Abstract
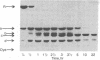
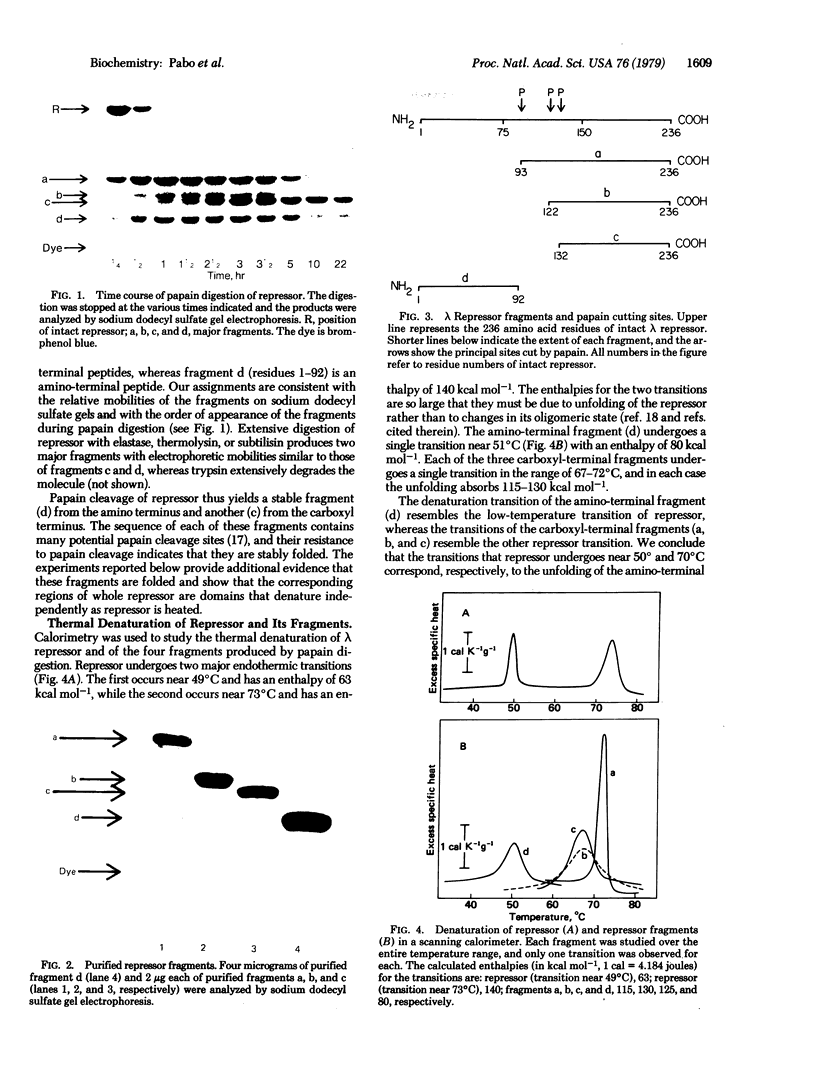
Papain digestion of the lambda phage repressor produces two fragments that are relatively resistant to further digestion. One includes the amino terminus (residues 1-92) and the other the carboxyl terminus (residues 132-236). Calorimetry shows that the amino-terminal fragment denatures near 50 degrees C and that the carboxyl-terminal fragment denatures near 70 degrees C. Intact repressor undergoes two denaturations, one near 50 degrees C and another near 70 degrees C. These and other data show that lambda repressor consists of two domains joined by a "connector" 40 amino acids long that is sensitive to proteases. The amino-terminal domain binds DNA, and the carboxyl-terminal domain oligomerizes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brack C., Pirrotta V. Electron microscopic study of the repressor of bacteriophage lambda and its interaction with operator DNA. J Mol Biol. 1975 Jul 25;96(1):139–152. doi: 10.1016/0022-2836(75)90187-4. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. Isolation of amino-terminal fragment of lactose repressor necessary for DNA binding. Biochemistry. 1977 Mar 8;16(5):938–943. doi: 10.1021/bi00624a020. [DOI] [PubMed] [Google Scholar]
- Hill R. L. Hydrolysis of proteins. Adv Protein Chem. 1965;20:37–107. doi: 10.1016/s0065-3233(08)60388-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis S. D., Shafer J. A. Ionization of a nitrophenol-containing reporter group at the active site of papain. Biochemistry. 1974 Feb 12;13(4):690–698. doi: 10.1021/bi00701a009. [DOI] [PubMed] [Google Scholar]
- Lieb M. LambdacI mutants: intragenic complementation and complementation with a cI promoter mutant. Mol Gen Genet. 1976 Aug 2;146(3):291–297. doi: 10.1007/BF00701253. [DOI] [PubMed] [Google Scholar]
- Lieb M. Studies of heat-inducible lambda bacteriophage. I. Order of genetic sites and properties of mutant prophages. J Mol Biol. 1966 Mar;16(1):149–163. doi: 10.1016/s0022-2836(66)80269-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P., Bailone A., Devoret R. Prophage lambda induction of Escherichia coli K12 envA uvrB: a highly sensitive test for potential carcinogens. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3700–3704. doi: 10.1073/pnas.73.10.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Hill B. Lac repressor and lac operator. Prog Biophys Mol Biol. 1975;30(2-3):227–252. doi: 10.1016/0079-6107(76)90011-0. [DOI] [PubMed] [Google Scholar]
