Abstract
The object of this study was to evaluate the single oral dose toxicity of platycodin D, a saponin from the root of Platycodon grandiflorum in male and female mice. Platycodin D was administered to female and male mice as an oral dose of 2000, 1000, 500, 250 and 125 mg/kg (body wt.). Animals were monitored for the mortality and changes in body weight, clinical signs and gross observation during 14 days after treatment, upon necropsy, organ weight and histopathology of 14 principle organs were examined. As the results, no platycodin D treatment related mortalities, clinical signs, changes on the body and organ weights, gross and histopathological observations against 14 principle organs were detected up to 2000 mg/kg in both female and male mice. Therefore, LD50 (50% lethal dose) and approximate LD of playtcodin D after single oral treatment in female and male mice were considered over 2000 mg/kg - the limited dosages recommended by KFDA Guidelines [2009-116, 2009], respectively.
Keywords: Platycodin D, Single oral dose toxicity, Mouse, Histopathology
INTRODUCTION
Platycodin radix, the roots of Platycodon grandiflorum, has been used traditionally as an expectorant and a remedy for bronchitis, tonsillitis, laryngitis and suppurative dermatitis in China, Korea and Japan (Han et al., 2000). Platycodin D is a major pharmacological constituent of Platycodi radix (Han et al., 2002), and it has been shows the anti-diabetic (Kim et al., 2000; Han et al., 2002; Zhao et al., 2005), antiinflammatory (Kim et al., 2001; Chung et al., 2008; Hong et al., 2008), anti-cancer (Kim et al., 2008; Yu and Kim, 2010), antinociceptive (Choi et al., 2002, 2004) and immunomodulatory (Xie et al., 2009, 2010) activities. However, no detailed toxicological assessment of Platycodin D has been reported even if mouse single dose toxicity test.
The objective of the present study, therefore, was to obtain the primary safety information about platycodin D, a saponin from the root of Platycodin radix, and further clarifies their safety for clinical use. In order to observe the 50% lethal dose (LD50), approximate lethal dosage (ALD), test articles were once orally administered to female and male mice at dose levels of 2000, 1000, 500, 250 and 125 mg/kg (body wt.) according to the recommendation of KFDA Guidelines (2009). The mortality and changes on body weight, clinical signs and gross observation were monitored during 14 days after oral administration of platycodin D with organ weights and histopathology of principle organs.
MATERIALS AND METHODS
Experimental animals. Each of thirty female and male ICR mice (6-wk old upon receipt, SLC, Japan) was used after acclimatization for 8 days. Animals were allocated five per polycarbonate cage in a temperature (20~25℃) and humidity (45~50%) controlled room. Light : dark cycle was 12 hrs : 12 hrs, and feed (Samyang, Korea) and water were supplied free to access. All animals were overnight fasted before dosing and terminal necropsy. Animals were marked by picric acid. This study was carried out with prior approval of the Animal Ethical Committee, The University of Daegu Haany University (Gyeongsan, Korea).
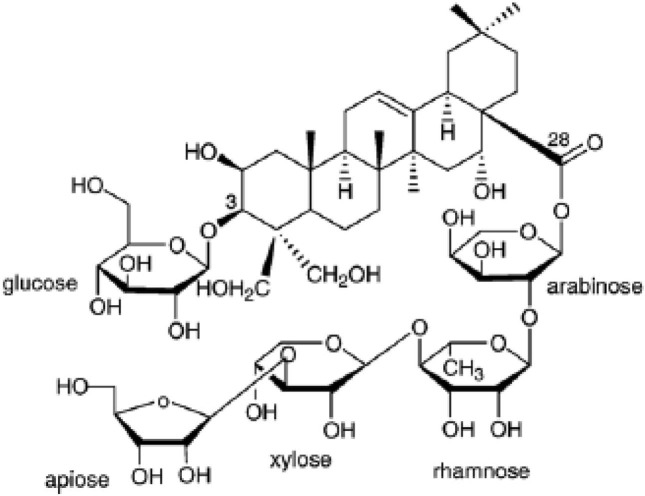
Preparation and administration of Platycodin D. The Platycodin D, gift from Glucan Corp. Ltd. (Korea), was extracted from Platycodi radix by previous method (Zhao et al., 2005). The raw sample (100 kg) of Platycodin radix was extracted with methanol and partitioned sequentially with n-hexane, chloroform, ethyl acetate and n-butanol. The n-butanol fraction was then subjected to Diaion HP-20 resin (Mitsubishi, Japan), and the fractions eluted at 60~80% of methanol were collected to obtain 90 g of crude saponins. The crude saponins were further purified by repeated silica gel (Merck, Germany) chromatography to obtain the purified platycodin D. The process was repeated several times until a sufficient quantity of platycodin D was obtained. The purified platycodin D was identified on the basis of Rf, FAB-MS (= 1225.38), and [13C]-NMRspectra compared with the authentic platycodin D (Fig. 1). Prepared platycodin D is light yellow powder, and stored in a desiccator to protect from light and humidity. Platycodin D is well dissolved (clear light yellow solution) at least 200 mg/ml concentrations in distilled water. The test article was single orally administered at a dosage volume of 20 ml/kg using distilled water as vehicle at 2000, 1000, 500, 250 and 125 mg/kg dose levels.
