Abstract
Screening of estrogenic activity on dichloro diphenyl trichloroethane (DDT), dichloro diphenyl dichloro ethylene (DDE), dieldrin, heptachlor, aldrin, chlordane, lindane, polybrominated diphenyl ethers (PBDE) and parabens was compared using Organization for Economic Cooperation and Development (OECD) test guideline 455 (TG455). The estrogenic activity of DDT was 58,000-fold (PC50, 1.67 × 10−6 M) less than 17β-estradiol(E2) (PC50, 2.88 × 10-11 M) but DDE, dieldrin, heptachlor, aldrin, chlordane, lindane and PBDE did not show any estrogenic activity in this assay system. In the case of paraben compounds, the rank of relative transcriptional activation (logRTA) was butyl paraben −1.63752 (PC50, 1.25 × 10−7M) > isobutyl paraben −2.34008 (PC50, 6.3 × 10−7M) > ethyl paraben −2.64016 (PC50, 1.26 × 10−6 M) > isopropyl paraben −2.73993 (PC50, 1.58 × 10−6M) > propyl paraben −2.84164 (PC50, 2.0 × 10−6 M). Our data suggest that OECD test guideline TG455 may be useful as a screening tool for potential endocrine disruptors.
Keywords: Estrogenic activity, Endocrine disruptors, OECD test guideline 455, DDT, Parabens
INTRODUCTION
Endocrine disruptors (EDCs) may be interfered the endocrine system in wildlife and humans reproduction (Colborn et al., 1993; Vos et al., 2000; Bonefeld-Jorgensen et al., 2004). They mimic, compete, block or alter the activities of endogenous hormones. Previous studies have been reported that endocrine disruptors interrupt interactions of ligandreceptor binding that act as receptor agonist or antagonist (Hunter et al., 1999; Damdimopoulos et al., 2008). For rapid alarming health and environmental consequences involved fast and reliable approaches are needed. Based on these situation, the Organization for Economic Cooperation and Development (OECD) conceptual framework for testing and assessment of man-made compounds comprised five levels and in vitro screening methods for alternative animal study ranked as level 2 (OECD, 2003). Recently, the stably transfected transcriptional activation assay (STTA) developed by Chemicals Evaluation and Research Institute (CERI) have been accepted as the OECD test guideline 455 (TG455) in 2009 (OECD, 2009). The STTA assay is to evaluate the ability of chemicals to function as an estrogen receptor alpha (ERα) ligand and activate an ERα agonistic responses.
Here, we validated the estrogenic activity of persistent organic pollutants DDT, DDE, dieldrin, heptachlor, aldrin, chlordane, lindane, PBDE) and parabens using STTA assay. Previous studies have been indicated that these compounds showed weak or potent estrogenic activity using in vitro and in vivo screening assay systems (Okubo et al., 2001; Vo and Jeung et al., 2009).
MATERIALS AND METHODS
Cell line and cell culture conditions. The hERα-HeLa- 9903 cell line (HeLa9903) which is stably transfected a human ERα gene containing a firefly luciferase gene as a reporter gene was provided from the CERI. The experiments were performed with 5~28 passages of the cells, which were seeded at 1 × 104 cells per well on 96 well plates and incubated cells for 3 hr at 37℃ with 5% CO2. The reagents of cell culture used commercial products except Dextran-coated charcoal treated fetal bovine serum (DCCFBS, provided from CERI). The cells were maintained and tested in 10% DCC-FBS-EMEM including 7.5% NaHCO3, 4 mM L-glutamine and 10% DCC-FBS.
Test chemicals. Reference chemicals: 10 mM 17α-estradiol (Sigma, St. Louis, MO, USA), 10 mM 17β-estradiol (E2, Sigma, St. Louis, USA), and 100 mM corticosterone (Wako, Japan) were prepared in dimethyl sulfoxide (DMSO, Sigma) as the stock solution. Test chemicals: dichloro diphenyl trichloroethane (DDT, Supelco, St. Louis), dichloro diphenyl dichloro ethylene (DDE, Sigma, St. Louis, USA), dieldrin (Chem Service, USA), heptachlor (Supelco, St. Louis, USA), aldrin (Chem Service, USA), chlordane (Chem Service, USA), lindane (Supelco, St. Louis, USA), polybrominated diphenyl ethers (PBDE, Chem Service, USA), butyl paraben (Sigma, St. Louis, USA), ethyl paraben (Aldrich, St. Louis, USA), propyl paraben (Aldrich, St. Louis, USA), isobutyl paraben (Wako, Japan), isopropyl paraben (Wako, Japan) were obtained in commercially available. All test chemicals were prepared at log-serial dilutions (10−5~10−10 M) with DMSO.
