Abstract
There has been growing concern about the toxicity of phthalate esters. Phthalate esters are being used widely for the production of perfume, nail varnish, hairsprays and other personal/cosmetic uses. Recently, exposure to phthalates has been assessed by analyzing urine for their metabolites. The parent phthalate is rapidly metabolized to its monoester (the active metabolite) and also glucuronidated, then excreted. The objective of this study is to evaluate the toxicity of phthalic acid (PA), which is the final common metabolic form of phthalic acid esters (PAEs). The individual PA isomers are extensively employed in the synthesis of synthetic agents, for example isophthalic acid (IPA), and terephthalic acid (TPA), which have very broad applications in the preparation of phthalate ester plasticizers and components of polyester fiber, film and fabricated items. There is a broad potential for exposure by industrial workers during the manufacturing process and by the general public (via vehicle exhausts, consumer products, etc). This review suggests that PA shows in vitro and in vivo toxicity (mutagenicity, developmental toxicity, reproductive toxicity, etc.). In addition, PA seems to be a useful biomarker for multiple exposure to PAEs in humans.
Keywords: Phthalic acid, Phthalates, Common metabolite, Plasticizer
INTRODUCTION
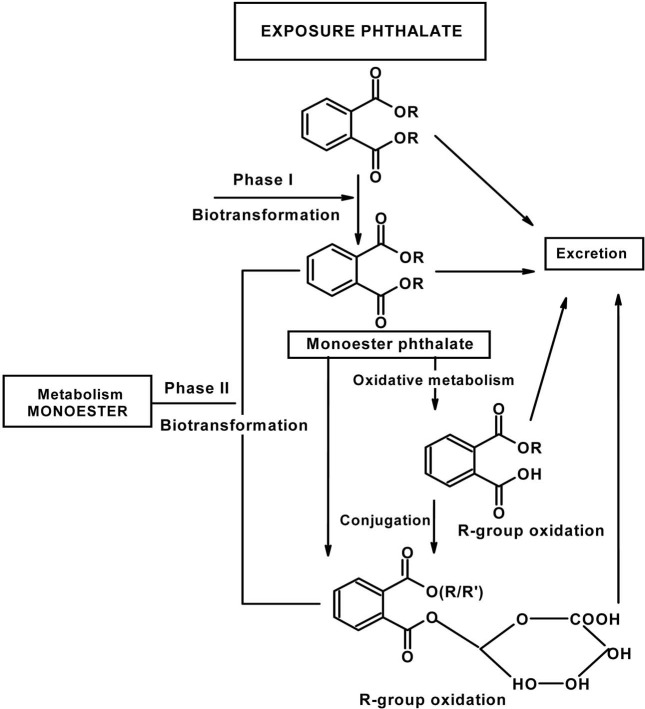
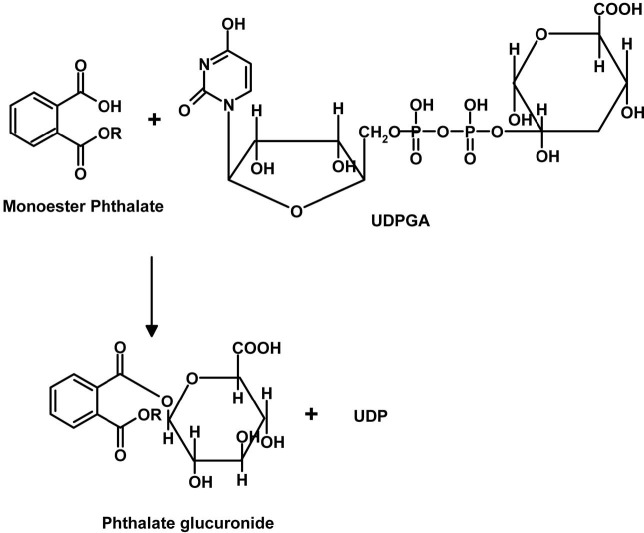
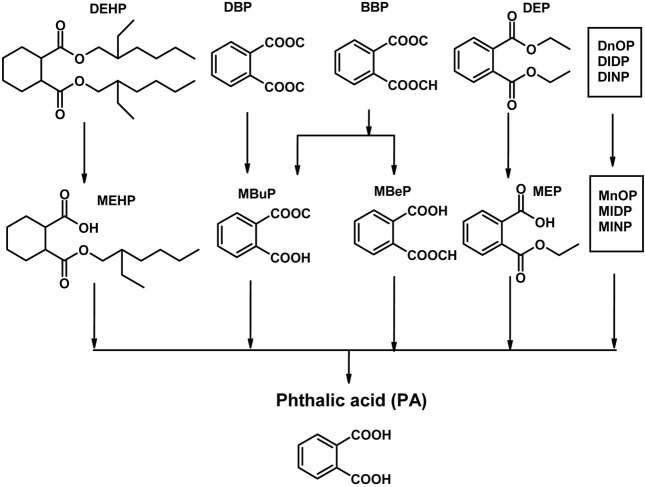
Plasticizers such as dialkyl phthalates (PA diesters) and phenol derivatives used for flexibility and durability of plastics, are man-made chemicals which are widely distributed environmental contaminants due to their use in consumer products (e.g. PVC, cosmetics, perfumes), food packaging, toys and medical devices (Huber et al., 1996). Since phthalates are not chemically bound to PVC, they are released from vinyl products with time and use, thus becoming regular contaminants in ambient air, drinking water and food products, all of which are potential sources of exposure for the general population (Bauer and Herrmann, 1997; Sharman et al., 1994; Mayer et al., 1972). They are produced in high volume and exposure to humans is poorly defined, particularly children. Phthalates are animal carcinogens and can cause fetal death, malformations and reproductive toxicity in laboratory animals. They interfere with or disrupt endocrine activity and systems, e.g. estrogenic and antiestrogenic activities are reported (Cooper and Kavlock, 1997; McLachlan, 2001; Okubo et al., 2003). Accordingly, they were termed endocrine disruptors (ED) or endocrine disrupting chemicals (EDCs). In addition, they are released directly into the environment during production and after disposal of PVC and other phthalate-containing products. Phthalates bioaccumulate in invertebrates, fish and plants but do not biomagnify, because higher animals efficiently metabolize and excrete phthalates (Staples et al., 1997). They are ubiquitous contaminants in food, indoor air, soils and sediments. The most commonly used phthalate compounds are di-n-butyl phthalate (DBP) and di-iso-octyl phthalate (DIOP), but others like bis(2-ethylhexyl) phthalate (DEHP), di-isobutyl phthalate (DIBP), and diallyl phthalate (DAP) are or have been used as well. Phthalates are rapidly metabolized in humans by phase 1 biotransformation to their respective monoesters, which, depending on the phthalate, can be further metabolized via oxidation of their lipophilic, aliphatic side chain. Metabolism of most diesters of PA in humans occurs initially by phase I biotransformation in which phthalate monoesters are formed, followed by a phase II biotransformation in which phthalate monoesters react with glucuronic acid to form their respective glucuronide conjugates. Phase II conjugation increases water solubility and facilitates urinary excretion of phthalate and reduces the potential biological activity because the putative biologically active species is the monoester metabolite (Fig. 1). For example, dibutyl phthalate (DBP) and DEHP are known to be metabolized to the bioactive monoesters monobutyl phthalate (MBP) and mono-2-ethylhexyl phthalate (MEHP), upon phase 1 biotransformation, which are excreted in urine (Heindel and Powell., 1992). Phthalate monoesters can be excreted unchanged or they can undergo phase II biotransformation, which is catalyzed by uridine 5'-diphosphoglucuronyl transferase (UGT) to produce glucuronide-conjugated monoesters. Alternatively, phthalate monoesters may be further metabolized to produce more hydrophilic oxidative products (Albro et al., 1973; Albro and Thomas, 1973; Albro and Moore, 1974; ATSDR, 1997, 2000; Barr et al., 2003; Silva et al., 2003) and their glucuronide conjugates (Fig. 2). The glucuronide conjugate and the free monoester can be excreted in urine and feces or, alternatively, can undergo ω or (ω-1) hydroxylation or β elimination to form several oxidative metabolites (Albro et al., 1973; ATSDR, 2002). Phthalates undergo rapid metabolism and in addition to forming specific metabolites share phthalic acid (PA) as a common metabolite (Albro et al., 1984) (Fig. 3). PA was recovered from the urine in almost quantitative yield after subcutaneous injection in the dog and in man (Shemiakin and Schukina, 1944) but was almost completely metabolized when orally administered to the dog. McBain et al., (1968) reported that rats orally dosed with 14C-PA excreted this compound in the urine but gave no quantitative data. Albro et al. (1973) have shown that PA was one of the metabolic products excreted in the urine when di-(2-ethylhexyl) phthalate was administered orally to the rat. PA administered orally to the rat is not appreciably metabolized and is not retained in the organs or tissues (Williams and Blanchifield, 1974). In several studies, phthalate monoesters metabolites have been used as markers of exposure (Blount et al., 2000; Silva et al., 2004). These biomarkers represent an integrative measure of phthalate exposure from multiple sources and pathways. However, for several high molecular weight phthalates (for instance DEHP and DOP), no specific metabolites have been unequivocally identified (Kato et al., 2005). Until specific biomarkers of exposure to isomeric phthalates are available, indirect measures of exposure (e.g., PA) to these phthalates might be valuable.
