Abstract
Gryllus bimaculatus (Gb) was orally administered at doses of 0, 0.04, 0.2, 1 and 5 g/kg bw/day for 13 consecutive weeks. There were no observed clinical signs or deaths related to treatment in all the groups tested. Therefore, the approximate lethal oral dose of G. bimaculatus was considered to be higher than 5 g/kg in rats. Throughout the administration period, no significant changes in diet consumption, ophthalmologic findings, organ weight, clinical pathology (hematology, clinical chemistry, coagulation, and urinalysis) or gross pathology were detected. Minor changes were found in hematological parameters for the 5 g/kg Gb-treated group (triglyceride reduction of 35.8%), but all changes were within normal physiological ranges. Microscopic examination did not identify any treatment-related histopathologic changes in the organs of Gb-treated rats in the high dose group. From these results, one can conclude that the no-observed adverse effect level (NOAEL) of G. bimaculatus is higher than 5 g/kg bw/day in rats.
Keywords: G. bimaculatus, 13-Week toxicity
INTRODUCTION
Cricket (Gryllus bimaculatus) water extract has been used in oriental medicine as a crude drug for treating fever and hypertension and the cricket is presently reared as food (Ahn et al., 2005). The main components of Gryllus bimaculatus are protein, fat including essential fatty acids- oleic acid, linoleic acid and γ-linoleic acid, ash, and moisture (Ahn et al., 2000). Recently, the extracts from Gryllus bimaculatus, were found to cause a significant decrease in blood ethanol concentrations by enhancing liver mitochondrial alcohol metabolizing enzymes (Ahn et al., 2004). In other countries, like grasshopper, the cricket (Gryllus bimaculatus) is eaten after roasting, but, it was not consumed as a food in Korea. Safety evaluation data on these products is limited.
The acute toxicity of G. bimaculatus in Sprague-Dawley rats was tested and it was practically non-toxic with an oral LD50 value of > 5 g/kg (Kim et al., 2002). A genotoxic evaluation of the biocomponents of cricket (Gryllus bimaculatus) was assessed using three mutagenicity tests: the Ames test, the chromosome aberration test in Chinese hamster ovary cells in vitro, and the micronucleus (MN) test in vivo, involving different test systems (bacteria, mammalian cells and mice bone marrow; Ahn et al., 2005).
On the other hand, there is little safety evaluation data on these products in terms of toxicology. Therefore, the present study examined the subacute toxicity of G. bimaculatus extract administered orally at 0, 0.04, 0.2, 1 and 5 g/kg for 13 consecutive weeks.
MATERIALS AND METHODS
Materials. The cricket, G. bimaculatus was purchased from an insect farm, Jungsun City, located in Kangwon-do, South Korea. They were freezed-dried in the Department of Agricultural Biology, National Academy of Agricultural, Korea.
Test preparation of Gb. Dried G. bimaculatus was homogenized in a blender to a powder at 4℃, dissolved in phosphate buffered saline (Sigma-Aldrich Inc., St. Louis, MO) and then orally administered at doses of 0, 0.04, 0.2, 1 and 5 g/kg bw/day over a 13-week period.
Animals. Specific pathogen-free SD rats (4 weeks old, weighing 165 ± 5 g, male and female), purchased from Samtako Co. Ltd. (Osan, Korea), were housed in an environmentally- controlled room at 23 ± 1℃, with relative humidity of 55 ± 10%, air ventilation of 10~18 cycles/hr, a 12-hr light/dark cycle of 150~300 lux, with feed and water available ad libitum and acclimated one week before the repeat-dose toxicity study began.
All procedures were conducted in accordance with the Korean Food and Drug Administration (KFDA) “Testing Guidelines for Safety Evaluation of Drugs” (Notification No. 2005-60, issued by the KFDA on Oct 21, 2005).
Ten animals of both sexes in each group were weighed and then administered Gb at a dose of 0.04, 0.2, 1 or 5 g/kg/day or its vehicle over a 13-week period.
The parameters examined included clinical signs and mortality, body weight, food consumption, urine analysis, hematological analysis, serum biochemical analysis, organ weight, ophthalmic observation and histopathological findings (Song et al., 2006).
Body weight. Animals were observed three times daily for clinical signs. Changes in body weight were recorded weekly and group means were calculated.
Food consumption. Daily food consumption was determined by subtracting leftover feed from provided feed. Food consumption was measured daily for the 1st week and weekly thereafter.
Urine sampling. During the final week of testing (week 13), rats were transferred to metabolic cages for 24 hr and urine was collected to determine specific gravity, pH, leukocyte content, nitrite, protein, glucose, ketone, urobilinogen, bilirubin and hemoglobin levels using commercial kits (Roche Diagonisctics GmbH, Mannheim, Germany).
Blood sampling and plasma assay. After 13 weeks of treatment, blood (~3 ml) was collected from the posterior vena cava under light CO2 inhalation and used for serum chemistry measurements. The parameters examined included total protein, albumin, total bilirubin, glucose, glutamic pyruvic transaminase (GPT), glutamic oxaloacetic transaminase (GOT), γ-glutamyl transferase (GGT), alkaline phosphatase (ALP), lactic dehydrogenase (LDH), total cholesterol, blood urea nitrogen (BUN), creatinine, triglyceride, uric acid, sodium, potassium and chloride. All were evaluated using an autoanalyzer (Hitachi 7060 automatic clinical analyzer, Tokyo).
Organ weights. Absolute and relative (organ-to-body weight ratios) weights were determined after sacrifice at 13 weeks; tissues included brain, pituitary gland, adrenal glands, liver, spleen, kidneys, heart, thymus, lung, stomach, thyroid gland and testes (or ovary).
Pathology and histopathology. The organs and tissues in the cranial, thoracic, and abdominal cavities of euthanized rats, were examined grossly for ophthalmic observation. Each organ was excised and fixed in phosphate-buffered formalin. After paraffin embedding, the excised organs and tissues were prepared for microscopic examination by sectioning and staining with hematoxylin and eosin.
Statistical analysis. Mean and standard deviation of all parameters were determined for each of the 5 groups. A Student’s t-test was used to establish the significant differences between the control and treatment groups. p < 0.05 was considered statistically significant.
