Abstract
Herbal medicines are widely used in many countries for the treatment of many diseases. Although the use of herb extracts as alternative medicine is growing, their toxicological properties have not been thoroughly investigated. In this study, we have investigated the effects of water and ethanol extracts of 18 herbs on the hepatic lipid metabolism and steatogenic hepatotoxicity. Ethanol extracts of Cirsium japonicum, Carthamus tinctorius, Rehmanniae glutinosa (preparata), Polygala tenuifolia, Foeniculum vulgare, Polygonum multiflorum, and Acorus gramineus and water extracts of Polygonum multiflorum and Rehmanniae glutinosa induced lipid accumulation in Sk-hep1 human hepatoma cells as determined by Nile red staining. These extracts increased the luciferase activity of sterol regulatory element (SRE) and decreased that of peroxisome proliferator response element (PPRE), indicating the possibilities of enhanced fatty acid synthesis and decreased fatty acid oxidation. To identify the components responsible for the fat accumulation, we tested 50 chemicals isolated from the nine herbs. Apigenin, luteolin, pectolinarin and lupeol from Cirsium japonicum, 8-methoxypsoralen and umbelliferone from Foeniculum vulgare and pomonic acid and jiocerebroside from Rehmanniae glutinosa significantly increased the accumulation of lipid droplets. These results suggest that ethanol extracts of Cirsium japonicum, Carthamus tinctorius, Rehmanniae glutinosa (preparata), Polygala tenuifolia, Foeniculum vulgare, Polygonum multiflorum, and Acorus gramineus and water extracts of Polygonum multiflorum and Rehmanniae glutinosa can cause fatty liver disease by decreasing β-oxidation of fatty acid and increasing lipogenesis.
Keywords: Herbal medicine, Steatosis, Nile red, PPARα, SREBP, In vitro screening
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a term used to describe the accumulation of fat in hepatocytes not caused by alcohol. The standard for the diagnosis of NAFLD is fat deposition in more than 5% of the hepatocytes (Tiniakos, 2009). It is mainly associated with insulin resistance and metabolic disorder. NAFLD is usually observed in obese or type 2 diabetes mellitus patients. The prevalence of NAFLD is 10~24% of the general population globally (Angulo, 2002). But the prevalence in obese individuals is 4.5-fold higher than that in non-obese individuals (Bellentani et al., 2000). NAFLD is also caused by drugs such as amiodarone, tetracycline and valproic acid (Fromenty et al., 1990; Deboyser et al., 1989; Eadie et al., 1988).
Many molecular mechanisms can lead to lipid accumulation in hepatocytes, including excessive fatty acid uptake in liver, increased de novo fatty acid synthesis and triglycerides, decreased rates of β-oxidation of fatty acids, and decreased export of triglycerides from liver. Peroxisome proliferator-activated receptor α (PPARα) is a key regulator in fatty acid catabolism. It is expressed in the liver and other tissues. PPARα regulates many genes involved in mitochondrial or peroxisomal fatty acid β-oxidation like acyl-CoA synthetase. In mice lacking expression of PPARα, hepatic steatosis is induced by high-fat diet or fasting, indicating that PPARα can prevent the accumulation of lipids by increasing the fatty acid β-oxidation (Kersten et al., 1999). Sterol regulatory element-binding protein-1c (SREBP-1c) is an important molecule in de novo lipogenesis. SREBP-1c is a transcription factor that belongs to bHLH-Zip family; it activates transcription of acetyl-CoA carboxylase, fatty acid synthase and stearoyl-CoA desaturase in the nucleus. Inactivation of the SREBP-1 in the livers of ob/ob mice attenuates fatty liver (Yahagi et al., 2002), demonstrating that SREBP-1 plays a crucial role in the regulation of lipogenesis in NAFLD.
Herbal medicines are widely used in many countries to treat many diseases. Although the use of herb extracts as alternative medicine is growing, their toxicological properties have not been thoroughly investigated. In this study, we established in vitro screening assays to identify steatogenic effects of herbal medicine and to provide mechanism-based assessments by testing representative molecules for different mechanisms of toxicity.