Fig. 1. General metabolic pathway for phthalates.
Fig. 2. Glucuronidation (phase II biotransfomation) of monoester phthalates to their glucuronides. (UDPGA, uridine5' - diphosphoglucuronic acid; UDP, uridine 5’ - diphosphate).
Fig. 3. Metabolism of PA esters (PAEs).
General description of PA. PA (Table 1), also called benzene dicarboxylic acid with formula C6H4(COOH)2, is the name of any of three isomers. The ortho form (1,2-benzene carboxylic acid; Fig. 4) is called simply PA. It is a white crystal decomposing at 191℃ and slightly soluble in water and ether. This compound is mainly produced and marketed in the form of its anhydride produced by the oxidation of orthoxylene and naphthalene. Its wide application is based on the ortho-related carboxylic acid groups as their dehydration is highly reactive with broad processing conditions to produce various downstream products. It is used to make simple esters widely used as plasticizers. It is used for making unsaturated polyester resins, alkyl resins, polyester polyols, dyes and pigments, halogenated anhydrides, polyetherimide resins, isatoic anhydride and insect repellents.
Table 1.
General Information on PAs
| Phthalic acid (PA) | Isophthalic aicd (IPA) | Terephthalic acid (TPA) | ||
|---|---|---|---|---|
| CAS No. | [88-99-3] | [121-91-5] | [100-21-0] | |
| SYNONYM(s) | 1,2-Benzendicarboxylic acid Benzene-1,2-Dicarboxylic acid o-Benzendicarboxylic acid o-Dicarboxybenzene ortho-PA Sunffal 20 | Benzene-1,3-dicarboxylic acid meta-PA | Benzene-1,4-dicarboxylic acid para-PA TPA PTA | |
| USE | Fixative for perfume. Industrial intermediate | Components of polyester fiber, film and fabricated items | Components of polyester fiber, film and fabricated items | |
| M.W | 166.14 g/mol | 166.14 g/mol | 166.14 g/mol | |
| Chemical Formula | C8H6O4/C6H4(COOH)2 | C6H4(COOH)2 | C6H4(COOH)2 | |
| Appearance | White Crystals | White crystaline solid | white crystals or powder | |
| Melting Point | Approx 230 | 345~348℃, sublimes | 402℃ (675 K), sublimes | |
| Density | 1.59 g/cm3 | 1.526 g/cm3 | 1.522 g/cm3 | |
| Water Solubility | Slight | Insoluble in water | Insoluble in water | |
| Other solvents (DMF, alkali) | Soluble | |||
| Acidity (pKa) | pKa1 = 2.92, pKa2 = 5.41 | pKa1 = 3.46, pKa2 = 4.46 | pKa1 = 3.51 (25℃), pKa2 = 4.82 (16℃) | |
Fig. 4. Chemical structure of PAs.
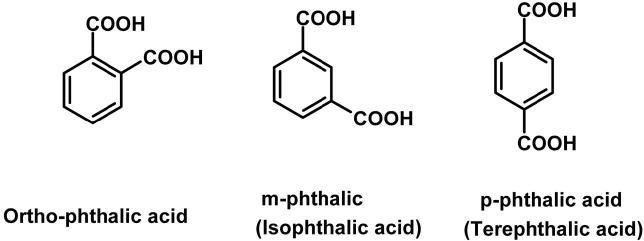
Isomers of PA. The meta form (Fig. 4) is isophthalic acid (IPA)(1,3-benzene carboxylic acid; Table 1). It is a white crystal subliming at 345℃, and slightly soluble in water, alcohol and acetic acid (insoluble in benzene). It is produced by oxidizing meta-xylene with chromic acid, or by fusing potassium meta-sulphobenzoate or meta-brombenzoate with potassium formate. IPA has excellent performance characteristics for coatings including excellent hardness, corrosion and stain resistance, hydrolytic stability of coatings and gel coats, excellent thermal stability and low resin color. It is a key ingredient for such products as marine, automotive and corrosion resistant pipes and tanks. Polyesters containing IPA are also used extensively in industrial coating applications for home appliances, automobiles, aluminum siding and metal office furniture. It is used as an intermediate for polyesters, polyurethane resins and plasticizers. The para form (Fig. 4), known as terephthalic acid (1,4-benzenecarboxylic acid; Table 1) is a combustible white powder that is insoluble in water, alcohol and ether (soluble in alkalies), and sublimes at 300℃. It can be produced by oxidizing caraway oil, a mixture of cymene and cuminol or by oxidizing para-diderivatives of benzene with chromic acid. TPA has been used mainly as a raw material for polyester fiber but lately it has been exploited for various uses in the non-fiber field for PET (polyethylene terephthalate)- bottles, PET-films, engineering of plastics and as poultry feed additives. PA derivatives are also widely used to make dyes, medicines, synthetic perfumes, pesticides? and other chemical compounds.
TOXICOLOGICAL CHARACTERISTICS OF PAs
Acute Toxicity.
PA: Following oral and intraveneous administration of PA to mouse and rats, changes in motor activity, muscle contraction or spasticity, lungs, thorax or respiration (cyanosis) were observed. PA has low acute toxicity as presented in Table 2. O-PA was reported to be a moderate irritant to the skin and mucous membranes of humans (BIBRA working group, 1989). It has been listed as a contact allergen in humans but no empirical support for this classification is found. The salts of O-PA have low acute oral toxicity in rats, but single intraperitoneal injections of the acid or its sodium salt to mice affected the central nervous system, liver and oxygen supply to the tissues. The toxicological overview of PA is summarized in Table 3.
Table 2.
Acute toxicity of PAs
| Compound | Species | Route of administration | LD50 (mg/kg body weight | Ref. |
|---|---|---|---|---|
| PA (ortho-PA) | Mouse | i.p. | 550 | - |
| Mouse | Oral | 2530 | - | |
| Rat | N/R | 1100 | GISAAA, 1967 | |
| Rat | Oral | 7900 | - | |
| TPA (Tere-PA) | Mouse | Oral | > 5000 | Hoshi et al., 1968 |
| 6400 | Moffitt et al., 1975 | |||
| 1470 | Moffitt et al., 1975 | |||
| Rat | > 5000 | |||
| > 15380 | Amoco Co., 1990; | |||
| 1960 | Amoco Co., 1975; | |||
| 18800 | ||||
| Mouse | i.p. | 1430 | ||
| [1240-1650] | Hoshi et al., 1968 | |||
| 880 | Grigas et al., 1971 | |||
| 1900 | ||||
| Rat | 1210 | - | ||
| 2250 | ||||
| Mouse | i.v. | 770 | - | |
| Na2TPA | Mice | Oral | 6300(5000) | Hoshi et al., 1968 |
| [5600-7150] | ||||
| s.c. | 8600(6800) | |||
| [7760-9550] | ||||
| i.p. | 4600(3600) | |||
| [4260-4870] | ||||
| i.v. | 1300 or more | |||
| (1000 or more) | ||||
| IPA (Iso-PA) | Rat | Oral | > 5,000 | IITRI, 1990 |
| 13,000 | Industrial BIO-TEST, 1975 | |||
| 10,900 | Industrial BIO-TEST, 1975 | |||
| 12,200 | Industrial BIO-TEST, 1958 | |||
| 10,400 | Marhold, 1986 | |||
| Rat | Inhalation | LC50 | ||
| > 11.37 g/m3 | Industrial BIO-TEST, 1958 | |||
| Rabbit | Dermal | 2,000 | IITRI, 1990. | |
| 23,000 | Industrial BIO-TEST, 1958 | |||
| Rat | i.p. | 13,000 | Calandra, 1975 | |
| Mouse | 4200 | - | ||
Table 3.
Toxicological data of PA
| Type | Species | Dose | Route/Period | Remarks | Ref. |
|---|---|---|---|---|---|
| Multiple dose toxicity | Rat | 102 mg/kg | Oral/26-week | Changes in serum composition (e.g., TP, bilirubin, cholesterol) | GISAAA, 1967 |
| Mutagenicity | Mice | 40 or 80 mg/kg | i.p./5-day | - Induction of dominant lethal mutations | Jha et al., 1998 |
| 50, 100, 150, 200, 300 mg/kg | i.p | - Increase in abnormal sperm head | |||
| Genotoxicity | CHO cells | - | - | Cytotoxicity. Chromosomal alterations. | Phillips et al., 1982 |
| Reproductive toxicity | Rat | 29810 mg/kg | Oral/Female 7~16 days After conception | - Fetotoxicity (except death, e.g., stunted fetus). | TOLED5, 1997 |
| - Developmental abnormalities (musculoskeletal system) | |||||
| Reproductive toxicity | WISH cells | - | - | 17beta-estradiol actions (PA binds to ER with high affinity) | Pavan et al., 2001 |
| Rat | - | - | Induction of testicular atrophy | Sharpe, 1998 | |
| Developmental toxicity | Rat | 1.25, 2.5, 5.0% (1021, 1763, 2981 mg/Kg) | Oral/7~16 days of pregnancy | - Decreased weight of male fetuses | Ema et al., 1997. |
| - Number of ossification center of the caudal vertebrae. | |||||
| Teratogenicity | Chicken embryo | 0.05 ml/eggs or less | - | Low teratogenic effect. (malformation of the developing chicken embryo) | Verrett et al., 1969 |
Terephthalic acid (TPA): TPA is primarily used for the production of polyester fibers, films, polyethylene terephthalate (PET) solid-state resins and polyethylene terephthalate engineering resins. Acute toxicity of TPA (Table 2) is very low and its LD50 is more than 5,000 mg/kg by oral administration. The sodium salt of TPA is less toxic than the acid itself after intraperitoneal injection, with an LD50 of the salt being 3600 mg/kg compared to 1430 mg/kg of the acid. This can be explained by the acidity of TPA (Hoshi et al., 1968).