Fig. 1. Structure of platycodin D. Platycodin D is a triterpenoid bidesmoside, composed of an aglycone moiety, 3-Glc and 28-OApi- Xyl-Rha-Ara.
Abnormal behavior, clinical sign and body weight. All abnormal clinical signs and behaviors were recorded before and after dosing at least twice a day based on the functional observational battery test (Irwin, 1968; Dourish, 1987). Body weights were measured on the day of dosing (Day 0) prior to treatment, 1, 2, 7, 13 and 14 days after dosing. In addition, to reduce the differences from individual body weight differences of animals at treatment, body weight gains during Day 0~Day 7, Day 7~Day 13 and Day 0~Day 14 was also calculated based on measured body weight at each point.
Necropsy. All unscheduled died animals were grossly observed immediately after finding them and all survived animals were subjected to terminal necropsy. Animals were asphyxiated by carbon dioxide and gross necropsy was performed in all animals at Day 14 after overnight fasting (about 18 h, water was not restricted).
Organ weight measurements and sampling. The absolute organ weight was measured and then relative organ weight (% for body weight) was calculated. The following organs were collected for histopathological observation.
Measured and sampled organs: lung, heart, thymus, left kidney, left adrenal gland, spleen, left testis or ovary, liver, splenic lobe of pancreas, brain, left epididymis or total uterus and left popliteal lymph node.
Histopathology. Samples were fixed in 10% neutral buffered formalin. After 18 hrs of fixation, paraffin embedding was conducted and 4 μm sections were prepared by routine histological methods. Representative sections of each specified organs were stained with hematoxylin-eosin for light microscopical examination.
Statistical analyses. Multiple comparison tests for different dose groups were conducted. Variance homogeneity was examined using the Levene test. If the Levene test indicated no significant deviations from variance homogeneity, the obtain data were analyzed by one way ANOVA test followed by Scheffe test to determine which pairs of group comparison were significantly different. In case of significant deviations from variance homogeneity were observed at Levene test, a non-parametric comparison test, the Mann- Whitney U test was conducted to determine the specific pairs of group comparison, which are significantly different. LD50 and 95% confidence limits were calculated by Probit method. Statistical analyses were conducted using SPSS for Windows (Release 14.0K, SPSS Inc., USA) and a p-value of less than 0.05 was considered to be a significant difference. In addition, degree of clinical signs, gross and histopathological findings were subdivided into 3 degrees: 3+ Severe, 2+ moderate, 1+ slight.
RESULTS
Mortalities. No unscheduled or Platycodin D-treatment related mortalities were detected in all dose levels tested in this study. At termination, all of animals (5/5; 100%) were survived in all dose levels tested including vehicle control.
Clinical signs. In this study, no Platycodin D treatment related abnormal clinical signs were observed during observation periods regardless of male and female mice.
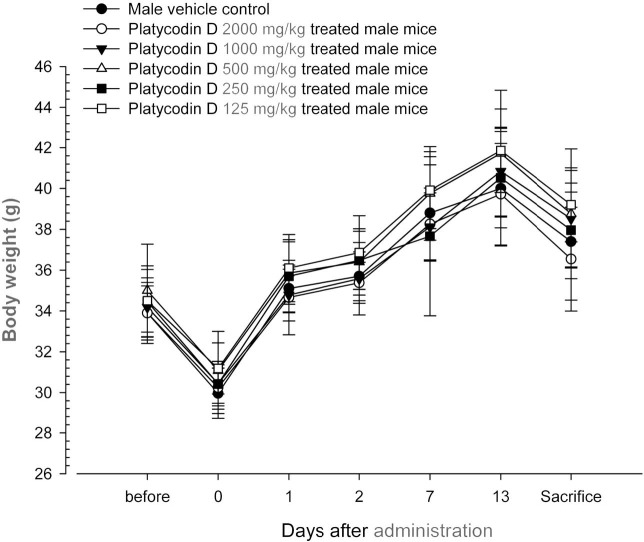
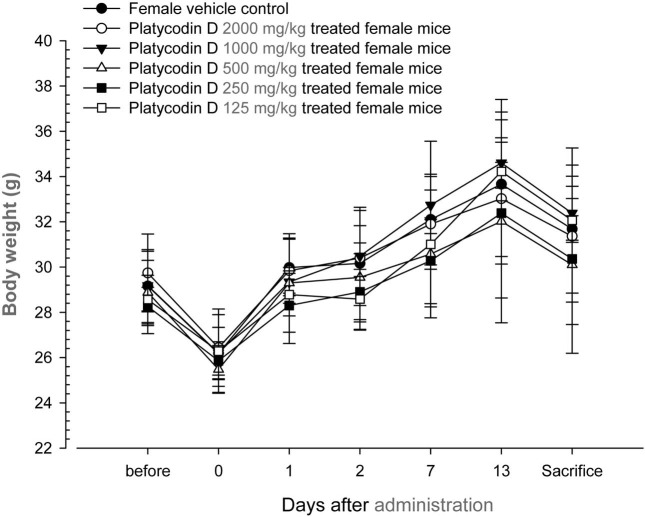
Changes on body weights and gains. No significant changes in body weight were detected as compared to that of vehicle control in all dose levels tested except for significant (p < 0.05) increase of body weight gains during Day 7~ Day 14 detected in platycodin D 1000 mg/kg treated male group (Table 1, Fig. 2 and 3).