RESULTS
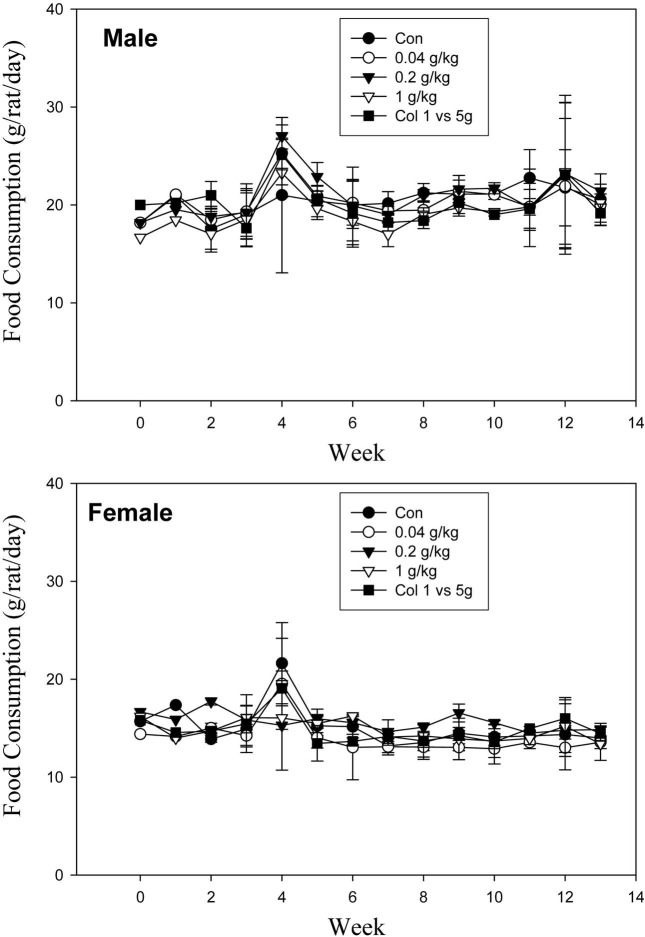
Clinical signs and food consumption. No deaths or adverse clinical signs were observed due to the ingestion of G. bimaculatus at doses of 0.04, 0.2, 1.0 and 5.0 g/kg/day (Table 1). Food consumption was similar for all study groups (Fig. 1).
Table 1.
Mortality of Sprague-Dawley rats treated orally with G. bimaculatus over a 13-week period
| Sex | Dosage (g/kg bw) | Weeks | Total mortality | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Start | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |||
| Male | CONa | 0/10b | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| 0.04 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| 0.2 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| 1.0 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| 5.0 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| Female | CON | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| 0.04 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| 0.2 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| 1.0 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
| 5.0 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | |
aCON: vehicle control group treated with PBS buffer.
Fig. 1. Food consumption of male and female SD rats, treated orally with G. bimaculatus powder over a 13-week period *Significantly different from the untreated controls (P < 0.05).
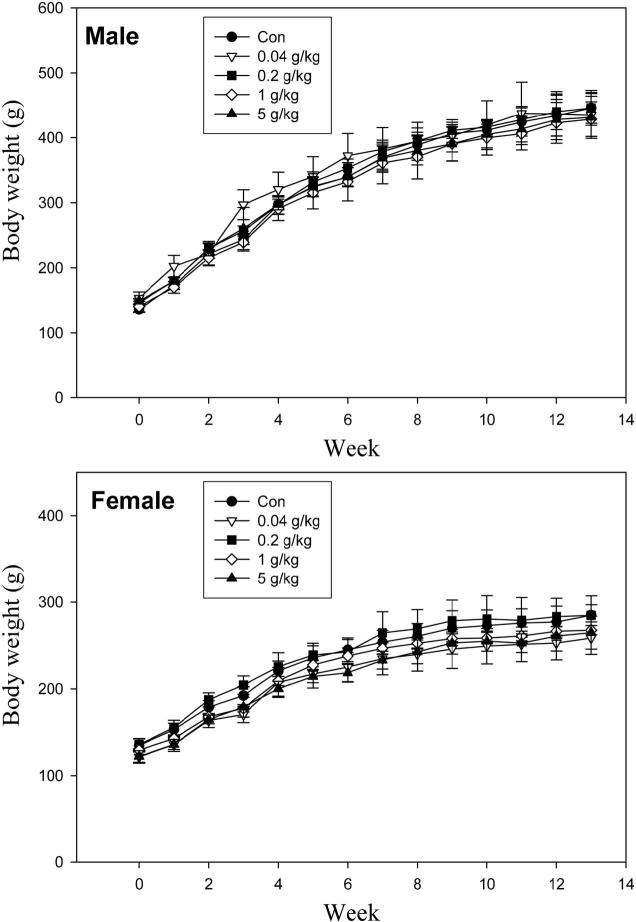
Body weight changes. There were no toxicologically significant differences in mean body weight between any of the treatment groups (Fig. 2). During the 13-week administration period, the body weights of the male and female SD rats in the 3 treatment groups were comparable across the control and treated groups. The mean weekly body weights versus time are presented in Fig. 2. No statistically significant differences were observed between the 5.0 g/kg G. bimaculatus-treated group and the control group.
Fig. 2. Body weight increases of male and female SD rats, treated orally with G. bimaculatus powder over a 13-week period. *Significantly different from the untreated controls (P < 0.05).
Urinalysis. No significant differences were observed between treatment and control group (Table 2).
Table 2.
Urinalysis data of G. bimaculatus treated groups at the end of the administration period
| Item | Urinalysis values | Male | Female | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CONa | 0.04 | 0.2 | 1 | 5 | CONa | 0.04 | 0.2 | 1 | 5 | ||
| Specific gravity | 1.000 | 0 | 5 | 0 | 0 | 4 | 1 | 0 | 2 | 1 | 1 |
| 1.005 | 9 | 0 | 2 | 5 | 1 | 2 | 1 | 0 | 1 | 1 | |
| 1.010 | 0 | 4 | 1 | 3 | 2 | 4 | 7 | 0 | 5 | 7 | |
| 1.015 | 0 | 5 | 0 | 0 | 3 | 3 | 2 | 4 | 4 | 2 | |
| 1.020 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 6 | 0 | 0 | |
| 1.025 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| PH | 6.0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 5 | 0 | 0 |
| 6.5 | 0 | 5 | 0 | 0 | 4 | 1 | 0 | 2 | 1 | 1 | |
| 7.0 | 1 | 1 | 0 | 0 | 2 | 4 | 0 | 3 | 6 | 4 | |
| 7.5 | 9 | 4 | 8 | 8 | 3 | 5 | 10 | 0 | 3 | 5 | |
| 8.0 | 0 | 0 | 2 | 2 | 0 | 0 | 10 | 0 | 0 | 0 | |
| Leucocyte | 10~25 mg/dl | 10 | 6 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| 75 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 500 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Nitrite | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| + | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Protein | 25 mg/dl | 10 | 8 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| 50 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 75 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Glucose | normal | 10 | 10 | 9 | 10 | 10 | 10 | 10 | 9 | 9 | 10 |
| 50 mg/dl | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | |
| 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ketone | - | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| 5 mg/dl | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Urobili nogen | normal | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 0 |
| 1 mg/dl | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Bilirubin | - | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| 1 mg/dl | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Blood | - | 10 | 10 | 10 | 9 | 9 | 10 | 10 | 8 | 10 | 10 |
| 1+ | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2+ | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | |
| Hemoglobin | - | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 9 | 10 |
| 1+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
aCON: vehicle control group treated with PBS buffer.