Isophthalic acid (IPA): IPA is mainly used for the synthesis of resins and coatings (70%) and in packaging of fibers and plastics (30%). Exposures to workers may occur via inhalation and dermal contact. Because IPA present in consumer products is bound to a polymer matrix, the potential for exposure to consumers is low. Additionally, because IPA is not persistent in the environment, the potential for environmental exposures is low. Both IPA and TPA have similar physicochemical properties, as well as similar metabolic and toxicological properties. IPA exhibits low acute toxicity by the oral, dermal, and inhalation routes. Acute oral LD50 (Table 2) ranging from 10,400 to 13,000 mg/kg have been reported in rats (Marhold, 1986; Industrial Bio-Test, 1958, 1975). Necropsy of animals showed pale and discolored kidneys (Industrial Bio-Test, 1975) and no deaths were reported in rats receiving a single oral dose of 5,000 mg/kg IPA (IITRI, 1990). Clinical signs (irritability, salivation, discoloration around nose and mouth, diarrhea, wet and/or discolored inguinal fur, discolored paws), appear within 24 hours after exposure, but generally resolved within 48 hours. No deaths or treatment-related effects were observed in rats exposed to 11,400 mg/m3 IPA dust for 4 hours (Industrial Bio-Test, 1958). In rabbits, no deaths were reported following a single dermal doses of 2000 or 23,000 mg/kg of IPA (IITRI, 1990; Industrial Bio-Test, 1958). Following intraperitoneal injection, LD50 values of 4,200 and 13,000 mg/kg have been reported for IPA in mice and rats, respectively (Calandra, 1975).
Toxicological data of PA.
General toxicity of PA: In limited studies, repeated feeding of the acid caused effects on the blood in rats and rabbits. There were no adverse effects on fetal development in mice given a single injection of O-PA during pregnancy. Rabbit sperm motility was inhibited by the potassium salt. Phthalic anhydride, the anhydrous form of the acid, gave no evidence of carcinogenicity in long-term feeding studies in rats and mice. O-PA did not cause mutations in bacterial tests (including Ames), but a mutagenic effect was seen in fruit flies (ref). The sodium salt did not induce chromosome damage in mammalian cells in culture (BIBRA working group, 1989). The effects of several phthalic acid monoesters on serum lipid composition were studied in rats. Wistar-rats were given diet containing 2% of mono-n-butylphthalate, monoisobutyl-phthalate, monooctyl-phthalate, or mono-2-ethylhexyl-phthalate (MEHP). No esterified fatty acids were increased in all treated animals, but almost doubled in controls versus MEHP fed animals. Triglycerides and total cholesterol were decreased, but free cholesterol did not change significantly. Fatty acid composition of serum phospholipids showed increases in the percentage of palmitic acid; oleic acid in serum cholesteryl esters was increased in treated rats with linoleic acid except for those given monooctyl-phthalate. For serum triglycerides, oleic acid increased and linoleic acid decreased except among those given MEHP. This study demonstrates that the monoesters of phthalic acid induce liver enlargement and changes in serum lipid components (Oishi and Hiraga, 1982). Several studies have focused on the effects of prostaglandins on bone formation and of prostaglandin synthase in osteoblastic cells (Kawaguchi et al., 1995; Pilbeam et al., 1995; Raisz, 1995; Raisz et al., 1993), suggesting the possibility that PA could influence prostanoid output. In addition, an inhibitory influence by PA esters on arachidonate metabolism has been demonstrated in rat peritoneal leucocytes or in human peritoneal T lymphocytes (Tavares and Vine, 1985; Carozzi et al., 1993).
Mutagenicity of PA: Germ cell mutagenicity of PA was evaluated by employing dominant lethal mutation and sperm head abnormality assays in male Swiss albino mice. For the dominant lethal mutation assay, adult male mice received a single intraperitoneal (i.p.) injection of either 40 or 80 mg/kg b.w. of PA for 5 consecutive days. For the sperm head abnormality assay, mice were treated with 50, 100, 150, 200 and 300 mg/kg b.w. as a single i.p. injection. Treatment of adult male mice with PA resulted in induction of dominant lethal mutations and induced dose-dependent increases in abnormal sperm following exposure of male mice during meiotic and postmeiotic stages of spermatogenesis. The results obtained indicate that PA is a germ cell mutagen (Jha et al., 1998). The mutagenic potential of PA (PA) was tested in the Ames assay. PA exhibited no mutagenicity in any of the strains of Salmonella typhimurium tested, with or without S9 metabolic activation (Agarwal et al., 1985). PA was tested for clastogenic activity in cultured Chinese hamster ovary (CHO) cells and showed no cytotoxicity and no chromosomal alterations (Phillips et al., 1982).
Teratogenicity of PA: The toxicity and potential teratogenicity to the developing chicken embryo was determined for PA. Compound was injected into either the yolk or air cell of fresh fertile White Leghorn eggs before incubation up to 0.05 ml/egg. PA had a low teratogenic effect but significant incidence of specific malformations with respect to the controls (Verrett et al., 1969).
Developmental toxicity of PA: PA possesses no developmental toxicity even at a dose which induces toxicity in rats. Pregnant rats were given PA at a dose of 0 (control), 1.25, 2.5 or 5.0% in the diet on days 7~16 of pregnancy. Average daily intakes of PA were 1,021 mg/kg for the 1.25% group, 1,763 mg/kg for the 2.5% group, and 2,981 mg/kg for the 5.0% group. Maternal toxicity occurred in the 2.5 and 5.0% groups as can be seen by significant decreases in the maternal body weight gain and food consumption during the administration period. No significant changes in maternal parameters were found in the 1.25% group. Neither deaths nor clinical signs of toxicity were noted in any group. No significant changes induced by PA were detected in the incidence of postimplantation loss and number and sex ratio of live fetuses. Significant decreases in the weight of male fetuses and number of ossification centers of the caudal vertebrae were found in the 5.0% group. Morphological examinations of fetuses revealed no evidence of teratogenesis. Thus, it appears unlikely that PA may be responsible for the production of developmental toxicity of PAEs (Ema et al., 1997).
Reproductive toxicity of PA: PA displaces [3H]estradiol from its binding sites, enhances the intracellular cyclic AMP concentration without influencing adenylyl cyclase activity and stimulates or inhibits prostaglandin output, probably depending on the intracellular nucleotide levels. The effects on prostanoid release are counteracted by addition of the protein-synthesis inhibitor cycloheximide, or when the diffusion of PA through the cell membrane is prevented. On the basis of our previous demonstration, that 17beta-estradiol exerts similar effects in WISH cells. Pavan et al. (2001) suggest that the molecular mechanisms underlying PA and steroid-hormone responses in this cell line are the same. This was the first demonstration that PA binds to the estrogen receptor with high affinity and mimics hormone physiological actions (Pavan et al., 2001). PA isomers are suspected as potent androgen receptor antagonists and might cause abnormalities in male reproductive systems (Japan Environment Agency, 1998; Sharpe, 1998). Induction of testicular atrophy by PA and six of its esters (PAEs) were compared in male Wistar rats. No harmful effects on the testes (i.e. concentration of testosterone, dihydrotestosterone, or zinc) were observed in rats fed high levels (2% PA) for 1 week (Oishi and Hiraga, 1980).
Toxicological data of TPA.