MATERIALS AND METHODS
Herb extracts preparation. Herb extracts were provided by the National Center for Standardization of Herbal Medicines in Korea. The dry powders of herbs (Leonurus sibiricus, Cirsium japonicum, Carthamus tinctorius, Cyperus rotundus, Eucommia ulmoides, Phlomis umbrosa, Rehmannia glutinosa, Rheum rhabarbarum, Polygala tenuifolia, Artemisia capillaris, Foeniculum vulgare, Polygonum multiflorum, Acorus gramineus, Kalopanax pictus, Achyranthes japonica, and Lycium chinense) were extracted with 70% ethanol or water at 95℃ for 3 hr. This procedure was repeated twice.
Cell culture. Sk-hep1 human hepatoma cells (ATCC, Manassas, VA) were maintained in Dulbecco’s Modified Eagle Medium (Gibco BRL, Grand Island, NY, USA) containing heat inactivated 10% fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin. Cells were incubated at 37℃ with 5% CO2.
Cell viability assay. Cell viability was measured with MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Cells were seeded in 96 well plates. After being stabilized, cells were incubated with or without each herb extract or chemical for 24 hr. 1 mg/ml MTT solution was added to each well and incubated for 2 hr. The formazan crystals were dissolved in DMSO, and the absorbance was measured at 540 nm. The concentration required for 50% inhibition of the growth (IC50) was determined by nonlinear regression analysis using the Graphpad PRISM™ statistics software package (Ver. 5.0; San Diego, CA).
Nile red assay. Cells were seeded in 96 black well plates. On the following day, cells were incubated with or without herb extract. Cells were fixed with 4% paraformaldehyde and stained with 1 μg/ml Nile red (Sigma-Aldrich, St. Louis, MO) solution. The fluorescence was measured with a microplate fluorescence reader (Molecular Devices, Sunny-Vale, CA) at excitation 488 nm and emission 580 nm.
Transient transfection and reporter gene assay. Skhep1 cells were seeded in 12 well plates. Cells were transiently transfected with PPARα, PPRE-luc, SRE-luc plasmid and renilla plasmid as an internal control by using the Lipofectamine 2000 (Invitrogen, Carlsbad, CA) as recommended by the manufacturer. Cells were incubated with or without each herb extract. Luciferase activities were measured by using the Dual-Luciferase assay detection kit (Promega, Madison, WI) with a Centro LB960 luminometer (Berthold Technologies, Oak Ridge, TN).
Statistics. All data were expressed as mean ± SD. Statistical analysis was performed using Student’s t-test. Differences between groups were considered to be statistically significant at p < 0.05.
RESULTS
Cytotoxicity of medicinal herb extracts in Sk-hep1 cells. To establish the optimal concentration for Nile red staining, we incubated Sk-hep1 cells with media containing 0~1000 μg/ml of water or ethanol extracts of 18 herbs for 24 h and then determined cell viability using the MTT colorimetric assay. Most extracts of medicinal herbs showed little or no cytotoxicity except ethanol extracts from Carthamus tinctorius, Rheum rhabarbarum, Artemisia capillaris and Polygonum multiflorum, which had IC50s around 500 μg/ml (Table 1).
Table 1.