Data are number of animals presenting the value of each item of urinalysis.
Hematology and blood chemistry. Some dose-dependent changes were observed between the treated and control groups with respect to the hematological parameters at the end of the experiment. An increase in partial thromboplastin time count was observed in the male rats in the treated groups; (control, 118.3 ± 30.8 sec; 0.04 g/kg, 91.5 ± 44.1 sec; 0.20 g/kg, 96.7 ± 13.3 sec; 1.0 g/kg, 122.7 ± 40.4 sec; 5.0 g/kg, 132.6 ± 28.6 sec) and the same trends were seen in females (Table 3 and Table 4) but without significant differences. Hematocrit, MCV, MCHC and Factor I (indicators of RBS function and status) were significantly different between some of the treated groups versus the control group. Minor changes were found in hematological parameters (eosinophils, neutrophils, lymphocytes and basophils) for some Gb-treated (one side) male or female rat groups. But, effects of Gb were not considered adverse because all changes in hematological data were within the normal physiological range (Table 3 and Table 4).
Table 3.
Hematological findings of male rats treated orally with G. bimaculatus for 13 weeks
| Item | Unit | CONa | 0.04 | 0.2 | 1 | 5 (g/kg bw/day) |
|---|---|---|---|---|---|---|
| WBC | 103/mm3 | 6.4 ± 1.6 | 8.5 ± 2.0 | 10.4 ± 4.3 | 8.8 ± 1.3 | 7.4 ± 2.1 |
| RBC | 106/mm3 | 8.8 ± 0.3 | 8.6 ± 0.4 | 9.0 ± 0.3 | 8.9 ± 0.4 | 9.6 ± 0.6 |
| Hgb | g/dl | 16.7 ± 0.3 | 16.3 ± 0.9 | 16.3 ± 0.6 | 16.5 ± 0.8 | 17.5 ± 1.3 |
| Hct | % | 54.1 ± 0.9 | 54.0 ± 2.8 | 54.2 ± 2.5 | 54.2 ± 2.7 | 56.8 ± 4.1 |
| MCV | fl | 61.6 ± 3.1 | 62.3 ± 1.4 | 60.2 ± 2.3 | 60.4 ± 1.6 | 58.9 ± 1.0 |
| MCH | pg | 19.0 ± 0.9 | 18.8 ± 0.6 | 18.2 ± 0.6 | 18.4 ± 0.6 | 18.2 ± 0.4 |
| MCHC | g/dl | 30.9 ± 0.4 | 30.2 ± 0.5 | 30.2 ± 0.5 | 30.5 ± 0.3 | 30.9 ± 0.3 |
| PLT | 103/mm3 | 1023.0 ± 195.2 | 915.4 ± 110.2 | 956.8 ± 168.0 | 852.7 ± 155.4 | 849.8 ± 148.9 |
| PTT | sec | 118.3 ± 30.8 | 91.5 ± 44.1 | 96.7 ± 13.3 | 122.7 ± 40.4 | 132.6 ± 28.6 |
| Thrombin time | sec | 181.8 ± 0 | 74.4 ± 18.8 | 131 ± 0.1 | 136.1 ± 43.6 | 106.5 ± 3.7 |
| Factor I | mg/dl | 124.3 ± 10.7 | 211.4 ± 42.5 | 163.6 ± 60.7 | 169.8 ± 49.3 | 197.6 ± 39.3 |
| PT | sec | 3.5 ± 2.8 | 2.2 ± 0.8 | 2.1 ± 0.6 | 2.3 ± 0.6 | 2.2 ± 0.9 |
| Neutrophil | % | 9.4 ± 4.3 | 9.8 ± 4.2 | 8.6 ± 3.4 | 14.0 ± 5.7 | 19.9 ± 4.5* |
| Lymphocyte | % | 76.0 ± 5.2 | 80.2 ± 2.4 | 82.1 ± 4.1 | 81.7 ± 5.4 | 76.1 ± 5.0 |
| Monocyte | % | 1.4 ± 0.5 | 1.7 ± 1.1 | 3.1 ± 0.6 | 1.4 ± 1.0 | 1.1 ± 0.4 |
| Eosinophil | % | 12.6 ± 9.0 | 7.8 ± 3.2 | 5.6 ± 2.4 | 2.2 ± 1.1* | 2.2 ± 1.8 |
| Basophil | % | 0.6 ± 0.3 | 0.4 ± 0.1 | 0.5 ± 0.2 | 0.4 ± 0.1 | 0.5 ± 0.1 |
Abbreviations: WBC, white blood cell, RBC, red blood cell, Hgb, hemoglobin, Hct, hematocrit, MCV, mean corpuscular volume, MCH, mean corpuscular hemoglobin, MCHC, mean corpuscular hemoglobin concentration, PLT, platelet. PTT, partial thromboplastin time, PT, prothrombin time.
aCON: PBS (as a vehicle) treated with murine normal dieta.
Each value represents mean ± S.D. Statistically significant from control (*P<0.05).
Table 4.