General toxicity of TPA: A 15 week oral repeat dose study of terephthalic acid (TPA) in rats determined an LOAEL of 3,837 mg/kg b.w./day for male rats and 4,523 mg/kg/day for female rats. The NOAEL was 1,220 mg/kg b.w./day for male rats and 1,456 mg/kg b.w/day for female rats (Amoco Co., 1970). TPA has a potentiating effect on biologically active substances such as tetracycline–type antibiotics (Peterson et al., 1959; Price and Zolli, 1959), thiamine, and sulfonamides (Hoshi et al., 1967). TPA content in tissues was low, even though higher content was found in liver and kidney than in plasma (Hoshi and Kuretani, 1968). Furthermore, it was found not to be metabolized but to be rapidly excreted, almost quantitatively in urine unchanged (Hoshi and Kuretani, 1967). It has been reported that TPA is neither biologically active nor toxic. TPA did not show any toxic indications in mice fed a 0.5% TPA diet (Hoshi et al., 1968). TPA at 20 mg/kg/day lowered serum cholesterol and triglyceride levels in rats (Hall et al., 1993). TPA is not metabolized in the body, does not accumulate in the tissues and mainly excreted in urine unchanged (Hoshi and Kuretani, 1967; Wolkowski-Tyl et al., 1982; Moffitt et al., 1975). TPA has been considered to be a non-genotoxic chemical and it is a mild respiratory tract and eye irritant. Workers exposed to TPA exhibited decreased pulmonary function (Li et al., 1999). Exposure of male weanling Fischer 344 rats to 4.0% TPA in the diet (positive controls) for two weeks (postnatal days 28-42) resulted in a 50% incidence of bladder calculi, aciduria, elevated urinary excretion of calcium (Ca) and magnesium (Mg), and slightly elevated serum levels of Ca and Mg relative to controls (Wolkowski-Tyl and Chin, 1983).
Carcinogenicity of TPA: Two-year feeding studies showed increase of calculi, bladder hyperplasia and tumors in rats. These effects were seen at doses of 2% TPA (1,000 mg/kg/day) and higher in the diet. The induction of bladder tumors is believed to be a result of injury to the bladder epithelium from calculi formation (CIIT, 1983)(Table 4). Calcium terephthalate (CaTPA) is the principal component of urinary tract calculi induced in rats by dietary administration of TPA (Chin et al., 1981). Masui et al. (1988) speculated that different kinds of crystals might have different effects on bladder epithelium. The calculi induced by TPA had a strong promoting activity on urinary bladder carcinogenesis and the precipitate containing calcium terephthalate (CaTPA) may also have weak promoting activity on urinary bladder carcinogenesis (Cui et al., 2006). As summarized for the reproductive toxicity study above, oral doses of 930~1,219 mg/kg-day of TPA administered in the diet for 90 days were fetotoxic (Gibson, 1982). The most likely route of potential exposure to IPA is via inhalation during manufacture and use. Therefore, the IPA inhalation study is likely to be more relevant than the oral TPA study for assessing fetotoxicity.
Table 4.
Toxicological data of TPA
| Type | Species | Dose | Route/Period | Remarks | Ref. |
|---|---|---|---|---|---|
| Repeated dose | Rat | 0.05, 0.16, 0.5, 1.6, 5.0% | Oral/15 weeks | - NOAEL: 1.6% (males; 1220 mg/kg, females; 1456 mg/kg) | Amoco Co, 1970 |
| - LOAEL: 5.0% (males; 3837 mg/kg, females; 4523 mg/kg) | |||||
| *Bladder calculi formation, hyperplasia of the bladder epithelium | |||||
| - | Rat | 20 mg/kg/day | - | Lowered serum cholesterol and triglyceride | Hall et al., 1993 |
| - | Rat | 4.0% TPA | Diet/2weeks (P.D.28-42) | Bladder calculi, Aciduria, Urinary excretion of Ca↑ and Mg↑ Serum levels of Ca and Mg↑ (slightly) | Wolkowski-Tyl and Chin, 1983 |
| Carcinogenicity | Rat | 1000 mg/kg/day | Diet/2-year | Increased incidence of bladder calculi, Bladder hyperplasia, and bladder tumors. | CIIT, 1983 |
Toxicological data of IPA.
General toxicity of IPA: In repeated dose studies (Table 5), the target organ is the kidney. In Wistar rats exposed to up to 0.5% IPA in feed (corresponding to a dose of approximately 250 mg/kg/day) for 13 weeks, no adverse effects were observed (Vogin, 1972). Levels of 1.6% (approximately 800 mg/kg/day) in feed produced small increases in the incidence of crystalluria (1/25 males, 2/25 females) and renal pathology (mild hydronephrosis, pelvic calcification, 5/25 males). This study identified an NOAEL and LOAEL of 250 and 800 mg/kg/day, respectively based on kidney effects in rats (Table 5). IPA, 5-carboxy-, 5-hydroxy-, 5- methoxy-, 5-fluoro-, 5-bromo-, 5-cyano-, and 5-methylisoPA were competitive inhibitors with L-glutamate for bovine liver glutamate dehydrogenase. The extent of inhibition by the derived compounds was not much greater than that obtained with the parent compound, IPA. A plot of pKi versus pH showed the presence of an ionizable group (pKa 7.4~7.8) at the enzyme active site which interacted with the substituent at the 5 position of the substituted isophthalates (Boots et al., 1976).
Table 5.
Toxicological data of IPA
| Type | Species | Dose | Route/Period | Remarks | Ref. |
|---|---|---|---|---|---|
| Repeated dose | Rat | 0.5, 1.6, 5.0% (250, 800, 2,500 mg/kg/day) | Oral/13-week | - NOAEL: 250 mg/kg/day, | Vogin, 1972 |
| - LOAEL: 800 mg/kg/day | |||||
| *Slight increase in the incidence of crystalluria, mild hydronephrosis, and pelvic calcification | |||||
| Rat | 1.0, 5.0, 10.0 mg/m3 | Inhalation/4-week (6 hours/day 5 days/week) | NOAEL > 10.0 mg/m3 | IITRI, 1988 | |
Carcinogenicity of IPA: Chronic dietary studies on the structural analogs TPA are available. A two-year feeding study (0, 20, 142 or 1,000 mg/kg/day) showed increased incidence of calculi, bladder hyperplasia and tumors in rats. These effects were seen only at the highest dose of 1,000 mg/kg/day and only in females (CIIT 1983). In a similar study by Gross (1974), bladder and ureter tumors were reported for both males and females. The difference in male tumor response may be partially explained by the higher doses used in the Gross study (500, 1,000 and 2,500 mg/kg/ day). The induction of bladder tumors is believed to be a result of injury to the bladder epithelium from calculi formation. Bladder calculi cannot occur unless the solubility of the stone components is exceeded. Based on urinary solubility of Ca-TPA, normal urine would become saturated with Ca-TPA at a TPA concentration of approximately 8 to 16 mM. Assuming that the average volume of urine excreted by humans is 1.5 liters/day, the amount of terepthalic acid that would have to be absorbed to produce the minimum saturating concentration of TPA is 2,400 mg/day. It is unlikely that humans would ingest enough TPA to induce bladder calculi and if therefore of low concern to human health. Based on similar findings from repeat dose studies with IPA and TPA (crystalluria), it is expected that IPA would respond similarly to that of TPA with respect to carcinogenicity.
EFFECTS ON HUMANS
Biomarkers of exposure to phthalates. It has recently been shown that plasticizers are present in indoor air dust, which may lead to human exposure via the inhalation route. Exposure in the rubber industry is multitudinous and has given rise to several occupational health concerns, including cancer, cardiovascular disease, pulmonary function abnormalities, hypertension, deterioration of intellectual and psychomotor function, nervous system dysfunction and reproductive disorders (Roth, 1999). Urinary levels of PA, a common metabolite of phthalates, were investigated across factories and departments in the contemporary rubber manufacturing industry. This study demonstrated that rubber workers in the contemporary rubber industry are exposed to phthalates as measured by PA. In this case, biological monitoring seems a reasonable approach. However, in the case of PA, attention should be given to individual background levels as this could lead to a substantial overestimation of the occupational contribution to total phthalate exposure (Vermeulen et al., 2005). Phthalates are ubiquitous environmental chemicals and as such, the high frequency of detectable levels is not surprising. Hence, more than 75% of the general US population screened in the NHANES survey had detectable levels of specific phthalate metabolites (Silva et al., 2004). It has been hypothesized that despite the rapid metabolism and elimination of most phthalates, a very stable background concentration may in theory be reached through chronic low-level exposures from dietary ingestion as well as other commonly used products (Duty et al., 2003; Silva et al., 2004). Urinary PA may be useful for the evaluation of phthalic-anhydride exposure even at low levels (Pfaffli, 1986). The toxicity of PAEs has been demonstrated to occur in stored polyvinyl chloride blood bags and in the tissues of patients after extensive blood transfusion (Guess et al., 1967; Jaeger and Rubin, 1970a, b, 1972). Pretreatment for the determination of PA, mono-(2-ethylhexyl) phthalate (MEHP) and di-(2-ethylhexyl)phthalate (DEHP) in human serum or plasma and the determination of these compounds in blood products by high-performance liquid chromatography was studied. About 0.1% of DEHP in a flexible bag was found to have migrated into human platelet plasma. Most of the MEHP and PA detected in human platelet plasma was not derived from the flexible bag but was produced by enzymatic hydrolysis of the migrating DEHP. The amount of DEHP eluted into blood products from the flexible bag differed, depending upon storage time, storage temperature, etc (Shintani, 1985). The study group consisted of 10 stable patients on continuous ambulatory peritoneal dialysis (CAPD) for at least 6 months using a plasticizer-containing PVC PD system. In serum, dialysate and urine, PA was the predominant metabolite of DEHP, but concentrations of MEHP were low. Unlike healthy subjects, peritoneal dialysis (PD) patients do not eliminate DEHP mainly in the form of MEHP or MEHP metabolites. The fact that concentrations of PA in urine exceed by far the respective serum concentrations indicates that PA is secreted by the kidney (Mettang et al., 1999). Although PD patients seem to be exposed to other sources of phthalates in addition to dialysis, use of plasticizer-free devices may help to reduce potentially immunosuppressive exposure to phthalate esters (Mettang et al., 2000). Salivary concentrations of 14 phthalate metabolites were measured in 39 anonymous adult volunteers using isotope-dilution, automated solid phase extraction-high performance liquid chromatography- tandem mass spectrometry. Seven phthalate metabolites were detected (limit of detection, < 1 ng/ml): PA in 18% of saliva samples tested, MMP in 8%, MEP in 38%, MBP in 85%, MiBP in 79%, MBzP in 44% and MEHP in 46% (Silva et al., 2005). These results are in agreement with urine being the matrix of choice for biomonitoring exposure to phthalates and other non-persistent chemicals (Needham et al., 2005) and suggest that saliva may be a good surrogate matrix for blood.