Table 1.
Body weight gains after oral treatment of platycodin D
| Group | Intervals | ||
|---|---|---|---|
| Day 0a~Day 7 | Day 7~Day 13 | Day 0~Day 14b | |
| Male | |||
| Vehicle control | 8.84 ± 1.24 | 1.20 ± 0.38 | 7.44 ± 1.86 |
| 2000 mg/kg | 8.02 ± 1.39 | 1.46 ± 0.98 | 6.30 ± 2.33 |
| 1000 mg/kg | 7.68 ± 0.82 | 2.72 ± 0.83* | 8.08 ± 1.44 |
| 500 mg/kg | 8.68 ± 0.44 | 1.96 ± 1.64 | 7.68 ± 1.85 |
| 250 mg/kg | 7.24 ± 4.26 | 2.86 ± 5.30 | 7.54 ± 1.24 |
| 125 mg/kg | 8.74 ± 0.88 | 1.94 ± 0.48 | 8.02 ± 1.07 |
| Female | |||
| Vehicle control | 5.92 ± 2.02 | 1.56 ± 1.58 | 5.50 ± 2.23 |
| 2000 mg/kg | 5.46 ± 2.74 | 1.12 ± 1.78 | 4.92 ± 2.94 |
| 1000 mg/kg | 6.54 ± 0.79 | 1.86 ± 0.90 | 6.18 ± 1.13 |
| 500 mg/kg | 5.10 ± 1.80 | 1.44 ± 1.73 | 4.62 ± 2.87 |
| 250 mg/kg | 4.42 ± 1.18 | 2.10 ± 1.63 | 4.50 ± 1.32 |
| 125 mg/kg | 4.72 ± 0.83 | 3.22 ± 1.11 | 5.78 ± 0.83 |
Values are expressed as mean ± SD of five mice, g.
aDay of treatment after overnight fasted.
bDay of sacrifice after overnight fasted.
*p < 0.05 compared with male vehicle control.
Fig. 2. Body weight changes in male mice after once oral administration of platycodin D. No meaningful changes were detected in all platycodin D treated groups as compared with vehicle control. Before means 1 day before administration; Day 0 means at administration; All animals at sacrifice and Day 0 overnight fasted; Values are expressed as mean ± SD of five mice, g.
Fig. 3. Body weight changes in female mice after once oral administration of platycodin D. No meaningful changes were detected in all platycodin D treated groups as compared with vehicle control. Before means 1 day before administration; Day 0 means at administration; All animals at sacrifice and Day 0 overnight fasted; Values are expressed as mean ± SD of five mice, g.
Changes on the organ weight. No meaningful changes on the absolute and relative organ weight of principle organs were observed in all platycodin D treated female and male mice as compared with each equal gender of vehicle control, except for significant (p < 0.05) increases of absolute and relative uterus weights restricted to 250 mg/kg of platycodin D treated female mice and decreases of relative brain weight in platycodin D 250 mg/kg treated male mice as compared with equal genders of vehicle control in the present study (Table 2, 3).
Table 2.
Changes on the absolute organ weights after oral treatment of platycodin D
| Dose (mg/kg) | Organs: Male | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung | Heart | Thymus | Kidney L | Adrenal gland L | Spleen | Testis L | Liver | Pancreas S | Brain | Epididymis L | Lymph node La | |
| 0 | 0.214 ± 0.016 | 0.174 ± 0.008 | 0.056 ± 0.007 | 0.328 ± 0.029 | 0.007 ± 0.001 | 0.114 ± 0.020 | 0.108 ± 0.017 | 1.669 ± 0.208 | 0.191 ± 0.011 | 0.476 ± 0.012 | 0.040 ± 0.005 | 0.028 ± 0.004 |
| 2000 | 0.211 ± 0.011 | 0.165 ± 0.008 | 0.063 ± 0.011 | 0.330 ± 0.042 | 0.010 ± 0.003 | 0.107 ± 0.016 | 0.114 ± 0.012 | 1.638 ± 0.147 | 0.178 ± 0.035 | 0.486 ± 0.017 | 0.039 ± 0.004 | 0.025 ± 0.011 |