Hematological findings of female rats treated orally with G. bimaculatus for 13 weeks
| Item | Unit | CONa | 0.04 | 0.2 | 1 | 5 (g/kg bw/day) |
|---|---|---|---|---|---|---|
| WBC | 103/mm3 | 6.6 ± 2.3 | 6.3 ± 0.7 | 6.6 ± 2.0 | 5.4 ± 1.6 | 4.1 ± 0.3 |
| RBC | 106/mm3 | 9.0 ± 0.3 | 8.6 ± 0.4 | 8.2 ± 0.8* | 8.3 ± 0.7* | 7.8 ± 0.3* |
| Hgb | g/dl | 16.7 ± 0.6 | 16.2 ± 0.9 | 15.2 ± 2.0 | 15.7 ± 1.5 | 14.8 ± 0.5* |
| Hct | % | 53.4 ± 2.9 | 50.0 ± 3.7 | 48.9 ± 6.4 | 48.1 ± 5.2* | 49.8 ± 2.4* |
| MCV | fl | 59.3 ± 2.7 | 57.7 ± 1.6 | 58.9 ± 2.5 | 57.7 ± 1.6 | 63.3 ± 3.6 |
| MCH | pg | 18.5 ± 0.4 | 18.7 ± 0.4 | 18.4 ± 0.6 | 18.8 ± 0.5 | 18.7 ± 0.3 |
| MCHC | g/dl | 31.3 ± 1.1 | 32.4 ± 0.7* | 31.2 ± 0.9 | 32.6 ± 0.9* | 29.7 ± 1.8* |
| PLT | 103/mm3 | 774.7 ± 281.4 | 922.6 ± 215.8 | 812.3 ± 204.0* | 884.1 ± 253.4 | 850.7 ± 150.2* |
| PTT | sec | 86.1 ± 56.7 | 96.0 ± 17.9 | 118.0 ± 35.5 | 103.4 ± 25.1 | 105.5 ± 40.6 |
| Thrombin time | sec | 134.6 ± 58.0 | 155.0 ± 55.1 | 128.7 ± 56.2 | 122.8 ± 44.3 | 68.7 ± 6.0 |
| Factor I | mg/dl | 100.8 ± 34.6 | 95.4 ± 18.3* | 105.0 ± 29.3 | 97.7 ± 36.3 | 110.0 ± 41.2 |
| PT | sec | 2.8 ± 0.8 | 4.6 ± 3.4 | 3.1 ± 1.3 | 6.9 ± 4.4 | 2.5 ± 1.1 |
| Neutrophil | % | 14.1 ± 5.3 | 12.9 ± 5.3 | 8.2 ± 4.5* | 11.9 ± 5.4 | 10.9 ± 5.8 |
| Lymphocyte | % | 81.9 ± 5.9 | 83.0 ± 6.0* | 85.9 ± 6.2* | 82.0 ± 7.8 | 83.2 ± 4.8 |
| Monocyte | % | 1.6 ± 0.7 | 1.3 ± 0.6 | 2.3 ± 1.4 | 3.2 ± 3.7 | 2.8 ± 1.1 |
| Eosinophil | % | 1.7 ± 0.5 | 2.2 ± 0.8 | 2.0 ± 1.7 | 2.4 ± 1.5 | 1.8 ± 1.3 |
| Basophil | % | 0.4 ± 0.1 | 0.3 ± 0.1 | 1.4 ± 3.0 | 0.3 ± 0.10* | 1.0 ± 1.1 |
Abbreviations: WBC, white blood cell, RBC, red blood cell, Hgb, hemoglobin, Hct, hematocrit, MCV, mean corpuscular volume, MCH, mean corpuscular hemoglobin, MCHC, mean corpuscular hemoglobin concentration, PLT, platelet. PTT, partial thromboplastin time, PT, prothrombin time.
aCON :PBS (as a vehicle) treated with murine normal dieta
Each value represents mean±S.D. Statistically significant from control (*P <0.05)
Serum biochemistry. In the sera of the Gb-treated groups, trigyceride levels were significantly lower than in the control after 13 weeks with dose-dependent changes in both males (control, 82.7 ± 18.9 mg/dl; 1.0 g/kg, 60.3 ± 11.1 mg/dl), and females (control, 93.7 ± 59.4 mg/dl; 1.0 g/kg, 54.4 ± 7.1 mg/dl). Serum glucose levels were lower vs control in the males (control, 403.0 ± 69.6 mg/dl; 1.0 g/kg, 293.5 ± 81.0 mg/dl), and females (controls, 309.1 ± 90.2 mg/dl; 1.0 g/kg, 147.8 ± 53.2 mg/dl). A significant increase in HDL cholesterol was observed in the 0.04 and 1.0 g/kg groups for both the males and females (male: control, 12.0 ± 2.2mg/dl; 0.04 g/kg, 15.5 ± 3.9mg/dl; 1.0 g/kg, 16.0 ± 2.2 mg/dl; female: control, 25.5 ± 8.3 mg/dl; 0.04 g/kg, 29.1 ± 3.7 mg/dl; 1.0 g/kg, 31.0 ± 3.8 mg/dl).
Alkaline phosphatase (ALP) levels of the treated groups were reduced in a dose-dependent manner in males (control, 133.5 ± 16.4 mg/dl; 1.0 g/kg, 109.9 ± 17.5 mg/dl), and females (controls, 169.0 ± 121.8 mg/dl; 1.0 g/kg, 93.8 ± 14.0 mg/dl). Also, uric acid levels of the treated groups were reduced in a dose-dependent manner in males (control, 8.7 ± 1.6 mg/dl; 1.0 g/kg, 6.8 ± 1.0 mg/dl), and females (control, 6.6 ± 2.0 mg/dl; 1.0 g/kg, 5.4 ± 0.8 mg/dl).
Calcium ion levels of the treated groups were reduced in a dose-dependent manner in males (control, 13.0 ± 0.3 nmol/l; 1.0 g/kg, 12.2 ± 0.5 nmol/l) and females (control, 13.0 ± 0.7 nmol/l; 1.0 g/kg, 11.6 ± 0.9 nmol/l), whereas inorganic phosphorus levels increased (male: control, 18.9 ± 3.3 nmol/l; 5.0 g/kg, 14.9 ± 2.6 nmol/l, female: control, 17.7 ± 5.7 mg/dl; 1.0 g/kg, 15.0 ± 2.0 mg/dl) (Tables 4 and 5). However, Gb was considered non-toxic because the all changes in serum biochemical data were within normal physiological range (Tables 5 and 6).
Table 5.