IC50 and steatogenic effects of medicinal herb extracts
| Herb extract | Extract | IC50 (μg/ml) | Nile red assay (% of control) | Herb extract | Extract | IC50 (μg/ml) | Nile red assay (% of control) |
|---|---|---|---|---|---|---|---|
| Leonurus sibiricus | H2O | > 1000 | 98.8 ± 6.5 | Polygala tenuifolia | H2O | > 1000 | 115.2 ± 40.0 |
| EtOH | > 1000 | 88.6 ± 29.4 | EtOH | 606 ± 340.0 | 179.9 ± 41.5* | ||
| Cirsium japonicum | H2O | > 1000 | 104.1 ± 6.8 | Artemisia capillaris | H2O | 858 ± 176.0 | 101.8 ± 15.1 |
| EtOH | > 1000 | 128.2 ± 10.7* | EtOH | 475 ± 133.0 | 40.1 ± 10.3* | ||
| Carthamus tinctorius | H2O | > 1000 | 86.6 ± 10.9* | Foeniculum vulgare | H2O | > 1000 | 76.0 ± 12.7* |
| EtOH | 521 ± 63.8 | 235.8 ± 43.1* | EtOH | 635 ± 30.4 | 272.3 ± 46.4* | ||
| Cyperus rotundus | H2O | > 1000 | 98.1 ± 1.7 | Polygonum multiflorum | H2O | > 1000 | 146.1 ± 11.5* |
| EtOH | > 1000 | 77.58 ± 26.3 | EtOH | 424 ± 273.0 | 156.2 ± 12.5* | ||
| Eucommia ulmoides | H2O | > 1000 | 96.3 ± 16.6 | Acorus gramineus | H2O | > 1000 | 105.5 ± 18.0 |
| EtOH | 644.4 ± 194.3 | 86.8 ± 10.1* | EtOH | 852 ± 243.0 | 194 ± 52.6* | ||
| Phlomis umbrosa | H2O | > 1000 | 87.1 ± 9.2* | Rehmannia glutinosa | H2O | > 1000 | 151.0 ± 23.9* |
| EtOH | > 1000 | 101.7 ± 8.9 | EtOH | > 1000 | 43.8 ± 7.0* | ||
| Rehmannia glutinosa (Dried) | H2O | > 1000 | 97.0 ± 12.1 | Kalopanax pictus | H2O | > 1000 | 102.0 ± 4.3 |
| EtOH | > 1000 | 98.7 ± 20.8 | EtOH | 714 ± 84.3 | 87.6 ± 13.0 | ||
| Rehmannia glutinosa (preparta) | H2O | > 1000 | 99.9 ± 9.2 | Achyranthes japonica | H2O | > 1000 | 99.0 ± 16.1 |
| EtOH | > 1000 | 132.4 ± 12.8* | EtOH | > 1000 | 88.1 ± 11.3 | ||
| Rheum rhabarbarum | H2O | 782.6 ± 111.5 | 60.0 ± 23.1* | Lycium chinense | H2O | > 1000 | 105.8 ± 17.2 |
| EtOH | 589.6 ± 175.8 | 73.8 ± 10.8* | EtOH | > 1000 | 95.8 ± 17.8 | ||
The IC50 value was obtained from MTT assay with various concentrations (0, 50, 100, 250, 500 and 1000 μg/ml) of each herb extract. Cells were also treated with 250 μg/ml of each herb extract for Nile red assay. Each value represents the mean ± SD of three independent experiments. *p < 0.05 compared with the control.
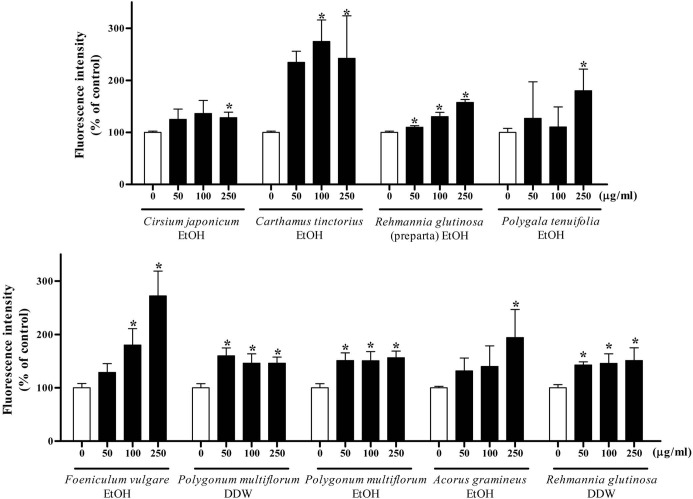
Effects of medicinal herb extracts on the steatogenic hepatotoxicity in Sk-hep1 cells. On the basis of the data obtained in the cytotoxicity assay, lipid droplet accumulation was determined after 24 h incubation with 250 μg/ml of water or ethanol extracts. We investigated the steatogenic effect of the water and ethanol extracts of 18 herbs using Nile red staining. Nile red is a fluorescent dye for the detection of intracellular lipid droplets. Seven ethanol extracts (Cirsium japonicum, Carthamus tinctorius, Rehmannia glutinosa preparata, Polygala tenuifolia, Foeniculum vulgare, Polygonum multiflorum, Acorus gramineus) and two water extracts (Polygonum multiflorum, Rehmanniae glutinosa) induced significant lipid accumulation in Sk-hep1 cells in a dose-dependent manner (Table 1, Fig. 1).