DISCUSSION
Phthalate exposure is believed to be ubiquitous given the widespread use of phthalates in plastics and cosmetic products; however, little is known about the particular sources and patterns of human exposure. Phthalates are non-persistent compounds that are metabolized rapidly. The metabolism of phthalates first produces phthalate monoesters, which can be metabolized further to oxidative products (ATSDR 2002; ATSDR 2001; ATSDR 1997; ATSDR 1995). Many metabolites are glucuronidated and excreted in the urine and feces (ATSDR 2002; ATSDR 2001; ATSDR 1997; ATSDR 1995). At high doses, some phthalates cause reproductive and developmental toxicities in animals exposed during the prenatal period (Ema and Miyawaki, 2001; Gray et al., 2000; Mylchreest et al., 1998; Parks et al., 2000). The active toxicants may be phthalate monoester metabolites (Ema et al., 2003; Ema and Miyawaki, 2002; Gray et al., 2000; Gray and Beamand, 1984; Mylchreest et al., 1998, 1999, 2000). In several studies, phthalate monoester metabolites have been used as markers of exposure (Blount et al., 2000; Silva et al., 2004). These biomarkers represent an integrative measure of phthalate exposure from multiple sources and pathways. Phthalate monoesters--primarily monoethylhexyl phthalate and monobutyl phthalate--are reproductive and developmental toxicants in animals. Accurate measures of phthalate exposure are needed to assess their human health effects. Phthalate monoesters have a biologic half-life of approximately 12 hr, and little is known about the temporal variability and daily reproducibility of urinary measures in humans (Hoppin et al., 2002). Mono(2- ethylhexyl) phthalate (MEHP), one of the metabolites of DEHP, consistently produces developmental, reproductive and hepatic toxicity in laboratory animals (ATSDR 2002; Gray and Beamand, 1984), raising concern about whether human exposure to DEHP approaches the levels of adverse effects found in toxicologic studies. Mono-(2-ethylhexyl) phthalate (MEHP) is a well-characterized Sertoli cell toxicant and is the active toxic metabolite of di-(2-ethylhexyl) phthalate (DEHP). DEHP is widely dispersed throughout the environment due to its use for the production of plastic products (Thomas and Thomas, 1984; Albro, 1987; Boekelheide, 1993). Although Sertoli cells are the direct target of MEHP, the primary consequence of MEHP exposure to rodents is a large increase in germ cell apoptosis (Richburg and Boekelheide, 1996). MEHP, the secondary metabolite of DEHP, which is more suitable biomarker, should be measured in the future. DEHP, DBP and their monoester metabolites appear to have the greatest potential fortoxicity. Phthalates undergo rapid metabolism and in addition to forming specific metabolites, share PA as a common metabolite (Albro et al., 1984). Repeated exposure may cause allergic skin rash, rhinitis, bronchitis and asthma (Chester et al., 1977; Maccia et al., 1976; NIOSH, 1981; Pauli et al., 1980). For a single oral dose of carbonyl labelled PA to rats, 95% of the radioactivity was recovered as PA in the feces and urine. The distribution between feces and the urine was relatively independent of the dose level with 70~80% of the radioactivity in the feces and 20~25% in the urine. No metabolites of PA could be detected in the feces or the urine but a small amount of the PA (0.15%) was converted to carbon dioxide 4 hours after dosing approximately 2% of the radioactivity was distributed throughout the organs and tissues with most of this radioactivity detected in the liver, kidney, spleen and testes. No radioactivity could be detected in these organs 24 hours after dosing (Williams and Blanchifield, 1974). Therefore, PA is not expected to accumulate in the body, based on information for a related acid (TPA). In one study on the metabolism of DEHP, a PA ester, PA was not metabolized by rats and was excreted unchanged in the urine (Forsberg et al., 1997). Bladder and kidney stones have been observed in animal studies following ingestion of very high concentrations of related acids (TPA and IPA). However, the dietary levels at which these effects were observed are very high and are not relevant to occupational exposures. In the case of PA, attention should be given to individual background levels as this could lead to a substantial overestimation of the occupational contribution to total phthalate exposure. In several studies, phthalate monoesters metabolites have been used as markers of exposure (Blount et al., 2000; Silva et al., 2004). However, for several high molecular weight phthalates (for instance DEHP and DOP) no specific metabolites have been unequivocally identified (Kato et al., 2005). Until specific biomarkers of exposure to isomeric phthalates are available, indirect measures of exposure (e.g. PA) to these phthalates might be valuable. Furthermore, in a recent study in the Swedish rubber industry, significant correlations were found between free urinary PA, on the one hand, and mono-ethyl phthalate, monon- butyl phthalate, mono-benzyl phthalate, and mono ethylhexyl phthalate, on the other (Bo A.G. Jonsson, personal communication). Similarly, in a study among anonymous volunteers a strong correlation (r = 0.85) was observed between the concentration of total PA and the sum of the concentration of 13 phthalate metabolites (Albro et al., 1984). From these studies, it can be concluded that indeed hydrolyzed or non-hydrolyzed PA can be used as an indicator of total phthalate body burden. Quantification of the PA produced by hydrolysis of urinary phthalate metabolites has been used as an indirect indicator of exposure to phthalates (Albro et al., 1984). The procedure involves basic hydrolysis of the urine sample (10 ml) with sodium hydroxide (NaOH), acidification of the urine with hydrochloric acid (HCl), liquid-liquid extraction of PA into diethyl ether, esterification of PA, and quantification of the derivatized PA by gas chromatography. One limitation of using PA as an indirect indicator of phthalate exposure is the lack of specificity of PA as a biomarker for any given phthalate. Because phthalates vary greatly in their toxicological properties, PA concentrations cannot be used for risk assessment purposes to estimate the likelihood, magnitude and uncertainty of health risks associated with environmental exposures to phthalates. However, PA concentrations may be useful for establishing the presence of phthalates in the body or as an indirect indicator to estimate the prevalence of total exposure to phthalates (Kato et al., 2005). The several data suggest that PA and secondary metabolites are excreted in the urine predominantly as glucuronide conjugates. Metabolites are believed to be responsible for the biologic activity attributed to phthalate exposure, and metabolite measurements may be more relevant in studies investigating associations between phthalate exposure and adverse health outcomes. Because no information about the biologic activity of the oxidative metabolites is available, are toxicological data on the metabolites of phthalates are urgently needed. Reliable biologic markers will enable application in epidemiologic studies as well as development of questionnaires that identify key predictors of phthalates. Because the toxicologic properties of phthalates vary, biologic markers may be useful for assessing exposure to phthalates and risk assessment.
Additionally, repeated-measures studies are needed to address the temporal variation of phthalate exposure over the course of days, weeks and throughout the year. However, given the good reliability for the four most common phthalates in this study, these biomarkers could be useful tools to estimate human exposure to phthalates, to determine the sources of phthalate exposure and to evaluate potential health effects associated with exposure. In view of these facts, it was concluded that PA shows toxicity in animals. And analysis of urinary PA seems to be useful for the evaluation of phthalic anhydride exposure even at low concentration levels. The analysis of PA may prove valuable when the working conditions of workers with suspected sensitization are assessed. The measurement of phthalate metabolites in people does not by itself mean that phthalates cause disease. Additional research is required to determine whether exposure to phthalates at the levels found in the general population is a cause for health concern.