- Naono S., Gros F. Control and selectivity of lambda DNA transcription in lysogenic bacteria. Cold Spring Harb Symp Quant Biol. 1966;31:363–375. doi: 10.1101/sqb.1966.031.01.047. [DOI] [PubMed] [Google Scholar]
- Ogata R. T., Gilbert W. An amino-terminal fragment of lac repressor binds specifically to lac operator. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5851–5854. doi: 10.1073/pnas.75.12.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim A. B., Noff D. Deletion mapping of trans dominant mutations in the lambda repressor gene. Virology. 1975 Apr;64(2):553–556. doi: 10.1016/0042-6822(75)90132-4. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Khechinashvili N. N. A thermodynamic approach to the problem of stabilization of globular protein structure: a calorimetric study. J Mol Biol. 1974 Jul 5;86(3):665–684. doi: 10.1016/0022-2836(74)90188-0. [DOI] [PubMed] [Google Scholar]
- Ptashne M., Backman K., Humayun M. Z., Jeffrey A., Maurer R., Meyer B., Sauer R. T. Autoregulation and function of a repressor in bacteriophage lambda. Science. 1976 Oct 8;194(4261):156–161. doi: 10.1126/science.959843. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L. Escherichia coli recA gene product inactivates phage lambda repressor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4714–4718. doi: 10.1073/pnas.75.10.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Mount D. W. Inactivation and proteolytic cleavage of phage lambda repressor in vitro in an ATP-dependent reaction. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2283–2287. doi: 10.1073/pnas.74.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer R. T., Anderegg R. Primary structure of the lambda repressor. Biochemistry. 1978 Mar 21;17(6):1092–1100. doi: 10.1021/bi00599a024. [DOI] [PubMed] [Google Scholar]
- Sauer R. T. DNA sequence of the bacteriophage gama cI gene. Nature. 1978 Nov 16;276(5685):301–302. doi: 10.1038/276301a0. [DOI] [PubMed] [Google Scholar]
- Sturtevant J. M. Heat capacity and entropy changes in processes involving proteins. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2236–2240. doi: 10.1073/pnas.74.6.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong T. Y., Hearn R. P., Wrathall D. P., Sturtevant J. M. A calorimetric study of thermally induced conformational transitions of ribonuclease A and certain of its derivatives. Biochemistry. 1970 Jun 23;9(13):2666–2677. doi: 10.1021/bi00815a015. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]