Fig. 1. Nile red staining of each herb extracts. Sk-hep1 cells were treated with various concentrations (0, 50, 100, 250 μg/ml) of each herb extract for 24 h. Nile red, a dye for the detection of intracellular lipid droplets by fluorescence was used to determine the steatogenic effects of herb extracts. Results are showed in the bars, which represent the percentage of control fluorescence intensity. Each bar represents the mean ± SD of three independent experiments. *p < 0.05 compared with control.
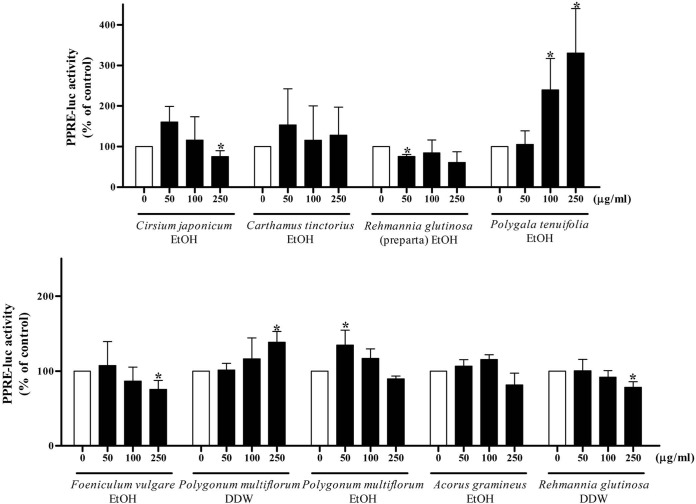
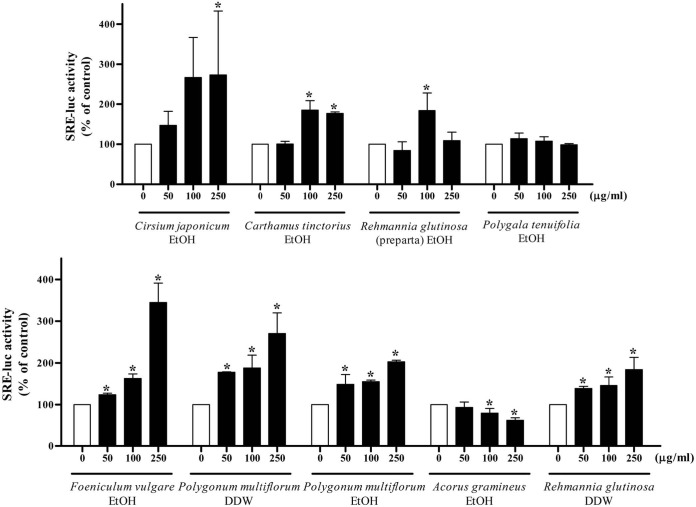
Effects of medicinal herb extracts on PPRE and SRE luciferase activities. To investigate whether the decrease of fatty acid oxidation or the increase of de novo lipogenesis might be involved in lipid accumulation induced by extracts of medicinal herbs, PPRE and SRE luciferase activity were measured following incubation with 9 extracts. Ethanol extracts of Cirsium japonicum and Foeniculum vulgare and water extract of Rehmanniae glutinosa decreased the PPRE activity significantly at a concentration of 250 μg/ml (Fig. 2). A dose-dependent increase in SRE activity was observed with ethanol extracts of Cirsium japonicum, Carthamus tinctorius, Foeniculum vulgare, and Polygonum multiflorum and water extracts of Polygonum multiflorum and Rehmannia glutinosa (Fig. 3).