Chemical treatment and determine the luciferase activity. The luciferase activities were determined in accordance with OECD TG 455. Cells were exposed with reference chemicals and serially diluted test chemicals, and then were incubated for 20~24 hrs (at 37℃ 5% CO2). The media on the cell plates were removed then added 50l of luciferase solution (Steady-Glo Luciferase Assay kit, Promega) and the activities were measured by a Luminometer (US Microplate Scintillation and Luminescence Counter, Packard).
Determine of relative transcriptional activation (RTA). The fold induction of E2 (1 nM), at least 4-fold grater than the mean vehicle control (VC), and the values of the PC50 (17α-estradiol, corticosterone and test compounds) were obtained from provided spreadsheet using GraphPad Prism software (GraphPad Software Inc., San Diego, CA). The RTA value of the estrogen activities were obtained from ratios of PC50 of test chemicals with PC50 of E2.
RESULTS AND DISCUSSION
Estrogenic activities of persistent organic pollutants by STTA. The estrogenic activities of persistent organic pollutants (DDT, DDE, dieldrin, heptachlor, aldrin, chlordane, lindane, PBDE) were determined by STTA assay. In the validation study for this assay system, the quality control and standard curve were coincident with criteria TG455 (Table 1 and Fig. 1). In this study, DDT showed a weak estrogenic activity which was 58,000-fold lower than that of E2. However, DDE, dieldrin, heptachlor, aldrin, chlordane, lindane and PBDE did not show any estrogenic activity (Table 2 and Fig. 2). As showed in Table 4, previous studies indicated that the relative estrogenic activity of DDT against E2 detected by 3 different assay systems (Coldham et al., 1997; Soto et al., 1995). DDT showed higher relative estrogenic activity in STTA assay as compare with that of Yeast and E-screen assays.
Table 1.
Performance and quality control criteria for the STTA assay
| Reference chemicals | Acceptable criteria Log[PC50M] | Result |
|---|---|---|
| 17β-estradiol (E2) | −11.4~−10.1 | −10.54~ |
| 17α-estradiol (α E2) | −9.6~−8.1 | −8.64~ |
| Corticosterone* | - | - |
*Corticosterone is used for negative control.
Fig. 1. The final concentration of each well for reference chemicals. E2 and α E2, positive control; cor, negative control.
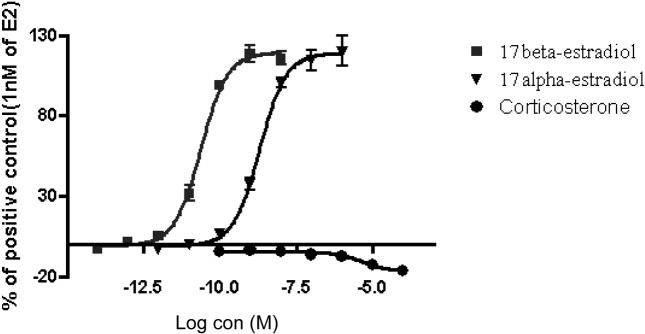
Table 2.
PC50 and logRTA values for STTA assay (persistent organic pollutants)
| Test chemicals | STTA assay | |
|---|---|---|
| PC50(M) | logRTA* | |
| 17β-estradiol | 2.88 × 10−11 | 2 |
| 17α-estradiol | 3.0 × 10−9 | −0.01773 |
| Dichloro diphenyl trichloroethane (DDT) | 1.67 × 10−6 | −2.76447 |
| Dichloro diphenyl dichloro ethylene (DDE) | Negative | Negative |
| Dieldrin | Negative | Negative |
| Heptachlor | Negative | Negative |
| Aldrin | Negative | Negative |
| Chlordane | Negative | Negative |
| Lindane | Negative | Negative |
| Polybrominated diphenyl ethers (PBDE) | Negative | Negative |
*Relative transcriptional activation is calculated as 100 × (PC50) of E2/(PC50) of test compound; a value of 100 indicates that the compound tested is a full agonist.
Abbreviations: RTA, Relative transcriptional activation; PC50, the concentration of chemical estimated to cause 50% of activity of the positive control response on a plate by plate basis.
Fig. 2. The final concentration of each well for persistent organic pollutants and reference chemicals.
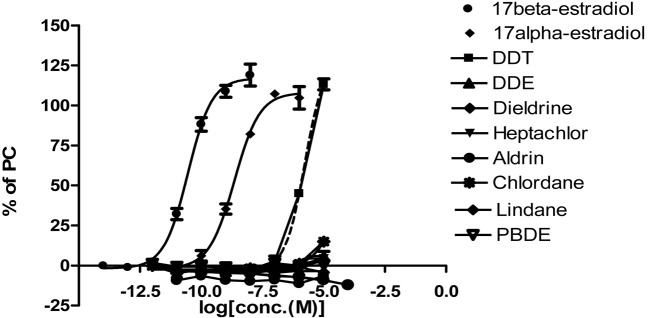
Table 4.