Biochemical serum values of male rats treated orally with. G. bimaculatus over a 13-week period
| Item | Unit | CONa | 0.04 | 0.2 | 1 | 5 |
|---|---|---|---|---|---|---|
| Toal protein | g/dl | 7.0 ± 0.1 | 6.9 ± 0.3 | 7.05 ± 0.2 | 7.2 ± 0.3 | 7.0 ± 0.2 |
| Bilirubin | mg/dl | below 0.1 | below 0.1 | below 0.1 | below 0.1 | below 0.1 |
| ALP | IU/l | 133.5 ± 16.4 | 112.7 ± 21.0 | 127.1 ± 34.9 | 109.9 ± 17.5* | 134.6 ± 45.2 |
| AST | IUl | 81.5 ± 14.0 | 80.9 ± 14.8 | 81.8 ± 16.6 | 90.9 ± 18.0 | 75.6 ± 8.5 |
| ALT | IU/l | 44.5 ± 12.1 | 41.8 ± 7.6 | 41.5 ± 10.9 | 44.4 ± 12.4 | 43.2 ± 8.5 |
| GGT | g/dl | below 2 | below 2 | below 2 | below 2 | below 2 |
| CK | IU/l | 157.9 ± 102.3 | 165.0 ± 102.8 | 137.4 ± 59.5 | 301.9 ± 234.9 | 161.9 ± 111.9 |
| LDH | IU/l | 528.3 ± 426.1 | 576.8 ± 491.0 | 424.5 ± 233.2 | 728.6 ± 369.9 | 329.5 ± 113.9 |
| Na | nmol/l | 139.6 ± 2.9 | 139.6 ± 5.4 | 140.8 ± 3.2 | 137.3 ± 4.3 | 140.6 ± 3.2 |
| K | nmol/l | 22.6 ± 4.1 | 23.5 ± 7.3 | 21.5 ± 5.2 | 24.9 ± 4.2 | 20.2 ± 4.3 |
| Cl | nmol/l | 97.4 ± 2.4 | 98.1 ± 2.1 | 97.9 ± 0.9 | 96.5 ± 3.3 | 97.5 ± 1.7* |
| Creatine | mg/dl | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.0 | 0.8 ± 0.1 |
| BUN | mg/dl | 19.4 ± 2.1 | 19.9 ± 3.1 | 19.6 ± 3.5 | 21.3 ± 2.8 | 21.1 ± 3.6 |
| Uric acid | mg/dl | 8.7 ± 1.6 | 6.9 ± 1.8 | 7.5 ± 1.2 | 6.8 ± 1.0* | 7.59 ± 1.61 |
| T.Chol | mg/dl | 84.2 ± 11.2 | 94.2 ± 17.7 | 88.5 ± 11.3 | 87.2 ± 10.0 | 89.7 ± 15.5 |
| H.Chol | mg/dl | 12.0 ± 2.2 | 15.5 ± 3.9* | 15.7 ± 2.5* | 16.0 ± 2.2* | 16.1 ± 3.3* |
| L.Chol | mg/dl | 15.9 ± 2.3 | 15.9 ± 3.6 | 16.0 ± 2.8 | 14.9 ± 2.6 | 14.7 ± 3.8 |
| TG | mg/dl | 82.7 ± 18.9 | 65.7 ± 9.8* | 68.8 ± 13.1 | 60.3 ± 11.1* | 64.7 ± 21.5 |
| Glucose | mg/dl | 403.0 ± 69.6 | 289.4 ± 112.1* | 338.7 ± 115.4 | 293.5 ± 81.0* | 392.3 ± 100.6 |
| Ca | nmol/l | 13.0 ± 0.3 | 12.6 ± 0.6 | 12.8 ± 0.5 | 12.2 ± 0.5* | 12.5 ± 0.4* |
| IP | mg/dl | 18.9 ± 3.3 | 19.5 ± 3.9 | 16.5 ± 4.2 | 17.9 ± 2.1 | 14.9 ± 2.6* |
Abbreviations: ALP: alkaline phosphatase, AST (GOT), glutamate oxaloacetate transaminase, ALT (GPT), glutamate pyruvate transaminase, GGT, γ-glutamyl transferase, CK: creatinine phosphokinase, LDH, lactate dehydrogenase, Na, Sodium, K, potassium, Cl, chloride, BUN, blood urea nitrogen, T. Chol: total cholesterol, H. Chol: HDL cholesterol, L. Chol: LDL cholesterol, TG, triglyceride, Ca, calcium, IP, inorganic phosphorus.
aCON: PBS (vehicle) treated with murine normal diet.
Each value represents mean ± S.D. Statistically significant from control (*P<0.05).
Table 6.
Biochemical serum values of female rats treated orally with G. bimaculatus over a 13-week period
| Item | Unit | CONa | 0.04 | 0.2 | 1 | 5 (g/kg bw/day) |
|---|---|---|---|---|---|---|
| Toal protein | g/dl | 7.2±0.3 | 6.9±0.2* | 6.88±0.3 | 7.0±0.2 | 6.6±0.3* |
| Bilirubin | mg/dl | below 0.1 | below 0.1 | below 0.1 | below 0.1 | below 0.1 |
| ALP | IU/l | 169.0±121.8 | 407.4±564.2 | 123.0±56.7 | 93.8±14.0* | 135.4±43.3 |
| AST | IUl | 90.7±27.0 | 144.5±196.1 | 88.5±11.2 | 89.2±14.7 | 93.5±24.4 |
| ALT | IU/l | 34.6±6.3* | 82.0±108.4 | 46.7±13.0 | 44.6±11.0 | 55.9±11.9 |
| GGT | g/dl | below 2 | below 2 | below 2 | below 2 | below 2 |
| CK | IU/l | 271.1±150.2 | 210.0±129.9 | 238.3±92.7* | 176.5±54.0 | 222.2±116.3 |
| LDH | IU/l | 717.2±497.3 | 1384.8±2538.9 | 634.3±152.9 | 634.8±289.0 | 819.5±582.7 |
| Na | nmol/l | 137.2±4.5 | 139.4±1.7 | 141.0±1.5 | 143.7±1.8* | 136.4±7.0 |
| K | nmol/l | 22.0±5.3 | 19.4±2.6 | 17.4±1.5* | 16.9±2.1 | 21.9±8.4 |
| Cl | nmol/l | 95.5±1.84 | 99.5±2.4 | 99.8±1.8* | 102.3±1.4* | 100.7±1.8* |
| Creatine | mg/dl | 0.8±0.1 | 0.7±0.1* | 0.7±0.1 | 0.7±0.0 | 0.7±0.1* |
| BUN | mg/dl | 31.7±5.6 | 28.1±3.3* | 30.6±5.3* | 32.3±5.9* | 31.4±5.8* |
| Uric acid | mg/dl | 6.6±2.0 | 6.2±1.3* | 5.5±1.1* | 5.4±0.8* | 4.7±0.9* |
| T.Chol | mg/dl | 94.3±23.6 | 91.1±11.9 | 94.0±17.8 | 108.7±17.1* | 108.9±25.8* |
| H.Chol | mg/dl | 25.5±8.3 | 29.1±3.7* | 25.9±3.6* | 31.0±3.8* | 29.1±6.8* |
| L.Chol | mg/dl | 9.1±2.1 | 9.3±2.5* | 9.6±1.9* | 9.9±2.2* | 12.7±3.1* |
| TG | mg/dl | 93.7±59.4 | 63.2±10.3* | 65.6±11.3* | 54.4±7.1* | 60.2±16.4* |
| Glucose | mg/dl | 309.1±90.2 | 166.2±115.3* | 160.5±68.0* | 147.8±53.2* | 190.6±80.3* |
| Ca | nmol/l | 13.0±0.7 | 12.4±0.6* | 12.1±0.6* | 11.6±0.9* | 11.4±0.8* |
| IP | mg/dl | 17.7±5.7 | 19.4±3.5 | 15.6±1.7* | 15.0±2.0* | 15.6±3.9 |
Abbreviations: ALP: alkaline phosphatase, AST (GOT), glutamate oxaloacetate transaminase, ALT (GPT), glutamate pyruvate transaminase, GGT, γ-glutamyl transferase, CK: creatinine phosphokinase, LDH, lactate dehydrogenase, Na, Sodium, K, potassium, Cl, chloride, BUN, blood urea nitrogen, T. Chol: total cholesterol, H. Chol: HDL cholesterol, L. Chol: LDL cholesterol, TG, triglyceride, Ca, calcium, IP, inorganic phosphorus.
aCON: PBS (vehicle) treated with murine normal diet.