| 1000 | 0.218 ± 0.016 | 0.173 ± 0.038 | 0.049 ± 0.010 | 0.342 ± 0.032 | 0.008 ± 0.003 | 0.125 ± 0.027 | 0.119 ± 0.008 | 1.773 ± 0.182 | 0.207 ± 0.022 | 0.472 ± 0.024 | 0.039 ± 0.006 | 0.027 ± 0.005 |
| 500 | 0.218 ± 0.015 | 0.173 ± 0.007 | 0.055 ± 0.015 | 0.364 ± 0.049 | 0.008 ± 0.002 | 0.118 ± 0.014 | 0.115 ± 0.010 | 1.723 ± 0.215 | 0.198 ± 0.023 | 0.485 ± 0.021 | 0.039 ± 0.007 | 0.023 ± 0.004 |
| 250 | 0.224 ± 0.019 | 0.174 ± 0.009 | 0.062 ± 0.012 | 0.331 ± 0.053 | 0.008 ± 0.003 | 0.107 ± 0.017 | 0.111 ± 0.008 | 1.687 ± 0.160 | 0.205 ± 0.018 | 0.445 ± 0.049 | 0.040 ± 0.002 | 0.031 ± 0.012 |
| 125 | 0.212 ± 0.010 | 0.171 ± 0.012 | 0.061 ± 0.011 | 0.348 ± 0.033 | 0.008 ± 0.001 | 0.121 ± 0.018 | 0.121 ± 0.010 | 1.727 ± 0.120 | 0.202 ± 0.016 | 0.499 ± 0.022 | 0.042 ± 0.003 | 0.030 ± 0.005 |
| Dose (mg/kg) | Organs: Female | |||||||||||
| Lung | Heart | Thymus | Kidney L | Adrenal gland L | Spleen | Ovary L | Liver | Pancreas S | Brain | Uterus | Lymph node L | |
| 0 | 0.190 ± 0.014 | 0.148 ± 0.009 | 0.074 ± 0.013 | 0.215 ± 0.017 | 0.008 ± 0.003 | 0.142 ± 0.021 | 0.035 ± 0.014 | 1.475 ± 0.188 | 0.174 ± 0.008 | 0.474 ± 0.011 | 0.128 ± 0.031 | 0.022 ± 0.006 |
| 2000 | 0.185 ± 0.022 | 0.154 ± 0.008 | 0.071 ± 0.015 | 0.221 ± 0.006 | 0.008 ± 0.002 | 0.129 ± 0.040 | 0.038 ± 0.006 | 1.517 ± 0.189 | 0.187 ± 0.022 | 0.483 ± 0.016 | 0.185 ± 0.092 | 0.020 ± 0.010 |
| 1000 | 0.200 ± 0.008 | 0.147 ± 0.008 | 0.067 ± 0.005 | 0.216 ± 0.010 | 0.008 ± 0.001 | 0.135 ± 0.035 | 0.056 ± 0.005 | 1.481 ± 0.128 | 0.167 ± 0.011 | 0.469 ± 0.016 | 0.173 ± 0.052 | 0.025 ± 0.006 |
| 500 | 0.193 ± 0.018 | 0.136 ± 0.012 | 0.071 ± 0.021 | 0.215 ± 0.018 | 0.008 ± 0.002 | 0.125 ± 0.050 | 0.036 ± 0.010 | 1.283 ± 0.200 | 0.168 ± 0.026 | 0.477 ± 0.025 | 0.156 ± 0.049 | 0.022 ± 0.008 |
| 250 | 0.195 ± 0.013 | 0.139 ± 0.010 | 0.070 ± 0.016 | 0.207 ± 0.020 | 0.009 ± 0.004 | 0.129 ± 0.019 | 0.045 ± 0.015 | 1.307 ± 0.098 | 0.165 ± 0.021 | 0.478 ± 0.026 | 0.192 ± 0.048* | 0.025 ± 0.005 |
| 125 | 0.193 ± 0.012 | 0.152 ± 0.010 | 0.078 ± 0.020 | 0.211 ± 0.022 | 0.008 ± 0.002 | 0.155 ± 0.020 | 0.042 ± 0.012 | 1.402 ± 0.156 | 0.187 ± 0.024 | 0.486 ± 0.015 | 0.159 ± 0.031 | 0.024 ± 0.011 |
Values are expressed as mean ± SD of five mice, g.
L, left sides; S, splenic lobes; a Popliteal lymph node.
*p < 0.05 compared with male vehicle control.
Table 3.
Changes on the relative organ weights after oral treatment of platycodin D
| Dose (mg/kg) | Organs: Male | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung | Heart | Thymus | Kidney L | Adrenal gland L | Spleen | Testis L | Liver | Pancreas S | Brain | Epididymis L | Lymph node La | |
| 0 | 0.574 ± 0.035 | 0.466 ± 0.035 | 0.149 ± 0.015 | 0.877 ± 0.042 | 0.018 ± 0.004 | 0.303 ± 0.041 | 0.288 ± 0.033 | 4.468 ± 0.515 | 0.510 ± 0.014 | 1.277 ± 0.068 | 0.108 ± 0.013 | 0.074 ± 0.010 |