Comparison of in vitro assay for dichloro diphenyl trichloroethane (DDT)
| Test chemicals | Yeast assay | E-screen assay | STTA assay |
|---|---|---|---|
| Coldhama logRP | Sotob logRPP | This study logRTA | |
| 17β- estradiol | 2 | 2 | 2 |
| Dichloro diphenyl trichloroethane (DDT) | −4.52 | −4 | −2.76 |
a: data from Coldham et al. (1997), b: data from Soto et al. (1995).
Abbreviations: E2, 17β-estradiol; RP, relative potency compared to E2 (100) by molar mass determined with the recombinant yeast bioassay; RPP, relative proliferative potency is the ratio between 17β-estradiol and xenoestrogen doses needed to produce maximal cell yields ×100; RTA, Relative transcriptional activation.
Estrogenic activities of parabens by STTA. The estrogenic activities of parabens (butyl-, ethyl-, propyl-, isobutyl-, and isopropyl- paraben) were determined by STTA assay. Butyl-, ethyl-, propyl-, isobutyl- and isopropyl paraben showed lower estrogenic activity than E2 (Table 3 and Fig. 3). The rank of logRTA on the parabens was butyl paraben −1.63752 (PC50, 1.25 × 10−7 M) > isobutyl paraben −2.34008 (PC50, 6.3 × 10−7 M) > ethyl paraben −2.64016 (PC50, 1.26 × 10−6 M) > isopropyl paraben −2.73993 (PC50, 1.58 × 10−6 M) > propyl paraben −2.84164 (PC50, 2.0 × 10−6 M). Butyl-, isobutyl- ethyl-, isopropyl-, and propyl paraben were shown weak estrogenic activities which were approximately 4,300-fold, 22,000-fold, 44,000-fold, 55,000-fold, 69,000-fold lower than E2. Butyl-, ethyl- and propyl paraben were all estrogenic in a yeast-based estrogen assay, with the most potent butyl paraben being 10,000 times less potent than E2 (Routledge et al., 1998; Terasaki et al., 2009). The relative estrogenic activity of parabens against E2 using 2 different assays were indicated in Table 5. Okubo et al. (2001) had shown that the relative estrogenic activity of parabens using MCF-7 cell proliferation assays. Butyl paraben, ethyl paraben and propyl paraben showed higher relative estrogenic activity in STTA assay as compare with that of MCF-7 cell proliferation assay.
Table 3.
PC50 and logRTA values for STTA assay (parabens)
| Test chemicals | STTA assay | |
|---|---|---|
| PC50 (M) | logRTA* | |
| 17β-estradiol | 2.88 × 10−11 | 2 |
| 17α-estradiol | 3.0 × 10−9 | −0.01773 |
| Butyl paraben | 1.25 × 10−7 | −1.63752 |
| Ethyl paraben | 1.26 × 10−6 | −2.64016 |
| Propyl paraben | 2.0 × 10−6 | −2.84164 |
| Isobutyl paraben | 6.3 × 10−7 | −2.34008 |
| Isopropyl paraben | 1.58 × 10−6 | −2.73993 |
*Relative transcriptional activation is calculated as 100 × (PC50) of E2/(PC50) of test compound, a value of 100 indicates that the compound tested is a full agonist.
Abbreviations: RTA, Relative transcriptional activation; PC50, the concentration of chemical estimated to cause 50% of activity of the positive control response on a plate by plate basis.
Fig. 3. The final concentration of each well for parabens and reference chemicals.
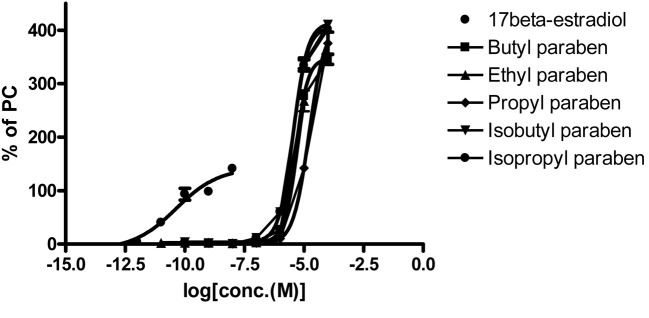
Table 5.
Comparison of in vitro assay for parabens
| Test chemicals | MCF-7 cell proliferation assay | STTA assay |
|---|---|---|
| Okuboa logRPP | This study logRTA | |
| 17β-estradiol | 2 | 2 |
| Butyl paraben | −3.82 | −1.64 |
| Ethyl paraben | −3.82 | −2.64 |
| Propyl paraben | −3.82 | −2.84 |
| Isobutyl paraben | −2.22 | −2.34 |
| Isopropyl paraben | −2.22 | −2.74 |
a: data from Okubo et al. (2001).