Each value represents mean ± S.D. Statistically significant from control (*P<0.05).
Pathology and organ weight. No significant treatmentrelated pathologies were observed. Any minor changes were few and dose-independent. At the end of the administration period, there were no treatment-related changes in absolute (Table 7) or relative organ weights (Table 8). There were some histopathological findings observed, however the histopathological alterations at the end of the administration period were not related to treatment as finding were dose independent. There were no detectable pathological findings for either sex. Furthermore, there were no adverse findings that presented in the 13-week repeated toxicity test (Table 9,Table 10).
Table 7.
Absolute organ weight of Sprague-Dawley rats treated orally G. bimaculatus over a 13--week period
| Sex | Organs | CON | 0.04 | 0.2 | 1 | 5 (g/kg bw/day) |
|---|---|---|---|---|---|---|
| Male | Adernal gland R. | 0.047 ± 0.012 | 0.050 ± 0.011 | 0.061 ± 0.014 | 0.536 ± 0.015 | 0.086 ± 0.082 |
| L | 0.043 ± 0.006 | 0.047 ± 0.011 | 0.051 ± 0.018 | 0.053 ± 0.013 | 0.043 ± 0.015 | |
| Kidney R. | 1.605 ± 0.322 | 1.605 ± 0.133 | 1.610 ± 0.181 | 1.408 ± 0.261 | 1.543 ± 0.143 | |
| L. | 1.542 ± 0.279 | 1.476 ± 0.255 | 1.589 ± 0.180 | 1.416 ± 0.237 | 1.527 ± 0.191 | |
| Heart | 1.442 ± 0.149 | 1.370 ± 0.168 | 1.409 ± 0.180 | 1.316 ± 0.148 | 1.307 ± 0.110 | |
| Liver | 13.353 ± 1.0690 | 13.030 ± 0.1620 | 12.874 ± 1.4520 | 9.486 ± 3.709 | 11.656 ± 1.0780 | |
| Lung | 2.068 ± 1.191 | 2.123 ± 0.454 | 2.273 ± 0.434 | 1.972 ± 0.257 | 1.930 ± 0.112 | |
| Spleen | 0.822 ± 0.087 | 0.782 ± 0.809 | 0.807 ± 0.108 | 0.744 ± 0.154 | 0.684 ± 0.127 | |
| Testis R. | 1.708 ± 0.188 | 0.771 ± 0.195 | 1.743 ± 0.130 | 1.694 ± 0.150 | 1.700 ± 0.274 | |
| L. | 0.583 ± 0.280 | 0.795 ± 0.183 | 1.730 ± 0.115 | 1.717 ± 0.198 | 1.706 ± 1.706 | |
| Stomach | 0.960 ± 0.224 | 0.740 ± 0.430 | 2.395 ± 0.468 | 2.216 ± 0.312 | 2.295 ± 0.236 | |
| Pancreas | 0.611 ± 0.082 | 0.627 ± 0.143 | 0.699 ± 0.078 | 0.463 ± 0.101 | 0.554 ± 0.110 | |
| Thymus | 0.362 ± 0.097 | 0.382 ± 0.111 | 0.382 ± 0.117 | 0.365 ± 0.145 | 0.321 ± 0.079 | |
| Female | Adernal gland R. | 0.053 ± 0.011 | 0.058 ± 0.012 | 0.046 ± 0.009 | 0.050 ± 0.009 | 0.046 ± 0.013 |
| L | 0.053 ± 0.012 | 0.053 ± 0.009 | 0.047 ± 0.009 | 0.048 ± 0.010 | 0.043 ± 0.015 | |
| Kidney R. | 1.041 ± 0.213 | 1.018 ± 0.127 | 1.033 ± 0.101 | 0.973 ± 0.075 | 1.042 ± 0.227 | |
| L. | 1.088 ± 0.136 | 1.018 ± 0.089 | 1.016 ± 0.103 | 1.070 ± 0.074 | 0.994 ± 0.070 | |
| Heart | 0.967 ± 0.036 | 0.863 ± 0.059 | 1.030 ± 0.210 | 0.870 ± 0.042 | 0.880 ± 0.071 | |
| Liver | 8.521 ± 1.524 | 8.269 ± 1.000 | 8.742 ± 0.961 | 7.405 ± 0.734 | 7.410 ± 0.837 | |
| Lung | 1.636 ± 0.169 | 1.515 ± 0.103 | 1.775 ± 0.252 | 1.592 ± 0.283 | 1.569 ± 0.238 | |
| Spleen | 0.637 ± 0.107 | 0.585 ± 0.085 | 0.654 ± 0.091 | 0.509 ± 0.070 | 0.535 ± 0.074 | |
| Ovary R. | 0.088 ± 0.015 | 0.080 ± 0.027 | 0.095 ± 0.019 | 0.082 ± 0.014 | 0.081 ± 0.030 | |
| L | 0.079 ± 0.023 | 0.076 ± 0.009 | 0.088 ± 0.024 | 0.073 ± 0.012 | 0.068 ± 0.020 | |
| Stomach | 1.965 ± 0.651 | 1.805 ± 0.488 | 1.997 ± 0.520 | 1.916 ± 0.332 | 1.970 ± 0.380 | |
| Pancreas | 0.566 ± 0.144 | 0.509 ± 0.055 | 0.536 ± 0.051 | 0.477 ± 0.085 | 0.526 ± 0.080 | |
| Thymus | 0.249 ± 0.062 | 0.243 ± 0.097 | 0.282 ± 0.148 | 0.165 ± 0.033 | 0.169 ± 0.033 | |
(g)
Each value represents mean ± S.D.
Statistically significant from control (*P < 0.05)
Table 8.