Fig. 2. PPRE reporter gene assay of each herb extract. Cells were transfected with PPARα and PPRE-luc plasmid. Cells were treated with 0, 50, 100 and 250 μg/ml of each herb extract for 24 h. The luciferase activity was normalized with the renilla activity. Results are expressed as the percentage of control. Each bar is representative of three independent experiments. *p < 0.05 compared with control.
Fig. 3. SRE reporter gene assay of each herb extract. After transfection with SRE-luc plasmid, cells were treated with 0, 50, 100 and 250 μg/ml of each herb extract for 24 h. The luciferase activity was normalized with the renilla activity. Results are expressed as the percentage of control. Each bar is representative of three independent experiments. *p < 0.05 compared with control.
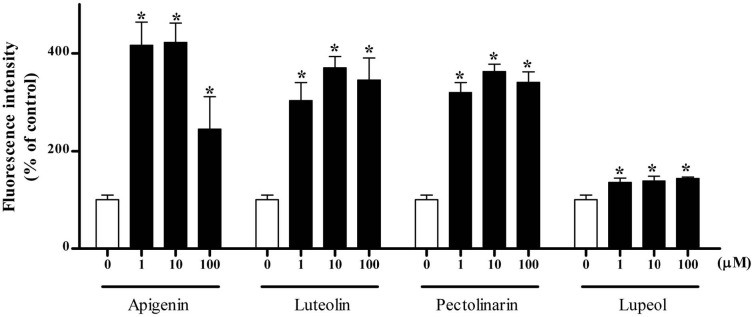
Effects of major components of medicinal herb extracts on lipid accumulation. To identify the components responsible for fat accumulation, 50 chemicals isolated from the 18 herbs were tested by Nile red staining. The steatogenic effects of various concentrations of the chemicals (1~ 100 μM) were examined in Sk-hep1 cells. Apigenin, luteolin, pectolinarin and lupeol from Cirsium japonicum induced significant accumulation of lipid droplets (Fig. 4). Umbelliferone from Foeniculum vulgare and pomonic acid and jiocerebroside from Rehmanniae glutinosa slightly increased the fat accumulation (data not shown). 8-methoxypsoralen from Foeniculum vulgare showed steatogenic effects at higher concentration (500 μM, data not shown).
Fig. 4. Nile red staining of the chemicals isolated from Cirsium japonicum. Cells were incubated with increasing concentrations (0, 1, 10, 100 μM) of each chemical (apigenin, luteolin, pectolinarin and lupeol) for 24 h in 10% serum media. Results are expressed as the percentage of control. Each bar shows the analysis of three independent experiments. *p < 0.05 compared with control.
DISCUSSION
In the current study, we investigated the effect of water and ethanol extracts of 18 herbs on hepatic lipid metabolism that can lead to steatogenic hepatotoxicity. PPRE and SRE luciferase activity were measured to identify the mechanisms of action. We demonstrated that 9 extracts of medicinal herbs induced lipid accumulation in Sk-hep1 cells. Herb extract-induced lipid accumulation was increased in a dose-dependent manner via reduced β-oxidation and increased de novo lipogenesis. Moreover, some components from these herbs enhanced lipid deposition in the cells. These results suggest the potential steatogenic effect of medicinal herb extracts used in alternative medicine.
As shown in Fig. 1 and 2, ethanol extracts of Cirsium japonicum and Foeniculum vulgare and water extract of Rehmanniae glutinosa induced lipid accumulation with significantly decreased PPRE activity at the highest concentration. In vivo and in vitro studies have demonstrated that PPARα plays an important role in lipid metabolism, and reduction of its activity thus results in triglyceride accumulation in the liver. In PPARα-deficient mice, hepatic triglyceride levels are elevated and hepatic steatosis is observed histologically (Costet et al., 1998). Such transgenic mice lack hepatic peroxisomal proliferation and have impaired expression of several PPARα target genes. Another study showed that PPARα target genes related to fatty acid oxidation are suppressed in obese mice (Liang and Tall, 2001). Thus, reduced PPRE activity by three herb extracts suggested the possibility of a steatogenic effect through the inhibition of fatty acid β-oxidation.