Abbreviations: E2, 17β-estradiol; RPP, relative proliferative potency is the ratio between 17β-estradiol and xenoestrogen doses needed to produce maximal cell yields ×100; RTA, Relative transcriptional activation.
In conclusion, STTA assay is useful and corresponds with other in vitro studies based on relative estrogenic activity. Our present results indicate that DDT and parabens (butyl-, ethyl-, propyl-, isobutyl- and isopropyl- paraben) act as weak estrogen agonist by human ERα mediated transcriptional activation.
Acknowledgments
The authors are grateful to CERI for the provide HeLa cells. This Study was supported by the 2011 research fund of the National Institute of Food & Drug Safety Evaluation/Korea Food & Drug Administration.
References
- 1.Bonefeld-Jorgensen E.C., Ayotte P. Toxicological properties of POPs and related health effects of concern for the arctic populations, in: AMAP assessment 2002: Human Health in the Arctic. AMAP. (2003):137.
- 2.Bonefeld-Jorgensen E.C. The human health effect programme in Greenland, a review. Sci. Total Environ. (2004);331:215–231. doi: 10.1016/j.scitotenv.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 3.Colborn T., vom Saal F.S., Soto A.M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. (1993);101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coldham N.G., Dave M., Sivapathasundaram S., McDonnell D.P., Connor C., Sauer M.J. Evaluation of a recombinant yeast cell estrogen screening assay. Environ. Health Perspect. (1997);105:734–742. doi: 10.1289/ehp.97105734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damdimopoulos A.E., Spyrou G., Gustafsson J.A. Ligands Differentially Modify the Nuclear Mobility of Estrogen Receptors alpha and beta. Endocrinology. (2008);149:339–345. doi: 10.1210/en.2007-0198. [DOI] [PubMed] [Google Scholar]
- 6.Hunter D.S., Hodges L.C., Vonier P.M., Fuchs-Young R., Gottardis M.M., Walker C.L. Estrogen receptor activation via activation function 2 predicts agonism of xenoestrogens in normal and neoplastic cells of the uterine myometrium. Cancer Res. (1999);59:3090–3099. [PubMed] [Google Scholar]
- 7.Krieger R.I. Handbook of Pesticide Toxicology, 1. Academic Press; San Diego, USA: (2001). [Google Scholar]
- 8.Nishihara T., Nishikawa J.I., Kanayama T., Dakeyama F., Saito K., Imagawa M., Takatori S., Kitagawa Y., Hori S., Utsumi H. Estrogenic activities of 517 chemicals by yeast two-hybrid assay. Journal of Health Science. (2000);46:282–298. [Google Scholar]
- 9.OECD, Organization for Economic Cooperation and Development. Draft summary report of the sixth meeting of the task force on endocrine disrupters testing and assessment (EDTA 6). Paris: (2003). [Google Scholar]
- 10.OECD, Organization for Economic Cooperation and Development. Ten new test guidelines and six updated test guidelines have been adopted by council on 7 September 2009. (2009)
- 11.Okubo T., Yokoyama Y., Kano K., Kano I. ERdependent estrogenic activity of parabens assessed by proliferation of human breast cancer MCF-7 cells and expression of ERalpha and PR. Food Chem. Toxicol. (2001);39:1225–1232. doi: 10.1016/S0278-6915(01)00073-4. [DOI] [PubMed] [Google Scholar]
- 12.Routledge E.J., Parker J., Odum J., Ashby J., Sumpter J.P. Some alkyl hydroxy benzoate preservatives (Parabens) are estrogenic. Toxicol. Appl. Pharmacol. (1998);153:12–19. doi: 10.1006/taap.1998.8544. [DOI] [PubMed] [Google Scholar]
- 13.Soni M.G., Carabin I.G., Burdock G.A. Safety assessment of esters of p-hydroxybenzoic acid (Parabens). Food Chem. Toxicol. (2005);43:985–1015. doi: 10.1016/j.fct.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Soto A.M., Sonnenschein C., Chung K.L., Fernandez M.F., Olea N., Srrano F.O. The E-screen assay as a tool to identify estrogens: An update on estrogenic environmental pollutants. Environ. Health Perspect. (1995);103:113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vo T.T., Jeung E.B. An evaluation of estrogenic activity of parabens using uterine Calbindin-D9K gene in an immature rat model. Toxicol. Sci. (2009);112:68–77. doi: 10.1093/toxsci/kfp176. [DOI] [PubMed] [Google Scholar]
- 16.Vos J.G., Dybing E., Greim H.A., Ladefoged O., Lambre C., Tarazona J.V., Brandt I., Vethaak A.D. Health effects of endocrine-disrupting chemicals on wildlife, with special reference to the European situation. Crit. Rev. Toxicol. (2000);30:71–133. doi: 10.1080/10408440091159176. [DOI] [PubMed] [Google Scholar]