Relative organ weight of Sprague-Dawley rats treated orally with G. bimaculatus over a 13-week period
| Sex | Organs Dosage (g/kg) | |||||
|---|---|---|---|---|---|---|
| CON | 0.04 | 0.2 | 1 | 5 | ||
| Male | Adernal gland R. | 0.011 ± 0.003 | 0.012 ± 0.003 | 0.014 ± 0.004 | 0.014 ± 0.036 | 0.023 ± 0.024 |
| L | 0.009 ± 0.001 | 0.012 ± 0.003 | 0.012 ± 0.005 | 0.014 ± 0.003 | 0.011 ± 0.005 | |
| Kidney R. | 0.367 ± 0.074 | 0.394 ± 0.022 | 0.379 ± 0.042 | 0.381 ± 0.065 | 0.403 ± 0.047 | |
| L. | 0.353 ± 0.063 | 0.364 ± 0.069 | 0.374 ± 0.037 | 0.383 ± 0.054 | 0.400 ± 0.046 | |
| Heart | 0.330 ± 0.032 | 0.337 ± 0.041 | 0.332 ± 0.039 | 0.357 ± 0.035 | 0.342 ± 0.029 | |
| Liver | 3.052 ± 0.120 | 3.174 ± 0.276 | 3.029 ± 0.282 | 2.555 ± 0.976 | 3.059 ± 0.326 | |
| Lung | 0.473 ± 0.038 | 0.518 ± 0.091 | 0.533 ± 0.079 | 0.535 ± 0.059 | 0.507 ± 0.046 | |
| Spleen | 0.188 ± 0.020 | 0.191 ± 0.021 | 0.199 ± 0.043 | 0.199 ± 0.043 | 0.178 ± 0.026 | |
| Testis R. | 0.391 ± 0.040 | 0.434 ± 0.037 | 0.410 ± 0.025 | 0.466 ± 0.047 | 0.445 ± 0.066 | |
| L. | 0.365 ± 0.072 | 0.440 ± 0.029 | 0.408 ± 0.030 | 0.408 ± 0.030 | 0.447 ± 0.035 | |
| Stomach | 1.960 ± 0.224 | 0.180 ± 0.096 | 0.561 ± 0.092 | 0.601 ± 0.080 | 0.605 ± 0.095 | |
| Pancreas | 0.611 ± 0.082 | 0.154 ± 0.034 | 0.165 ± 0.015 | 0.132 ± 0.031 | 0.015 ± 0.032 | |
| Thymus | 0.083 ± 0.036 | 0.095 ± 0.031 | 0.090 ± 0.030 | 0.102 ± 0.047 | 0.085 ± 0.025 | |
| Female | Adernal gland R. | 0.021 ± 0.005 | 0.249 ± 0.005 | 0.019 ± 0.004 | 0.023 ± 0.005 | 0.020 ± 0.005 |
| L | 0.021 ± 0.005 | 0.023 ± 0.004 | 0.018 ± 0.007 | 0.022 ± 0.005 | 0.019 ± 0.007 | |
| Kidney R. | 0.411 ± 0.077 | 0.438 ± 0.067 | 0.422 ± 0.044 | 0.440 ± 0.041 | 0.462 ± 0.095 | |
| L. | 0.431 ± 0.052 | 0.439 ± 0.054 | 0.415 ± 0.041 | 0.439 ± 0.039 | 0.442 ± 0.035 | |
| Heart | 0.384 ± 0.024 | 0.371 ± 0.027 | 0.426 ± 0.121 | 0.393 ± 0.035 | 0.392 ± 0.040 | |
| Liver | 3.354 ± 0.422 | 3.551 ± 0.441 | 3.563 ± 0.258 | 3.342 ± 0.243 | 3.290 ± 0.367 | |
| Lung | 0.649 ± 0.076 | 0.651 ± 0.050 | 0.724 ± 0.091 | 0.720 ± 0.131 | 0.695 ± 0.086 | |
| Spleen | 0.254 ± 0.051 | 0.251 ± 0.036 | 0.267 ± 0.032 | 0.230 ± 0.022 | 0.237 ± 0.027 | |
| Ovary R. | 0.035 ± 0.006 | 0.035 ± 0.012 | 0.039 ± 0.009 | 0.037 ± 0.007 | 0.036 ± 0.011 | |
| L | 0.031 ± 0.009 | 0.033 ± 0.006 | 0.036 ± 0.011 | 0.033 ± 0.006 | 0.030 ± 0.008 | |
| Stomach | 0.776 ± 0.237 | 0.771 ± 0.195 | 0.816 ± 0.212 | 0.864 ± 0.126 | 0.873 ± 0.159 | |
| Pancreas | 0.222 ± 0.048 | 0.219 ± 0.028 | 0.220 ± 0.024 | 0.216 ± 0.040 | 0.234 ± 0.041 | |
| Thymus | 0.098 ± 0.022 | 0.142 ± 0.013 | 0.159 ± 0.101 | 0.075 ± 0.015 | 0.075 ± 0.015 | |
(g)
Relative organ weight is calculated as organ weight/body weight (%).
Each value represents of mean ± S.D.
Statistically significant from control (*P < 0.05).
Table 9.
Histopathological findings of organs in male rats treated orally with G. bimaculatus for 13 weeks
| Histopathological findings | 0 (CONa) | 0.04 | 0.2 | 1 | 5 (g/kg bw/day) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ | |
| Kidney | ||||||||||||||||||||
| Chronic nephritis | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Pyelonephritis | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Microcalcification | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Nuclear enlargement (cellular atypia) | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Liver | ||||||||||||||||||||
| Vasculitis | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Necrosis | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Lung | ||||||||||||||||||||
| Epithelioid granulomas | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Foamy cell collection | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Spleen | ||||||||||||||||||||
| Hemosiderosis | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Extramedullary hematopoiesis | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Testis | ||||||||||||||||||||
| Necrosis | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Stomach | ||||||||||||||||||||
| Necrosis | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Pancreas | ||||||||||||||||||||
| Fat necrosis | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Thymus | ||||||||||||||||||||
| Agonal hemorrhage | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Lymphocytic necrosis | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
aCON: treated with PBS buffer.
-: Normal, +: Mild, ++: Moderate, +++: Severe.
Values are number of animals presenting the histopathological lesions.
The value of 0 of animals means non-significant finding.
Table 10.
Histopathological findings of organs in male rats treated orally with G. bimaculatus for 13 weeks
| Histopathological findings | 0 (CONa) | 0.04 | 0.2 | 1 | 5 (g/kg bw/day) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ | |
| Kidney | ||||||||||||||||||||
| Chronic nephritis | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Pyelonephritis | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Microcalcification | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Nuclear enlargement (cellular atypia) | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Liver | ||||||||||||||||||||
| Vasculitis | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Necrosis | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Lung | ||||||||||||||||||||
| Epithelioid granulomas | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Foamy cell collection | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Spleen | ||||||||||||||||||||
| Hemosiderosis | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Extramedullary hematopoiesis | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Testis | ||||||||||||||||||||
| Necrosis | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Stomach | ||||||||||||||||||||
| Necrosis | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Pancreas | ||||||||||||||||||||
| Fat necrosis | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Thymus | ||||||||||||||||||||
| Agonal hemorrhage | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Lymphocytic necrosis | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
aCON: treated with PBS buffer.