| 2000 | 0.580 ± 0.043 | 0.458 ± 0.041 | 0.172 ± 0.033 | 0.906 ± 0.104 | 0.025 ± 0.007 | 0.289 ± 0.043 | 0.311 ± 0.036 | 4.482 ± 0.246 | 0.486 ± 0.087 | 1.344 ± 0.102 | 0.106 ± 0.011 | 0.075 ± 0.021 |
| 1000 | 0.571 ± 0.041 | 0.458 ± 0.095 | 0.123 ± 0.023 | 0.881 ± 0.071 | 0.024 ± 0.004 | 0.323 ± 0.072 | 0.311 ± 0.022 | 4.605 ± 0.415 | 0.536 ± 0.058 | 1.246 ± 0.107 | 0.103 ± 0.012 | 0.072 ± 0.015 |
| 500 | 0.570 ± 0.052 | 0.451 ± 0.028 | 0.153 ± 0.028 | 0.958 ± 0.120 | 0.023 ± 0.003 | 0.306 ± 0.057 | 0.304 ± 0.016 | 4.434 ± 0.216 | 0.522 ± 0.065 | 1.264 ± 0.107 | 0.105 ± 0.014 | 0.062 ± 0.010 |
| 250 | 0.605 ± 0.043 | 0.463 ± 0.021 | 0.168 ± 0.024 | 0.886 ± 0.108 | 0.018 ± 0.002 | 0.295 ± 0.025 | 0.293 ± 0.029 | 4.438 ± 0.228 | 0.535 ± 0.038 | 1.191 ± 0.096* | 0.106 ± 0.009 | 0.076 ± 0.026 |
| 125 | 0.549 ± 0.030 | 0.447 ± 0.013 | 0.161 ± 0.024 | 0.906 ± 0.035 | 0.020 ± 0.002 | 0.302 ± 0.049 | 0.311 ± 0.023 | 4.405 ± 0.191 | 0.522 ± 0.053 | 1.284 ± 0.044 | 0.107 ± 0.012 | 0.076 ± 0.011 |
| Dose (mg/kg) | Organs: Female | |||||||||||
| Lung | Heart | Thymus | Kidney L | Adrenal gland L | Spleen | Ovary L | Liver | Pancreas S | Brain | Uterus | Lymph node L | |
| 0 | 0.601 ± 0.044 | 0.467 ± 0.022 | 0.233 ± 0.040 | 0.680 ± 0.043 | 0.027 ± 0.009 | 0.446 ± 0.043 | 0.109 ± 0.039 | 4.651 ± 0.334 | 0.552 ± 0.040 | 1.508 ± 0.156 | 0.413 ± 0.127 | 0.071 ± 0.022 |
| 2000 | 0.616 ± 0.099 | 0.504 ± 0.081 | 0.215 ± 0.032 | 0.710 ± 0.082 | 0.029 ± 0.007 | 0.445 ± 0.124 | 0.122 ± 0.025 | 4.838 ± 0.096 | 0.608 ± 0.091 | 1.574 ± 0.235 | 0.658 ± 0.333 | 0.061 ± 0.035 |
| 1000 | 0.614 ± 0.040 | 0.457 ± 0.042 | 0.207 ± 0.018 | 0.670 ± 0.042 | 0.025 ± 0.005 | 0.420 ± 0.105 | 0.175 ± 0.007 | 4.571 ± 0.289 | 0.516 ± 0.025 | 1.445 ± 0.061 | 0.548 ± 0.171 | 0.078 ± 0.018 |
| 500 | 0.642 ± 0.040 | 0.460 ± 0.048 | 0.252 ± 0.024 | 0.718 ± 0.068 | 0.025 ± 0.005 | 0.429 ± 0.098 | 0.112 ± 0.028 | 4.261 ± 0.282 | 0.590 ± 0.072 | 1.627 ± 0.195 | 0.485 ± 0.110 | 0.079 ± 0.027 |
| 250 | 0.650 ± 0.048 | 0.459 ± 0.059 | 0.223 ± 0.051 | 0.675 ± 0.054 | 0.030 ± 0.010 | 0.415 ± 0.045 | 0.133 ± 0.031 | 4.311 ± 0.269 | 0.554 ± 0.119 | 1.564 ± 0.152 | 0.631 ± 0.151* | 0.081 ± 0.015 |
| 125 | 0.605 ± 0.032 | 0.476 ± 0.033 | 0.244 ± 0.067 | 0.667 ± 0.066 | 0.028 ± 0.004 | 0.481 ± 0.055 | 0.128 ± 0.038 | 4.367 ± 0.368 | 0.580 ± 0.072 | 1.520 ± 0.052 | 0.465 ± 0.035 | 0.081 ± 0.032 |
Values are expressed as mean ± SD of five mice, % of body weight at sacrifice
L, left sides; S, splenic lobes; aPopliteal lymph node.
*p < 0.05 as compared with vehicle control.
Necropsy findings. No platycodin D-treatment related changes on the gross findings were observed as compared with equal gender of vehicle control except for some sporadic findings such as slight (1+) congestion spots of lung, atrophy of thymus, cyst in kidney, spleen atrophy or hypertrophy, and hypertrophy and focal hemorrhage of popliteal lymph node, which were sporadically detected throughout all experimental groups tested in the present including both gender of vehicle control (Table 4).
Table 4.