Fig. 3 shows that ethanol extracts of Cirsium japonicum, Carghamus tinctorus, Foeniculum vulgare, and Polygonum multiflorum and water extracts of Polygonum multiflorum and Rehmannia glutinosa increased the SRE activity in a dose-dependent manner. SREBP plays a crucial role in the regulation of hepatocyte lipid homeostasis and the promoter of the SREBP-1c has response elements for insulin, glucagon and liver X-activated receptors (LXR). In a study with transgenic mice overexpressing SREBP-1c in the liver, 2- to 4-fold increases of genes involved in lipid synthesis (e.g., acetyl-coA synthase, fatty acid synthase, stearoyl-coA desaturase 1) were observed (Horton et al., 1998). The increase of SREBP-1c target genes promotes fatty acid synthesis and triglyceride accumulation. Also, high levels of SREBP-1c were reported in the livers of ob/ob mice with insulin resistance (Shimomura et al., 2000). Consequently, these 6 extracts of medicinal herbs may facilitate the de novo lipogenesis in liver via SREBP-1c activation.
We tested 50 chemicals from medicinal herbs to identify whether these components had steatogenic effects. Apigenin, luteolin, pectolinarin and lupeol from Cirsium japonicum induced lipid accumulation in Sk-hep1 cells. Luteolin has been reported to have a therapeutic effect on CCl4-induced liver fibrosis by promoting extracellular matrix degradation and increasing the hepatic regenerative capability (Domitroviæ et al., 2009). Apigenin and lupeol are known to prevent ROS generation and lipid peroxidation and have hepato-protective effects (Choi et al., 2007; Sudhahar et al., 2007). However, there are no reports about their toxicological effects on lipid homeostasis. Our study suggests that these chemicals can cause steatosis based on the in vitro screening.
Nile red is a fluorescent dye that binds specifically to neutral lipid. McMillian et al. (2001) showed that treatment with free fatty acids increased the intensity of Nile red fluorescence in cells. This was confirmed by the quantification of total lipids and triglycerides. Several compounds known to cause hepatic lipid accumulation in vivo (e.g., ethionine, cyclosporin A and valproic acid) increased Nile red binding in HepG2 cells (Gómez-Lechón et al., 2007). The previous reports support using this cell-based assay with Nile red to identify the potential of medicinal herbs to induce steatosis.
NAFLD is very commonly found in humans, and the drug R&D process for medicinal herbs should therefore involve simple in vitro screening methods for hepatotoxicity. Animal studies to identify compounds with steatotic potential are slow, time-consuming and expensive. In this study, we suggest that in vitro screening using Nile red can be used to evaluate steatogenic effects. Also, the assessment of SRE and PPRE activities can provide clues to the mechanism of medicinal herb-induced fatty liver. Although in vitro screening may not reflect in vivo conditions, it can serve as the first screening step in the drug development of medicinal herbs in order to better avoid hepatotoxicity.
Acknowledgments
This work was supported by a grant from the Korea Food and Drug Administration for Studies on Standardization of Herbal Medicine (2009, 2010) and by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A100096). We thank the National Center for Standardization of Herbal Medicines in Korea for providing herbal extracts.