References
- 1.Agarwal D.K., Lawrence W.H., Nunez L.J., Autian J. Mutagenicity evaluation of phthalic acid esters and metabolites in Salmonella typhimurium cultures. J. Toxicol. Environ. Health. (1985);16:61–69. doi: 10.1080/15287398509530719. [DOI] [PubMed] [Google Scholar]
- 2.Albro P.W., Tomas R., Fishbein L. Metabolism of diethylhexyl phthalate by rats. Isolation and characterization of the urinary metabolites. J. Chromatogr. (1973);76:321–330. doi: 10.1016/S0021-9673(01)96915-8. [DOI] [PubMed] [Google Scholar]
- 3.Albro P.W., Thomas R.O. Enzymatic hydrolysis of di-(2-ethylhexyl) phthalate by lipases. Biochim. Biophys. Acta. Biochim. (1973);306:380–390. doi: 10.1016/0005-2760(73)90176-8. [DOI] [PubMed] [Google Scholar]
- 4.Albro P.W., Moore B. Identification of the metabolites of simple phthalate diesters in rat urine. J. Chromatogr. (1974);94:209–218. doi: 10.1016/S0021-9673(01)92368-4. [DOI] [PubMed] [Google Scholar]
- 5.Albro P.W., Jordan S., Corbett J.T., Schroeder J.L. Determination of total phthalate in urine by gas chromatography. Anal. Chem. (1984);56:247–250. doi: 10.1021/ac00266a029. [DOI] [PubMed] [Google Scholar]
- 6.Albro P.W. The biochemical toxicology of di-(2-ethylhexyl) and related phthalates: Testicular atrophy and hepatocarcinogenesis. Rev. Biochem. Toxicol. (1987);8:73–119. [Google Scholar]
- 7.Amoco Co. Fifteen Week Oral Toxicity of TerePA. Albino Rats. Conducted by Toxicological Evaluations. LSL Study#1358. (1970)
- 8.Amoco Co. Acute Oral Toxicity Study of TerePA in Rats. Conducted by Industrial Bio-Test Laboratories, Inc. IBT Study #601-06339. (1975)
- 9.Amoco Co. Acute Oral Toxicity Study of TerePA in Rats. Conducted by IIT Research Institute. IITRI Study #1557. (1990)
- 10.ATSDR. Toxicological profile for diethyl phthalate (DEP). Agency for Toxic Substances and Disease Registry; Atlanta, GA: (1995). [PubMed] [Google Scholar]
- 11.ATSDR. Toxicological profile for di-n-octyl phthalate (DNOP). Agency for Toxic Substances and Disease Registry; Atlanta, GA: (1997). [Google Scholar]
- 12.ATSDR. Toxicological pro.le for di(2-ethylhexyl)phthalate (DEHP).. U.S. Department of Health and Human Services, Public Health Service. ATSDR; Atlanta GA: (2000). [Google Scholar]
- 13.ATSDR. Toxicological profile for di-n-butyl phthalate (DBP). Agency for Toxic Substances and Disease Registry; Atlanta, GA.: (2001). [PubMed] [Google Scholar]
- 14.ATSDR. Toxicological Profile for Di(2-ethylhexyl)phthalate (DEHP). Agency for Toxic Substances and Disease Registry, Atlanta, GA.; Atlanta, GA: (2002). [PubMed] [Google Scholar]
- 15.Barr D.B., Silva M.J., Kato K., Reidy J.A., Malek N.A., Hurtz D., Sadowski M., Needham L.L., Calafat A.M. New directions in the quantitation of human exposure to phthalates. Environ. Health. Perspect. (2003) doi: 10.1289/ehp.6074.. Online, 24 February 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauer M.J., Herrmann R. Estimation of the environmental contamination by phthalic acid esters leaching from household wastes. Sci. Total Environ. (1997);208:49–57. doi: 10.1016/S0048-9697(97)00272-6. [DOI] [PubMed] [Google Scholar]
- 17.BIBRA working group. Ortho-PA and its sodium and potassium salts. Toxicity profile. The British Industrial Biological Research Association.; (1989). p. 5. [Google Scholar]
- 18.Blount B.C., Silva M.J., Caudill S.P., Needham L.L., Pirkle J.L., Sampson E.J., Lucier G.W., Jackson R.J., Brock J.W. Levels of seven urinary phthalate metabolites in a human reference population. Environ. Health Perspect. (2000);108:979–982. doi: 10.1289/ehp.00108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boekelheide K. Sertoli cell toxicants. In The Sertoli Cell (L.D. Russell and M.D. Griswold, Eds.) Cache River Press; Clearwater, FL: (1993). pp. 551–575. [Google Scholar]
- 20.Boots S.G., Franklin M.A., Dunlavey B., Costello J., Lipsitz C., Boots M.R., Rogers K.S. Synthesis of 5-substituted isoPAs and competitive inhibition studies with bovine liver glutamate dehydrogenase. Proc. Soc. Exp. Biol. Med. (1976);151:316–320. doi: 10.3181/00379727-151-39200. [DOI] [PubMed] [Google Scholar]
- 21.Calandra J.C. IBT No. 601-06339 - Acute Toxicity Studies with Five Test Materials - P.O.No. 99-8-4140. Industrial BIO-TEST Laboratories, Inc.; Northbrook, IL.: (1975). [Google Scholar]
- 22.Carozzi S., Nasini M.G., Schelotto C., Caviglia P.M., Santoni O., Pietrucci A. A biocompatibility study on peritoneal dialysis solution bags for CAPD. Adv. Peritoneal Dial. (1993);9:138–142. [PubMed] [Google Scholar]
- 23.Chin T.Y., Tyl R.W., Popp J.A., Heck H.D. Chemical urolithiasis: 1. Characteristics of bladder stone induction by terePA and dimethyl terephthalate in weanling Fischer-344 rats. Toxicol. Appl. Pharmacol. (1981);58:307–321. doi: 10.1016/0041-008X(81)90435-X. [DOI] [PubMed] [Google Scholar]
- 24.CIIT. Chronic Dietary Administration of TerePA. CIIT Docket 20124. (1983)
- 25.Chester E.H., Schwartz H.J., Payne C.B. Jr., Greenstein S. Phthalic anhydride asthma. Clin. Allergy. (1977);7:15–20. doi: 10.1111/j.1365-2222.1977.tb01419.x. [DOI] [PubMed] [Google Scholar]
- 26.Cooper R.L., Kavlock R.J. Endocrine disruptors and reproductive development: a weight-of-evidence overview. J. Endocrinol. (1997);152:159–166. doi: 10.1677/joe.0.1520159. [DOI] [PubMed] [Google Scholar]
- 27.Cui L., Shi Y., Dai G., Pan H., Chen J., Song L., Wang S., Chang H.C., Sheng H., Wang X. Modification of N-Methyl-N-Nitrosourea initiated bladder carcinogenesis in Wistar rats by terephthalic acid. Toxicol. Appl. Pharmacol. (2006);210:24–31. doi: 10.1016/j.taap.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Duty S.M., Silva M.J., Barr D.B., Brock J.W., Ryan L., Chen Z., Herrick R.F., Christiani D.C., Hauser R. Phthalate exposure and human semen parameters. Epidemiology. (2003);14:269–277. [PubMed] [Google Scholar]
- 29.Ema M., Miyawaki E., Harazono A., Kawashima K. Developmental toxicity evaluation of phthalic acid, one of the metabolites of phthalic acid esters, in rats. Toxicol. Lett. (1997);93:109–115. doi: 10.1016/S0378-4274(97)00078-7. [DOI] [PubMed] [Google Scholar]
- 30.Ema M., Miyawaki E. Effects of monobutyl phthalate on reproductive function in pregnant and pseudopregnant rats. Reprod. Toxicol. (2001);15:261–267. doi: 10.1016/S0890-6238(01)00131-9. [DOI] [PubMed] [Google Scholar]
- 31.Ema M., Miyawaki E. Effects on development of the reproductive system in male offspring of rats given butyl benzyl phthalate during late pregnancy. Reprod. Toxicol. (2002);16:71–76. doi: 10.1016/S0890-6238(01)00200-3. [DOI] [PubMed] [Google Scholar]
- 32.Ema M., Miyawaki E., Hirose A., Kamata E. Decreased anogenital distance and increased incidence of undescended testes in fetuses of rats given monobenzyl phthalate, a major metabolite of butyl benzyl phthalate. Reprod. Toxicol. (2003);17:407–412. doi: 10.1016/S0890-6238(03)00037-6. [DOI] [PubMed] [Google Scholar]
- 33.Forsberg K., et al. Quick selection guide to chemical protective clothing. 3rd ed. Van Nostrand Reinhold.; (1997). [Google Scholar]
- 34.Gray L.E., Ostby J., Furr J., Price M., Veeramachaneni D.N.R., Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol. Sci. (2000);58:350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- 35.Gray T.J., Beamand J.A. Effect of some phthalate esters and other testicular toxins on primary cultures of testicular cells. Food Chem. Toxicol. (1984);22:123–131. doi: 10.1016/0278-6915(84)90092-9. [DOI] [PubMed] [Google Scholar]