Necropsy findings after oral treatment of platycodin D
| Dose (mg/kg) | Male | Female | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2000 | 1000 | 500 | 250 | 125 | 0 | 2000 | 1000 | 500 | 250 | 125 | |
| Lung | ||||||||||||
| Normal | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 3/5 | 5/5 | 4/5 | 4/5 | 4/5 | 4/5 |
| Congestion | 1/5 | 1/5 | 1/5 | 1/5 | 1/5 | 1/5 | 2/5 | 0/5 | 1/5 | 1/5 | 1/5 | 1/5 |
| Thymus | ||||||||||||
| Normal | 4/5 | 5/5 | 4/5 | 3/5 | 3/5 | 4/5 | 4/5 | 5/5 | 3/5 | 3/5 | 3/5 | 4/5 |
| Atrophy | 1/5 | 0/5 | 1/5 | 2/5 | 2/5 | 1/5 | 1/5 | 0/5 | 2/5 | 2/5 | 2/5 | 1/5 |
| Kidney | ||||||||||||
| Normal | 5/5 | 5/5 | 5/5 | 5/5 | 4/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 4/5 | 5/5 |
| Cyst | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | 0/5 |
| Spleen | ||||||||||||
| Normal | 3/5 | 4/5 | 3/5 | 4/5 | 3/5 | 4/5 | 2/5 | 4/5 | 3/5 | 4/5 | 3/5 | 5/5 |
| Atrophy | 2/5 | 1/5 | 2/5 | 1/5 | 2/5 | 0/5 | 2/5 | 1/5 | 2/5 | 1/5 | 2/5 | 0/5 |
| Hypertrophy | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| LNa | ||||||||||||
| Normal | 3/5 | 4/5 | 1/5 | 4/5 | 4/5 | 5/5 | 2/5 | 1/5 | 5/5 | 3/5 | 3/5 | 5/5 |
| Hypertrophy | 2/5 | 1/5 | 3/5 | 1/5 | 1/5 | 0/5 | 3/5 | 4/5 | 0/5 | 2/5 | 2/5 | 0/5 |
| Focal hemorrhage | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
Observed animals/total observed animals (five mice).
aBilateral popliteal lymph node.
Histopathological findings. No meaningful changes on the histopathological findings of 14 principle organs were observed in platycodin D-treated groups as compared with equal gender of vehicle control except for some sporadic findings such as slight (1+) hypertrophy of lung alveolus wall with focal hemorrhage, decreases of lymphoid cells in the cortex of thymus and red pulps of spleen, focal inflammatory cell infiltration in liver, edematous changes on the uterus, and focal hyperplasia of lymphoid cells in the popliteal lymph node, which were sporadically detected throughout all experimental groups tested in the present study including both gender vehicle controls (Table 5).
Table 5.
Histopathological findings after oral treatment of platycodin D
| Dose (mg/kg) | Male | Female | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2000 | 1000 | 500 | 250 | 125 | 0 | 2000 | 1000 | 500 | 250 | 125 | |
| Lung | ||||||||||||
| Normal | 5/5 | 5/5 | 4/5 | 5/5 | 4/5 | 4/5 | 4/5 | 4/5 | 5/5 | 5/5 | 5/5 | 5/5 |
| Congestion | 0/5 | 0/5 | 1/5 | 0/5 | 1/5 | 1/5 | 1/5 | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Thymus | ||||||||||||
| Normal | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 4/5 | 5/5 | 5/5 |
| DE* | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 |
| Kidney | ||||||||||||
| Normal | 4/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 |
| AT-G* | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Spleen | ||||||||||||
| Normal | 5/5 | 4/5 | 5/5 | 3/5 | 4/5 | 4/5 | 5/5 | 4/5 | 5/5 | 5/5 | 5/5 | 5/5 |
| DE* | 0/5 | 1/5 | 0/5 | 2/5 | 1/5 | 1/5 | 0/5 | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Liver | ||||||||||||
| Normal | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 4/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 |
| IF* | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Testis/Uterus | ||||||||||||
| Normal | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 4/5 | 5/5 |
| ED* | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | 0/5 |
| LNa | ||||||||||||
| Normal | 5/5 | 5/5 | 4/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 |
| HP* | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
Observed animals/total observed animals (five mice).
aLeft popliteal lymph node.
*Abbreviations: DE, decreases of lymphoid cells; AT-G, focal atrophy of glomerulus; IF, focal inflammatory cell infiltration; ED, edematous changes; HP, focal lymphoid cell hyperplasia.
DISCUSSION
In the present study, we investigated the single oral dose toxicity of platycodin D on the mice as a part of the safety test. In order to observe 50% lethal dose (LD50) and approximate lethal dosage (LD), test substances were administered orally to female and male ICR mice at dose levels of 2000, 1000, 500, 250 and 125 mg/kg. We could not find any mortality, clinical signs, and changes in the body and organ weights except for significant (p < 0.05) increases of absolute and relative uterus weights restricted to 250 mg/kg of platycodin D treated female mice and decreases of relative brain weight in platycodin D 250 mg/kg treated male mice. In addition, no platycodin D-treatment related abnormal gross findings and changes in histopathology of principle organs were detected except for some sporadic accidental findings in both male and female mice.
In KFDA Guidelines (2009-116, 2009), the recommended highest dose of test materials were 2000 mg/kg or the maximum solubility, and they also recommended that in case of single dose toxicity in mouse, the dosage volume was below 20 ml/kg (Flecknell, 1996). In the present study, the highest dosage was selected as 2000 mg/kg in a volume of 20 ml, the recommended oral dose volume in mice (Flecknell, 1996) and the limited highest dosages recommended by KFDA Guidelines (2009-116, 2009), and 1000, 500, 250 and 125 mg/kg are selected using common ratio 2. In addition, each female and male vehicle control groups was added. Test material was orally administered using distilled water as vehicle in the present study.