-: Normal, +: Mild, ++: Moderate, +++: Severe.
Values are number of animals presenting the histopathological lesions.
The value of 0 of animals means non-significant finding.
DISCUSSION
G. bimaculatus belongs to Gryllidae, Ortoptera and is used as a natural food for humans and reptiles in Southeast Asia and Africa (Cui et al., 2002). Owing to the development of artificial rearing techniques, the number of insect rearing farms is increasing, suggesting that insect protein might be a future food resource because of its safety, economical cost, easy management and small area requirements. Accordingly, last year, an industrial entomology rearing law was announced. Therefore, more safety data will be needed for insect food approval in the future.
Recently, it was reported that the primary pharmacological activities of G. bimaculatus include selective hepatoprotective activities (Ahn et al., 2002; Kwon et al., 2004) and alcohol metabolizing enzyme induction in rats (Ahn et al., 2004).
The safety of Gb has been evaluated systematically in a series of acute and sub-acute toxicological tests showing only slight acute (single dose) toxicity with an oral LD50 value of > 5 g/kg (Kim et al., 2002). Another safety study on the extract of cricket was reported. G. bimaculatus at doses of 0 (vehicle), 0.025, 0.05, 0.1 and 0.2 mg/kg for 2 weeks (Hwang et al., 2004) were examined. On the other hand, a previous report showed that a dose of 0.2 mg/kg of a water/methanol cricket extract had protective effects on acute hepatic damage in ICR-mice induced by the administration of CCl4 (Ahn et al., 2002). The same extract dosage (0.2 mg/kg) was used to evaluate the alcohol-induced toxicity in Gb as an alcohol metabolizing enhancer via liver mitochondrial alcohol dehydrogenase and acetaldehyde dehydrogenase (Ahn et al., 2004). Therefore, the repeated upper oral dose in subchronic toxicity study would need to be more than 0.2 mg/kg, such as 0.5 g/kg or 1 g/kg for calculating of NOAEL. In this acute oral toxicity study of Gb in SD rats, Gb did not induce any remarkable toxic responses and the LD50 was previously reported as > 5 g/kg (Kim et al., 2002). After a dose range finding (DRF), the safety of Gb at doses of 0, 0.04, 0.2, 1 and 5 g/kg was examined over 13 consecutive weeks. Treatment at 5.0 g/kg/day with Gb resulted in a decrease in serum triglycerides and glucose in a dose-dependent manner in both sexes but all changes were within normal physiological range. There were no clinical signs or deaths related to treatment in any of the groups tested. Therefore, the approximate lethal oral dose of G. bimaculatus was considered to be > 5 g/kg/day in rats. Throughout the administration period, no significant changes in diet consumption, ophthalmologic findings, organ weight, clinical pathology (hematology, clinical chemistry, coagulation, and urinalysis) and gross pathology were detected. Minor changes were noted in hematological and biochemical serum parameters for the 0.04. 0.2, 1, 5 g/kg/day Gbtreated groups, but all changes were within the normal physiological range. A microscopic examination did not identify any treatment-related histopathology changes in the organs of the Gb-treated groups.
Overall, the no-observed adverse effect level (NOAEL) of G. bimaculatus is higher than 5.0 g/kg bw/day in rats.
Acknowledgments
This work supported by the National Academy of Agricultural Science, Basic research project (No. PJ006642), RDA, Republic of Korea.
References
- 1.Ahn M.Y., Ryu K.S., Park B.Y., Kim D.W., Kim I.S., Kim S.H. Effects of cricket supplements on the chicken meats and its eggs. Korean J. Poult. Sci. (2000);27:169–282. [Google Scholar]
- 2.Ahn M.Y., Lee Y.W., Ryu K.S., Kim I. K., Kim J.W., Lee Y.K., Kim E.S., Kim Y.S., Lee H.S. Protective effects of water/methanol extracts of cricket on the acute hepatic damages in the ICR-mice induced by administration of CCl4. Korean J. Food Sc. Technol. (2002);34:684–687. [Google Scholar]
- 3.Ahn M.Y., Lee Y.W., Ryu K.S., Lee H.S., Kim I.K., Kim J.W., Lim S.S. Effect of water and methanol extracts of cricket (Gryllus bimaculatus) on alcohol metabolism. Kor. J. Pharmacogn. (2004);35:175–178. [Google Scholar]
- 4.Ahn M.Y., Bae H.J., Kim I.S., You E.J., Kwack S.J., Kim H.S., Kim D.H., Ryu K.S., Lee H.S., Kim J.W., Kim I., Lee B.M. Genotoxic evaluation of the biocomponents of the cricket, Gryllus bimaculatus, using three mutagenicity tests. J. Toxicol. Environ. Health A. (2005);68:2111–2118. doi: 10.1080/15287390500182537. [DOI] [PubMed] [Google Scholar]
- 5.Cui Z., Ahn M.Y., Lee Y.B., Ryu K.S. Materia medica from insects. Shinilbooks.; Seoul: (2002). pp. 137–145. [Google Scholar]
- 6.Hwang S.Y., Sin J.S., Kwon W., Chai H.Y., Cho J.H., Lee N.J., Park J.B., Kim I., Ryu K.S., Yun C.Y., Kang J.K., Kim Y.B. Repeated-dose toxicity study for the extract of cricket, Gryllus bimaculatus, in rat. The Korean Journal of Laboratory Animal Science. (2004);20:113–209. [Google Scholar]
- 7.Kim I.S., Ahn M.Y., Ryu K.S., Lee B.M. Acute oral toxicity of G. bimaculatus in rats. J. Toxicol. Pub. Health. (2002);18:397–400. [Google Scholar]
- 8.Kwon W., Chai H.Y., Cho Y.M., Chio E.K., Sin J.S., Kim T.M., Kim I., Hwang S.Y., Yun C.Y. Protective effects of extract of cricket, Gryllus bimaculatus, against hepatotoxicity induced by 2,3,7,8-tetrachlorodibenzo-r-dioxin (TCDD) in rats. Korean J. Lab. Ani. Sci. (2004);53:1341–1357. [Google Scholar]
- 9.Song S.W., Jung W., Hong D.H. Thirteen-week repeated-dose toxicity studies of STB-HO-BM in rats. J. Toxicol. Pub. Health. (2006);22:135–144. [Google Scholar]