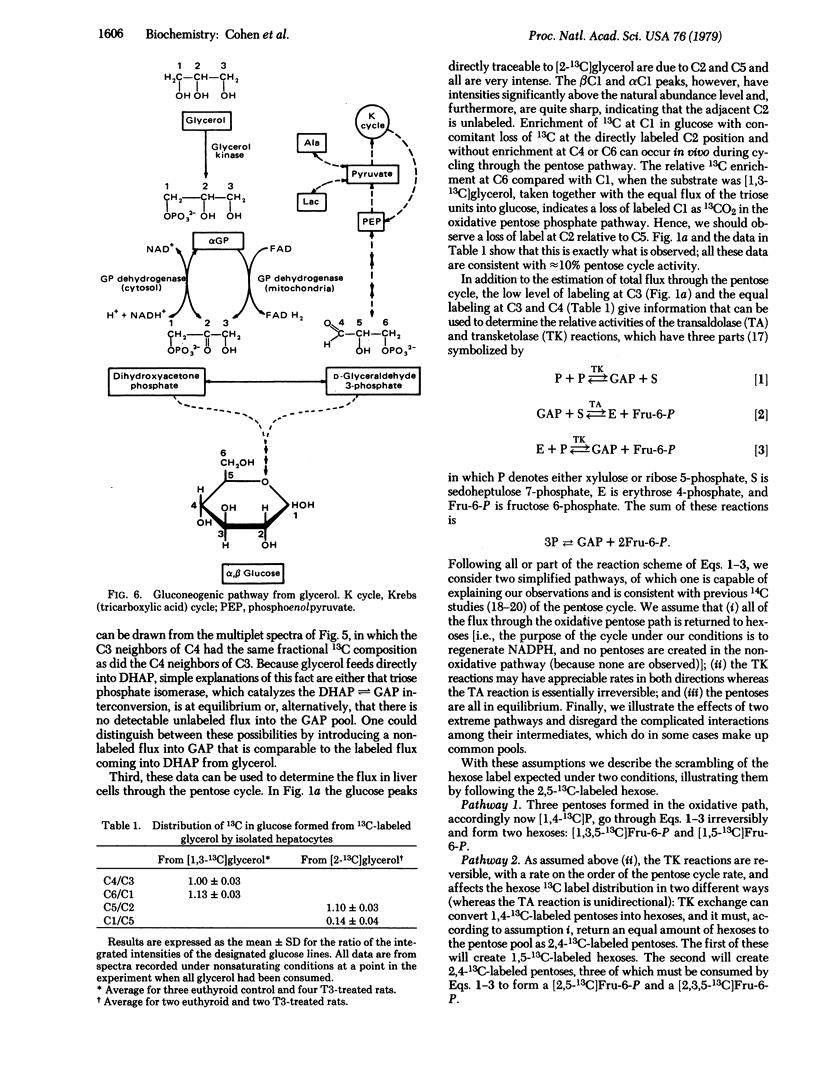
The gluconeogenic pathway from [2-13C]glycerol and [1,3-13C]glycerol has been followed in suspensions of isolated rat hepatocytes at 25°C by 13C NMR at 90.5 MHz. The flow of label through the major pathway from glycerol to L-glycerol 3-phosphate and into glucose was followed in cells from control and triiodothyronine-treated rats. Treatment increased the rates of glucose formation and glycerol consumption 2-fold and decreased the αGP level to 40%. We calculate that ≈60% of the flux is through the mitochondrial glycerol phosphate dehydrogenase in cells from triiodothyronine-treated rats, compared with ≈15% in cells from the controls. Equal distribution of label between the trioses of glucose was obtained and, because the C3-C4 spin-spin coupling gives the distribution of labeled carbons in the same molecule, it was possible to measure the amount of triose from unlabeled fructose incorporated into the glucose labeled at carbons 1, 3, 4, and 6. About 10% of the hexoses had flowed through the pentose cycle and back into the hexose pathway in cells from fasted rats. From the distribution of label at glucose carbons not labeled via the major pathway and from the carbon spin-spin splitting patterns observed, we conclude that transketolase is reversible whereas transaldolase is essentially irreversible in the nonoxidative pentose branch.