References
- 1.Angulo P. Non-alcoholic Fatty Liver Disease. New Engl. J Med. (2002);346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Bellentani S., Saccoccio G., Masutti F., Crocè L.S., Brandi G., Sasso F., Cristanini G., Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann. Intern. Med. (2000);132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 3.Choi S.I., Jeong C.S., Cho S.Y., Lee Y.S. Mechanism of apoptosis induced by apigenin in HepG2 human hepatoma cells: involvement of reactive oxygen species generated by NADPH oxidase. Arch. Pharm. Res. (2007);30:1328–1335. doi: 10.1007/BF02980274. [DOI] [PubMed] [Google Scholar]
- 4.Costet P., Legendre C., More J., Edgar A., Galtier P., Pineau T. Peroxisome proliferator-activated receptor alpha-isoform deficiency leads to progressive dyslipidemia with sexually dimorphic obesity and steatosis. J Biol. Chem. (1998);273:29577–29585. doi: 10.1074/jbc.273.45.29577. [DOI] [PubMed] [Google Scholar]
- 5.Deboyser D., Goethals F., Krack G., Roberfroid M. Investigation into the mechanism of tetracycline-induced steatosis: study in isolated hepatocytes. Toxicol. Appl. Pharmacol. (1989);97:473–479. doi: 10.1016/0041-008X(89)90252-4. [DOI] [PubMed] [Google Scholar]
- 6.Domitroviæ R., Jakovac H., Tomac J., Sain I. Liver fibrosis in mice induced by carbon tetrachloride and its reversion by luteolin. Toxicol. Appl. Pharmacol. (2009);241:311–321. doi: 10.1016/j.taap.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Eadie M.J., Hooper W.D., Dickinson R.G. Valproateassociated hepatotoxicity and its biochemical mechanisms. Med. Toxicol. Adverse Drug Exp. (1988);3:85–106. doi: 10.1007/BF03259935. [DOI] [PubMed] [Google Scholar]
- 8.Fromenty B., Fisch C., Labbe G., Degott C., Deschamps D., Berson A., Letteron P., Pessayre D. Amiodarone inhibits the mitochondrial beta-oxidation of fatty acids and produces microvesicular steatosis of the liver in mice. J. Pharmacol. Exp. Ther. (1990);255:1371–1376. [PubMed] [Google Scholar]
- 9.Gómez-Lechón M.J., Donato M.T., Martínez-Romero A., Jiménez N., Castell J.V., O'Connor J.E. A human hepatocellular in vitro model to investigate steatosis. Chem. Biol. Interact. (2007);165:106–116. doi: 10.1016/j.cbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Horton J.D., Bashmakov Y., Shimomura I., Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc. Natl. Acad. Sci. U.S.A. (1998);95:5987–5992. doi: 10.1073/pnas.95.11.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kersten S., Seydoux J., Peters J.M., Gonzalez F.J., Desvergne B., Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J. Clin. Invest. (1999);103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang C.P., Tall A.R. Transcriptional profiling reveals global defects in energy metabolism, lipoprotein, and bile acid synthesis and transport with reversal by leptin treatment in ob/ob mouse liver. J. Biol. Chem. (2001);276:49066–49076. doi: 10.1074/jbc.M107250200. [DOI] [PubMed] [Google Scholar]
- 13.McMillian M.K., Grant E.R., Zhong Z., Parker J.B., Li L., Zivin R.A., Burczynski M.E., Johnson M.D. Nile Red binding to HepG2 cells: an improved assay for in vitro studies of hepatosteatosis. In Vitr. Mol. Toxicol. (2001);14:177–190. doi: 10.1089/109793301753407948. [DOI] [PubMed] [Google Scholar]
- 14.Shimomura I., Matsuda M., Hammer R.E., Bashmakov Y., Brown M.S., Goldstein J.L. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol. Cell. (2000);6:77–86. [PubMed] [Google Scholar]
- 15.Sudhahar V., Kumar S.A., Varalakshmi P., Sundarapandiyan R. Mitigating role of lupeol and lupeol linoleate on hepatic lipemic-oxidative injury and lipoprotein peroxidation in experimental hypercholesterolemia. Mol. Cell Biochem. (2007);295:189–198. doi: 10.1007/s11010-006-9288-2. [DOI] [PubMed] [Google Scholar]
- 16.Tiniakos D.G. Liver biopsy in alcoholic and non-alcoholic steatohepatitis patients. Gastroenterol. Clin. Biol. (2009);33:930–939. doi: 10.1016/j.gcb.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Yahagi N., Shimano H., Hasty A.H., Matsuzaka T., Ide T., Yoshikawa T., Amemiya-Kudo M., Tomita S., Okazaki H., Tamura Y., Iizuka Y., Ohashi K., Osuga J., Harada K., Gotoda T., Nagai R., Ishibashi S., Yamada N. Absence of sterol regulatory element-binding protein-1 (SREBP-1) ameliorates fatty livers but not obesity or insulin resistance in Lep(ob)/Lep(ob) mice. J Biol. Chem. (2002);277:19353–19357. doi: 10.1074/jbc.M201584200. [DOI] [PubMed] [Google Scholar]