- 36.Gibson J.E. A ninety day study of terephthalic acidinduced urolithiasis and reproductive performance in Wistar and CD rats. Research Triangle Institute Experimental Pathology Laboratories, Inc. Chemical Industry Institute of Toxicology.; (1982). [Google Scholar]
- 37.GISAAA. Gigiena i Sanitariya. For English translation. See HYSAAV. (1967);32:12. [Google Scholar]
- 38.Grigas E.O., Ruiz R., Ariado D.M. Cardiopulmonary effects of antimalarial drugs. IV. Terephthalic acid and its dihydroxamic derivative. Toxicol. Appl.Pharmacol. (1971);18:469–486. doi: 10.1016/0041-008X(71)90139-6. [DOI] [PubMed] [Google Scholar]
- 39.Guess W.L., Jacob J., Autian J. A study of polyvinyl chloride blood bag assemblies I Alteration or contamination of ACD solutions. Drug Intelligence. (1967);1:120–127. [Google Scholar]
- 40.Hall I.H., Wong O.T., Reynolds D.J., Simlot R., Chang J.J. Terephthalic Acid in Sprague-Dawley rats as a hypolipidemic agent. Arch. Pharm. (1993);326:5–13. doi: 10.1002/ardp.19933260104. [DOI] [PubMed] [Google Scholar]
- 41.Heindel J.J., Powell C.J. Phthalate ester effects on rat Sertoli cell function-in vitro-effects of phthalate side-chain and age of animal. Toxicol. Appl. Pharmacol. (1992);115:116–123. doi: 10.1016/0041-008X(92)90374-2. [DOI] [PubMed] [Google Scholar]
- 42.Hoshi A., Yanai R., Kuretani K. Effect of terephthalic acid upon the sulfadimethoxine content of blood plasma. Chem. Pharm. Bull.,(Tokyo). (1967);15:1138–1144. doi: 10.1248/cpb.15.1138. [DOI] [PubMed] [Google Scholar]
- 43.Hoshi A., Kuretani K. Metabolism of terephthalic acid. 3. Absorption of terephthalic acid from the gastrointestinal tract and detection of its metabolites. Chem. Pharm. Bull. (1967);15:1979–1984. doi: 10.1248/cpb.15.1979. [DOI] [PubMed] [Google Scholar]
- 44.Hoshi A., Yanai R., Kuretani K. Toxicity of terephthalic acid. Chem. Pharm. Bull. (1968);16:1655–1660. doi: 10.1248/cpb.16.1655. [DOI] [PubMed] [Google Scholar]
- 45.Hoshi A., Kuretani K. Distribution of terephthalic acid in tissues. Chem. Pharm. Bull. (1968);16:131–135. doi: 10.1248/cpb.16.131. [DOI] [PubMed] [Google Scholar]
- 46.Hoppin J.A., Brock J.W., Davis B.J., Baird D.D. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ. Health Perspect. (2002);110:515–518. doi: 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huber W.W., Grasl-Kraupp B., Schulte-Hermann R. Hepatocarcinogenic potential of di(2-ethylhexyl)phthalate in rodents and its implications on human risk. Crit. Rev. Toxicol. (1996);26:365–481. doi: 10.3109/10408449609048302. [DOI] [PubMed] [Google Scholar]
- 48.IITRI, IIT Research Institute. Four-week Inhalation Toxicity Study of Isophthalic acid in Rats. Study No. 1301. (1988)
- 49.IITRI, IIT Research Institute. Acute Oral Toxicity Study of Isophthalic acid in Rats. Study No. 1553. (1990)
- 50.Industrial BIO-TEST Lab. Inc. Report to Amoco Corporation Range-Finding Toxicity Studies on There Test Materials. (1958)
- 51.Jaeger R.J., Rubin R.J. Plasticizers from plastic devices : Excretion, metabolism and accumulation by biological systems. Science. (1970a);178:460–462. doi: 10.1126/science.170.3956.460. [DOI] [PubMed] [Google Scholar]
- 52.Jaeger R.J., Rubin R.J. Contamination of blood stored in plastic packs. Lancet. (1970b);2:151. doi: 10.1016/s0140-6736(70)92734-0. [DOI] [PubMed] [Google Scholar]
- 53.Japan Environment Agency. http://www.env.go.jp/en/chemi/ed/speed98/sp98.html. Strategic Programs on Environmental Endocrine Disruptors '98 (SPEED '98). (1998)
- 54.Jha A.M., Singh A.C., Bharti M. Germ cell mutagenicity of phthalic acid in mice. Mutat. Res. (1998);422:207–212. doi: 10.1016/S0027-5107(98)00151-1. [DOI] [PubMed] [Google Scholar]
- 55.Kato K., Silva M.J., Needham L.L., Calafat A.M. Determination of total phthalates in urine by isotope-dilution liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. (2005);814:355–360. doi: 10.1016/j.jchromb.2004.10.056. [DOI] [PubMed] [Google Scholar]
- 56.Kawaguchi H., Pilbeam C.C., Harrison J.R., Raisz L.G. The role of prostaglandins in the regulation of bone metabolism. Clin. Orthop. Relat. Res. (1995);313:36–46. [PubMed] [Google Scholar]
- 57.Li Z., Zhang C., Wang K., Gu L., Shi A., Gong N., Xu X., He D., Xu J., Wang X. [Changes in the pulmonary function of factory workers exposure to terephthalic acid]. Wei Sheng Yan Jiu. (1999);28:1–3. [PubMed] [Google Scholar]
- 58.Maccia C.A., Bernstein I.L., Emmet E.A., Broaks S.M. In vitro demonstration of specific IgE in phthalic anhydride hypersensitivity. Am. Rev. Resp. Disease. (1976);113:701–704. doi: 10.1164/arrd.1976.113.5.701. [DOI] [PubMed] [Google Scholar]
- 59.Marhold J. Preheld Prumyslove Toxikologie; Organicke Latky. 317. Avecenum; Prague, Czechoslovakia: (1986). [Google Scholar]
- 60.Masui T., Shirai T., Imaida K., Uwagawa S., Fukushima S. Effects of urinary crystals induced by acetazolamide, uracil, and diethylene glycol on urinary bladder carcinogenesis in N-butyl-N-(4-hydroxybutyl)nitrosamine-initiated rats. Toxicol. Lett. (1988);40:119–126. doi: 10.1016/0378-4274(88)90152-X. [DOI] [PubMed] [Google Scholar]
- 61.Mayer F.L., Stalling D.L., Johnson J.L. Phthalate esters as environmental contaminants. Nature. (1972);238:411–413. doi: 10.1038/238411a0. [DOI] [PubMed] [Google Scholar]
- 62.McLachlan J.A. Environmental signaling: what embryos and evolutionteach us about endocrine disrupting chemicals. Endocr. Rev. (2001);22:319–341. doi: 10.1210/er.22.3.319. [DOI] [PubMed] [Google Scholar]
- 63.Mettang T., Alscher D.M., Pauli-Magnus C., Dunst R., Kuhlmann U., Rettenmeier A.W. Phthalic acid is the main metabolite of the plasticizer di(2-ethylhexyl) phthalate in peritoneal dialysis patients. Adv. Perit. Dial. (1999);15:229–233. [PubMed] [Google Scholar]
- 64.Mettang T., Pauli-Magnus C., Alscher D.M., Kirchgessner J., Wodarz R., Rettenmeier A.W., Kuhlmann U. Influence of plasticizer-free CAPD bags and tubings on serum, urine, and dialysate levels of phthalic acid esters in CAPD patients. Perit. Dial. Int. (2000);20:80–84. [PubMed] [Google Scholar]
- 65.Moffitt A.E., Clary J.J., Lewis T.R., Blanck M.D., Perone V.B. Absorption, distribution, and excretion of terePA and dimethylterephthalate. Amer. Ind. Hyg. Assoc. J. (1975);36:633–641. doi: 10.1080/0002889758507303. [DOI] [PubMed] [Google Scholar]
- 66.Mylchreest E., Cattley R.C., Foster P.M.D. Male reproductive tract malformations in rats following gestational and lactational exposure to di(n-butyl) phthalate: an antiandrogenic mechanism? Toxicol. Sci. (1998);43:47–60. doi: 10.1006/toxs.1998.2436. [DOI] [PubMed] [Google Scholar]
- 67.Mylchreest E., Sar M., Cattley R.C., Foster P.M.D. Disruption of androgen-regulated male reproductive development by di(n-butyl) phthalate during late gestation in rats is different from flutamide. Toxicol. Appl. Pharmacol. (1999);156:81–95. doi: 10.1006/taap.1999.8643. [DOI] [PubMed] [Google Scholar]
- 68.Mylchreest E., Wallace D.G., Cattley R.C., Foster P.M.D. Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to di(n-butyl) phthalate during late gestation. Toxicol. Sci. (2000);55:143–151. doi: 10.1093/toxsci/55.1.143. [DOI] [PubMed] [Google Scholar]
- 69.NIOSH, National Institute of Occupational Safety and Health. Occupational health guideline for phthalic anhydride. DHHS(NIOSH) Publication No 81-123, US Department of Labor. Occupational Safety and Health Administration.; (1981). [Google Scholar]
- 70.Needham L.L., Patterson D.G., Barr D.B., Grainger J., Calafat A.M. Uses of speciation techniques in biomonitoring for assessing human exposure to organic environmental chemicals. Anal. Bioanal. Chem. (2005);381:397–404. doi: 10.1007/s00216-004-2975-5. [DOI] [PubMed] [Google Scholar]