Keywords: pentose cycle, triiodothyronine, glycerol-3-phosphate dehydrogenase, alanine
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Kun E., Werner H. V. Regulatory role of reducing-equivalent transfer from substrate to oxygen in the hepatic metabolism of glycerol and sorbitol. Eur J Biochem. 1973 Mar 15;33(3):407–417. doi: 10.1111/j.1432-1033.1973.tb02697.x. [DOI] [PubMed] [Google Scholar]
- Burch H. B., Lowry O. H., Meinhardt L., Max P., Jr, Chyu K. Effect of fructose, dihydroxyacetone, glycerol, and glucose on metabolites and related compounds in liver and kidney. J Biol Chem. 1970 Apr 25;245(8):2092–2102. [PubMed] [Google Scholar]
- Carnicero H. H., Moore C. L., Hoberman H. D. Oxidation of glycerol 3-phosphate by the perfused rat liver. J Biol Chem. 1972 Jan 25;247(2):418–426. [PubMed] [Google Scholar]
- Cohen S. M., Ogawa S., Rottenberg H., Glynn P., Yamane T., Brown T. R., Shulman R. G. P nuclear magnetic resonance studies of isolated rat liver cells. Nature. 1978 Jun 15;273(5663):554–556. doi: 10.1038/273554a0. [DOI] [PubMed] [Google Scholar]
- Freedland R. A., Krebs H. A. The effect of thyroxine treatment on the rate of gluconeogenesis in the perfused rat liver. Biochem J. 1967 Sep;104(3):45P–45P. [PMC free article] [PubMed] [Google Scholar]
- Katz J., Rognstad R. The labeling of pentose phosphate from glucose-14C and estimation of the rates of transaldolase, transketolase, the contribution of the pentose cycle, and ribose phosphate synthesis. Biochemistry. 1967 Jul;6(7):2227–2247. doi: 10.1021/bi00859a046. [DOI] [PubMed] [Google Scholar]
- LEE Y. P., TAKEMORI A. E., LARDY H. Enhanced oxidation of alpha-glycerophosphate by mitochondria of thyroid-fed rats. J Biol Chem. 1959 Nov;234:3051–3054. [PubMed] [Google Scholar]
- Ogawa S., Shulman R. G., Glynn P., Yamane T., Navon G. On the measurement of pH in Escherichia coli by 31P nuclear magnetic resonance. Biochim Biophys Acta. 1978 Apr 11;502(1):45–50. doi: 10.1016/0005-2728(78)90130-5. [DOI] [PubMed] [Google Scholar]
- Rognstad R., Katz J. Effects of hormones and of ethanol on the fructose 6-P-fructose 1,6-P2 futile cycle during gluconeogenesis in the liver. Arch Biochem Biophys. 1976 Dec;177(2):337–345. doi: 10.1016/0003-9861(76)90447-1. [DOI] [PubMed] [Google Scholar]
- Short J., Brown R. F., Husakova A., Gilbertson J. R., Zemel R., Lieberman I. Induction of deoxyribonucleic acid synthesis in the liver of the intact animal. J Biol Chem. 1972 Mar 25;247(6):1757–1766. [PubMed] [Google Scholar]
- Tischler M. E., Hecht P., Williamson J. R. Determination of mitochondrial/cytosolic metabolite gradients in isolated rat liver cells by cell disruption. Arch Biochem Biophys. 1977 May;181(1):278–293. doi: 10.1016/0003-9861(77)90506-9. [DOI] [PubMed] [Google Scholar]
- Ugurbil K., Brown T. R., den Hollander J. A., Glynn P., Shulman R. G. High-resolution 13C nuclear magnetic resonance studies of glucose metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3742–3746. doi: 10.1073/pnas.75.8.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K., Rottenberg H., Glynn P., Shulman R. G. 31P nuclear magnetic resonance studies of bioenergetics and glycolysis in anaerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2244–2248. doi: 10.1073/pnas.75.5.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner H. V., Berry M. N. Stimulatory effects of thyroxine administration on reducing-equivalent transfer from substrate to oxygen during hepatic metabolism of sorbitol and glycerol. Eur J Biochem. 1974 Mar 1;42(2):315–324. doi: 10.1111/j.1432-1033.1974.tb03342.x. [DOI] [PubMed] [Google Scholar]
- Woods H. F., Krebs H. A. The effect of glycerol and dihydroxyacetone on hepatic adenine nucleotides. Biochem J. 1973 Jan;132(1):55–60. doi: 10.1042/bj1320055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. W. Effects of D- and L-thyroxine on enzymes in liver and adipose tissue of rats. Am J Physiol. 1968 Feb;214(2):378–383. doi: 10.1152/ajplegacy.1968.214.2.378. [DOI] [PubMed] [Google Scholar]