No platycodin D treatment related mortalities and clinical signs were detected up to 2000 mg/kg - the highest dosages used in the present study. Significant (p < 0.05) increase of body weight gains during Day 7~Day 14 detected platycodin D 1000 mg/kg treated male group were difficult to considered platycodin D treatment related toxicological signs because they did not showed any dosage-dependent changes, all animals including 2000 mg/kg treated male and female mice shows body weight increases ranged in normal age-matched mice (Plata and Murphy, 1972; Yamaguchi et al., 1983).
Decrease of relative brain weight detected in Platycodin D 250 mg/kg treated male mice, are not considered as platycodin D treatment related toxicological signs because they did not showed any dosage-dependent changes and no meaningful gross and histopathological changes were detected in the brain in the present study. The increased trends of testis and urine weights were detected in all platycodin D treated female or male mice as compared with equal genders of vehicle control, respectively. These increased trends of reproductive organs were considered as difficult to consider as platycodin D treatment related toxicological signs because significant changes were restricted to the relative uterus weights of platycodin D 250 mg/kg treated female mice as compared with female vehicle control and no meaningful histopathological changes were detected in the testis and uterus at sacrifice. However, it also difficult to exclude that platycodin D can be influenced on the reproductive organs like pytoestrogen. Pytoestrogens have been various influenced on the male and female reproductive organs as pharmacological effects (Roberts et al., 2000; Jaroenporn et al., 2006). It also already known that the uterus weights in the mice are generally changed with estrus cycles (Pineda, 1989).
The slight congestion of lung, atrophy of thymus, cyst in kidney, spleen atrophy or hypertrophy, and hypertrophy and focal hemorrhage of popliteal lymph node detected in the present study as gross findings, and hypertrophy of lung alveolus wall with focal hemorrhages, decreases of lymphoid cells in the cortex of thymus and red pulps of spleen, focal inflammatory cell infiltration in liver, edematous changes on the uterus, and hyperplasia of lymphoid cells in the popliteal lymph node detected as histopathological findings were considered as accidental findings not toxicological signs related to the platycodin D treatment because they were sporadically detected throughout the whole experimental groups tested in the present study including both genders of vehicle control. Especially, the edematous changes in uterus were considered as secondary changes from different physiological estrus cycles (Banks, 1986; Pineda, 1989). In addition, most of them were also generally observed in normal mice (Lee et al., 2005, 2006).
Although there are any available data on the absorption of platycodin D in mice, but platycodin D has been showed pharmacodynamic effects on various animal experiments after oral administration at the lower dosage than 2000 mg/kg (Kim et al., 2000; Han et al., 2000, 2002; Zhao et al., 2005; Xie et al., 2010) as direct evidences that oral administration of platycodin D can be absorbed by intestine.
Because no platycodin D treatment related mortalities were detected up to 2000 mg/kg in both male and female mice in the present study, the LD50 and approximate LD of platycodin D after single oral treatment in female and male mice were considered over 2000 mg/kg, and is likely to be safe in humans.
Acknowledgments
This research was supported by a grant from Daegu Haany University Ky-rin Fund, 2011 (2011-901-39).
References
- 1.Banks W.J., Banks W.J. Female reproductive system in Applied veterinary histology. Williams & Wilkins; Baltimore: (1986). pp. 506–526. [Google Scholar]
- 2.Chung J.W., Noh E.J., Zhao H.L., Sim J.S., Ha Y.W., Shin E.M., Lee E.B., Cheong C.S., Kim Y.S. Antiinflammatory activity of prosapogenin methyl ester of platycodin D via nuclear factor-kappaB pathway inhibition. Biol. Pharm. Bull. (2008);31:2114–2120. doi: 10.1248/bpb.31.2114. [DOI] [PubMed] [Google Scholar]
- 3.Choi S.S., Han E.J., Lee T.H., Lee J.K., Han K.J., Lee H.K., Suh H.W. Antinociceptive mechanisms of platycodin D administered intracerebroventricularly in the mouse. Planta Med. (2002);68:794–798. doi: 10.1055/s-2002-34396. [DOI] [PubMed] [Google Scholar]
- 4.Choi S.S., Han E.J., Lee T.H., Han K.J., Lee H.K., Suh H.W. Antinociceptive profiles of platycodin D in the mouse. Am. J. Chin. Med. (2004);32:257–268. doi: 10.1142/S0192415X04001916. [DOI] [PubMed] [Google Scholar]
- 5.Dourish C.T., Greenshaw A.J., Dourish C.T. Effects of drugs on spontaneous motor activity in Experimental psychopharmacology. Humana Press; Clifton: (1987). pp. 325–334. [Google Scholar]