- 71.Oishi S., Hiraga K. Testicular Atrophy Induced by phthalic acid Esters: Effect on Testosterone and Zinc Concentrations. Toxicol. Appl. Pharmacol. (1980);53:35–41. doi: 10.1016/0041-008X(80)90378-6. [DOI] [PubMed] [Google Scholar]
- 72.Oishi S., Hiraga K. Effects of Monoesters of Ophthalic acid on serum lipid composition of rats. Toxicol. Lett. (1982);14:79–84. doi: 10.1016/0378-4274(82)90012-1. [DOI] [PubMed] [Google Scholar]
- 73.Okubo T., Suzuki T., Yokoyama Y., Kano K., Kano I. Estimation of estrogenic and anti-estrogenic activities of some phthalate diesters and monoesters by MCF-7 cell proliferation assay in vitro. Biol. Pharm. Bull. (2003);26:1219–1224. doi: 10.1248/bpb.26.1219. [DOI] [PubMed] [Google Scholar]
- 74.Parks L.G., Ostby J.S., Lambright C.R., Abbott B.D., Klinefelter G.R., Barlow N.J., Gray L.E. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol. Sci. (2000);58:339–349. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- 75.Pauli G., Bessot J.C., Kopferschmitt M.C., Lingot G., Wending R., Ducos P., Limasset J.C. Meat wrapper's asthma: identification of the causal agent. Clin. Allergy. (1980);10:263–269. doi: 10.1111/j.1365-2222.1980.tb02106.x. [DOI] [PubMed] [Google Scholar]
- 76.Pavan B., Biondi C., Ferretti M.E., Lunghi L., Paganetto G. Phthalic acid mimics 17beta-estradiol actions in WISH cells. Toxicol. Lett. (2001);118:157–164. doi: 10.1016/S0378-4274(00)00279-4. [DOI] [PubMed] [Google Scholar]
- 77.Perteson E.H., Hendrix W.L., Braddy. L.D. The potentiation of erythromycin and oxytetracycline activity in birds by means of terephthalic acid. Poultry Sci. (1959);38:235–237. [Google Scholar]
- 78.Pfaffli P. Phthalic acid excretion as an indicator of exposure to phthalic anhydride in the work atmosphere. Int. Arch. Occup. Environ. Health. (1986);58:209–216. doi: 10.1007/BF00432103. [DOI] [PubMed] [Google Scholar]
- 79.Phillips B.J., James T.E., Gangolli S.D. Genotoxicity studies of di(2-ethylhexyl)phthalate and its metabolites in CHO cells. Mutat. Res. (1982);102:297–304. doi: 10.1016/0165-1218(82)90139-2. [DOI] [PubMed] [Google Scholar]
- 80.Pilbeam C.C., Raisz L.G., Voznesensky O., Alander C.B., Delman B.N., Kawaguchi H. Autoregulation of inducible prostaglandin G/H synthase in osteoblastic cells by prostaglandins. J. Bone Miner. Res. (1995);10:406–414. doi: 10.1002/jbmr.5650100311. [DOI] [PubMed] [Google Scholar]
- 81.Price K.E., Zolli Z. The influence of terephthalic acid on oxytetracycline serum levels in chicken studies on mode of action. I. Avian Diseases. (1958);157:157–169. [Google Scholar]
- 82.Raisz L.G. Physiologic and pathologic roles of prostagladins and other eicosanoids in bone metabolism. J. Nutr. (1995);125:2024S–2027S. doi: 10.1093/jn/125.suppl_7.2024S. [DOI] [PubMed] [Google Scholar]
- 83.Raisz L.G., Fall P.M., Gabbitas B.Y., McCarthy T.L., Kream B.E., Canalis E. Effects of prostaglandin E2 on bone formation in cultured fetal rat calvariae: role of insulinlike growth factor-1. Endocrinology. (1993);133:1504–1510. doi: 10.1210/en.133.4.1504. [DOI] [PubMed] [Google Scholar]
- 84.Richburg J.H., Boekelheide K. Mono-(2-ethylhexyl) phthalate rapidly alters both Sertoli cell vimentin filaments and germ cell apoptosis in young rat testes. Toxicol. Appl. Pharmacol. (1996);137:42–50. doi: 10.1006/taap.1996.0055. [DOI] [PubMed] [Google Scholar]
- 85.Roth V.S. Rubber industry epidemiology. Occup. Med. (1999);14:849–856. [PubMed] [Google Scholar]
- 86.Sharman M., Read W.A., Castle L., Gilbert J. Levels of di-(2-ethylhexyl)phthalate and total phthalate esters in milk, cream, butter and cheese. Food Addit. Contam. (1994);11:375–385. doi: 10.1080/02652039409374236. [DOI] [PubMed] [Google Scholar]
- 87.Sharpe R.M. Natural and anthropogenic environmental oestrogens: the scientific basis for risk assessment. Pure Appl. Chem. (1998);70:1685–1701. doi: 10.1351/pac199870091685. [DOI] [Google Scholar]
- 88.Shemiakin M.M., Schukina L.A. Experimental corroboration of the mechanism of biological action of quinones of the type of vitamin K. Nature. (1944);154:513. doi: 10.1038/154513a0. [DOI] [Google Scholar]
- 89.Shintani H. Determination of phthalic acid, mono-(2-ethylhexyl) phthalate and di-(2-ethylhexyl)phthalate in human plasma and in blood products. J. Chromatogr. (1985);337:279–290. doi: 10.1016/0378-4347(85)80041-4. [DOI] [PubMed] [Google Scholar]
- 90.Silva M.J., Malek N.A., Hodge C.C., Reidy J.A., Kato K., Barr D.B., Needham L.L., Brock J.W. Improved quantitative detection of 11 urinary phthalate metabolites in humans using liquid chromatography-atmospheric pressure chemical ionization .tandem mass spectrometry. J. Chromatogr. B. (2003);789:393–404. doi: 10.1016/S1570-0232(03)00164-8. [DOI] [PubMed] [Google Scholar]
- 91.Silva M.J., Barr D.B., Reidy J.A., Malek N.A., Hode C.C., Caudill S.P., Brock J.W., Needham L.L., Calafat A.M. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ. Health Perspect. (2004);112:331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Silva M.J., Reidy J.A., Samandar E., Herbert A.R., Needham L.L., Calafat A.M. Detection of phthalate metabolites in human saliva. Arch. Toxicol. (2005);79:647–652. doi: 10.1007/s00204-005-0674-4. [DOI] [PubMed] [Google Scholar]
- 93.Staples C.A., Peterson D.R., Parkerton T.F., Adams W.J. The environmental fate of phthalate esters: a literature review. Chemosphere. (1997);35:667–749. doi: 10.1016/S0045-6535(97)00195-1. [DOI] [Google Scholar]
- 94.Tavares I.A., Vine N.D. Phthalic acid esters inhibit arachidonate metabolism by rat peritoneal leucocytes. J. Pharm. Pharmacol. (1985);37:67–68. doi: 10.1111/j.2042-7158.1985.tb04936.x. [DOI] [PubMed] [Google Scholar]
- 95.Thomas J.A., Thomas M.J. Biological effects of di-(2-ethylhexyl) phthalate and other phthalic acid esters. Crit. Rev. Toxicol. (1984);13:283–317. doi: 10.3109/10408448409023761. [DOI] [PubMed] [Google Scholar]
- 96.TOLED5. (Elsevier Science Pub.B.V., POB211, 1000AE Amsterdam, Netherlands) V.1- 1977-. (1997);93:109. doi: 10.1016/S0378-4274(97)00078-7. http://www.ccohs.ca/products/databases/samples/rtecs.html. [DOI] [Google Scholar]
- 97.Vermeulen R., Jonsson B.A., Lindh C.H., Kromhout H. Biological monitoring of carbon disulphide and phthalate exposure in the contemporary rubber industry. Int. Arch. Occup. Environ. Health. (2005);78:663–669. doi: 10.1007/s00420-005-0017-z. [DOI] [PubMed] [Google Scholar]
- 98.Verrett M.J., Mutchler M.K., Scott W.F., Reynaldo E.F., McLaughlin. Teratogenic effects of captan and related compounds in the developing chicken embryo. Ann. N. Y. Acad. Sci. (1969);160:334–343. doi: 10.1111/j.1749-6632.1969.tb15853.x. [DOI] [PubMed] [Google Scholar]
- 99.Williams D.T., Blanchfield B.J. Retention, excretion, and metabolism of phthalic acid administered orally to the rat. Bull. Environ. Contam. Toxicol. (1974);12:109–112. doi: 10.1007/BF01713035. [DOI] [PubMed] [Google Scholar]
- 100.Wolkowski-Tyl R., Chin T.Y., Heck H.D. Chemical urolithiasis. III. Pharmacokinetics and transplacental transport of terephthalic acid in Fischer-344 rats. Drug. Metab. Dispos. (1982);10:486–490. [PubMed] [Google Scholar]
- 101.Wolkowski-Tyl R., Chin T.Y. Effects of selected therapeutic agents on urolithiasis induced by terephthalic acid in the male weanling Fischer 344 rat. Fundam. Appl. Toxicol. (1983);3:552–558. doi: 10.1016/S0272-0590(83)80103-1. [DOI] [PubMed] [Google Scholar]