- 6.Flecknell P. Laboratory Animal Anesthesia. 2nd Ed. Harcourt Brace & Company; New York: (1996). p. 269. [Google Scholar]
- 7.Hong J., Shin K.H., Lim S.S., Kwak J.H., Zee O., Ishihara K., Hirasawa N., Seyama T., Ohuchi K. Lead compounds for anti-inflammatory drugs isolated from the plants of the traditional oriental medicine in Korea. Inflamm. Allergy Drug Targets. (2008);7:195–202. doi: 10.2174/187152808785748100. [DOI] [PubMed] [Google Scholar]
- 8.Han L.K., Xu B.J., Kimura Y., Zheng Y., Okuda H. Platycodi radix affects lipid metabolism in mice with high fat diet-induced obesity. J. Nutr. (2000);130:2760–2764. doi: 10.1093/jn/130.11.2760. [DOI] [PubMed] [Google Scholar]
- 9.Han L.K., Zheng Y.N., Xu B.J., Okuda H., Kimura Y. Saponins from platycodi radix ameliorate high fat dietinduced obesity in mice. J. Nutr. (2002);132:2241–2245. doi: 10.1093/jn/132.8.2241. [DOI] [PubMed] [Google Scholar]
- 10.Irwin S. Comprehensive observational assessment: Ia. A systemic, quantitative procedure for assessing the behavioral and physiological state of the mouse. Psychopharmacologia. (1968);13:222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- 11.Jaroenporn S., Malaivijitnond S., Wattanasirmkit K., Trisomboon H., Watanabe G., Taya K., Cherdshewasart W. Effects of Pueraria mirifica, an herb containing phytoestrogens, on reproductive organs and fertility of adult male mice. Endocrine. (2006);30:93–101. doi: 10.1385/ENDO:30:1:93. [DOI] [PubMed] [Google Scholar]
- 12.Kim K.S., Seo E.K., Lee Y.C., Lee T.K., Cho Y.W., Ezaki O., Kim C.H. Effect of dietary Platycodon grandiflorum on the improvement of insulin resistance in obese Zucker rats. J. Nutr. Biochem. (2000);11:420–424. doi: 10.1016/S0955-2863(00)00098-X. [DOI] [PubMed] [Google Scholar]
- 13.Kim M.O., Moon D.O., Choi Y.H., Lee J.D., Kim N.D., Kim G.Y. Platycodin D induces mitotic arrest in vitro, leading to endoreduplication, inhibition of proliferation and apoptosis in leukemia cells. Int. J. Cancer. (2008);122:2674–2681. doi: 10.1002/ijc.23442. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y.P., Lee E.B., Kim S.Y., Li D., Ban H.S., Lim S.S., Shin K.H., Ohuchi K. Inhibition of prostaglandin E2 production by platycodin D isolated from the root of Platycodon grandiflorum. Planta Med. (2001);67:362–364. doi: 10.1055/s-2001-14317. [DOI] [PubMed] [Google Scholar]
- 15.Korea Food and Drug Administration. Testing Guidelines for Safety Evaluation of Drugs (Notification No. 2009-116, issued by the Korea Food and Drug Administration on August 24, 2009). (2009)
- 16.Lee H.S., Lee I.G., Ku S.K. Single oral dose toxicity study of water extracts of Picrorrhiza Rhizoma in mice. J. Toxicol. Pub. Health. (2006);22:117–126. [Google Scholar]
- 17.Lee J.H, Yang K.J., Shin H.D., Park B.R., Son C.W., Jang H.J., Park D.C., Lee H.S., Ku S.K. Single subcutaneous dose toxicity of Polycan®, a β-glucan originated from Aureobasidium in mice. Lab. Anim. Res. (2005);21:299–305. [Google Scholar]
- 18.Pineda M.H., McDonald L.E., Pineda M.H. Female reproductive system in Veterinary endocrinology and reproduction. Lea & Febiger; Philadelphia: (1989). pp. 303–354. [Google Scholar]
- 19.Plata E.J., Murphy W.H. Growth and haematologic properties of the BALB/wm strain of inbred mice. Lab. Anim. Sci. (1972);22:712–720. [PubMed] [Google Scholar]
- 20.Roberts D., Veeramachaneni D.N., Schlaff W.D., Awoniyi C.A. Effects of chronic dietary exposure to genistein, a phytoestrogen, during various stages of development on reproductive hormones and spermatogenesis in rats. Endocrine. (2000);13:281–286. doi: 10.1385/ENDO:13:3:281. [DOI] [PubMed] [Google Scholar]
- 21.Xie Y., Sun H.X., Li D. Platycodin D is a potent adjuvant of specific cellular and humoral immune responses against recombinant hepatitis B antigen. Vaccine. (2009);27:757–764. doi: 10.1016/j.vaccine.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 22.Xie Y., Sun H.X., Li D. Platycodin D improves the immunogenicity of newcastle disease virus-based recombinant avian influenza vaccine in mice. Chem. Biodivers. (2010);7:677–689. doi: 10.1002/cbdv.200900183. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi C., Fujita S., Obara T., Ueda T. Effects of room temperature on reproduction, body and organ weights, food and water intakes, and hematology in mice. Jikken. Dobutsu. (1983);32:1–11. doi: 10.1538/expanim1978.32.1_1. [DOI] [PubMed] [Google Scholar]
- 24.Yu J.S., Kim A.K. Platycodin D induces apoptosis in MCF-7 human breast cancer cells. J. Med. Food. (2010);13:298–305. doi: 10.1089/jmf.2009.1226. [DOI] [PubMed] [Google Scholar]
- 25.Zhao H.L., Sim J.S., Shim S.H., Ha Y.W., Kang S.S., Kim Y.S. Antiobese and hypolipidemic effects of platycodin saponins in diet-induced obese rats: evidences for lipase inhibition and calorie intake restriction. Int. J. Obes. (Lond.) (2005);29:983–990. doi: 10.1038/sj.ijo.0802948. [DOI] [PubMed] [Google Scholar]