Abstract
The aim of this study was to evaluate the single oral dose toxicity of Bupleuri Radix (BR) aqueous extracts, it has been traditionally used as anti-inflammatory agent, in male and female mice. BR extracts (yield = 16.52%) was administered to female and male ICR mice as an oral dose of 2,000, 1,000 and 500 mg/kg (body weight) according to the recommendation of Korea Food and Drug Administration (KFDA) Guidelines. Animals were monitored for the mortality and changes in body weight, clinical signs and gross observation during 14 days after dosing, upon necropsy; organ weight and histopathology of 14 principal organs were examined. As the results, no BR extracts treatment related mortalities, clinical signs, changes on the body and organ weights, gross and histopathological observations against 14 principal organs were detected up to 2,000 mg/kg in both female and male mice, except for soft feces and related body weight decrease detected in male mice treated with 2,000 mg/kg. Therefore, LD50 (50% lethal dose) and approximate LD of BR aqueous extracts after single oral treatment in female and male mice were considered over 2000 mg/kg, respectively. Although it was also observed that the possibilities of digestive disorders, like soft feces when administered over 2,000 mg/kg of BR extracts in the present study, these possibilities of digestive disorders can be disregard in clinical use because they are transient in the highest dosages male only.
Keywords: Bupleuri Radix, Single oral dose toxicity, Mouse, Histopathology
INTRODUCTION
As increase of the concern in the functional food and well being in life, the demands and consumption of functional food originated from natural sources are increased (Lee et al., 2003). However, the toxicological aspects about these natural origin-functional foods have been neglected because of the reasons that they have been used as various purposes for long times (Roh and Ku, 2010). Therefore, it is considered that more detail and systemic toxicological studies should be tested for prevention of abuse and potential toxicities even if they have been used as traditional folk medicine.
Bupleuri Radix (BR) is a dried root of Bupleurum falcatum Linne (Family: Umbelliferae), and it has been traditionally used as anti-inflammatory agent in Korea (Lee et al., 2009; Cho et al., 2010). Saikosaponins are isolated as active ingredients of BR and also showed potent anti-inflammatory activities (Yamamoto et al., 1975a,b; Abe et al., 1980). BR has been showed anti-inflammatory (Lin et al., 1990; Hattori et al., 1991), immunomodulatory (Sakurai et al., 1999; Guo et al., 2000), anti-ulcerative (Matsumoto et al., 2002), platelet activation inhibitory (Chang and Hsu, 1991), corticosterone secretory (Nose et al., 1989), hepatoprotective (Abe et al., 1982) and nephroprotective (Niikawa et al., 1990; Hattori et al., 1991) activities. However, no detailed toxicological assessment of BR has been reported even mouse single oral dose toxicity test.
The objectives of the present study, therefore, were to obtain the primary safety information about BR aqueous extracts, and further to clarify their safety for clinical use. In order to observe the 50% lethal dose (LD50) and approximate lethal dosage (ALD), test articles were once orally administered to female and male mice at dose levels of 2000, 1000, 500, 250 and 125 mg/kg (body wt.) according to the recommendation of KFDA Guidelines (2009). The mortality and changes on body weight, clinical signs and gross observation were monitored during 14 days after oral administration of BR extracts with organ weights and histopathology of principal organs.
MATERIALS AND METHODS
Experimental animals. Each of thirty female and male ICR mice (6-wk old upon receipt, SLC, Japan) was used after acclimatization for 7 days. Animals were allocated five per polycarbonate cage in a temperature (20~25℃) and humidity (45~50%) controlled room. Light : dark cycle was 12 hrs : 12 hrs, and feed (Samyang, Korea) and water were supplied free to access. All animals were overnight fasted before dosing and terminal necropsy. Animals were marked by picric acid. This study was carried out with prior approval of the Animal Ethical Committee, The University of Daegu Haany University (Gyeongsan, Korea)
Preparation and administration of BR aqueous extracts. Aqueous BR extracts (yield = 16.52%) were prepared by routine methods using rotary vacuum evaporator (Buchi Rotavapor R-144, Switzerland) and programmable freeze dryer (Freezone 1; Labconco Corp., MO, USA) from dried root of Bupleurum falcatum, which were purchased from Omniherb (Korea) after confirming the morphology under microscopy. In the present study, prepared herbs were boiled at 80℃, 3 hrs and then, evaporated and lysophilized. Powders of BR extracts are light brown color. BR extracts were stored in a refrigerator at -20℃ to protect from light and degeneration, and they are well soluble upto 200 mg/ml concentration levels in distilled water used as vehicle as clear light brown solution. The test article was single orally administered at a dosage volume of 20 ml/kg using distilled water as vehicle at 2,000, 1,000 and 500 mg/kg dose levels.
Abnormal behavior, clinical sign and body weight. All abnormal clinical signs and behaviors were recorded before and after dosing at least twice a day based on the functional observational battery test (Irwin, 1968; Dourish, 1987). Body weights were measured on the day of dosing (Day 0) prior to treatment, 1, 2, 7, 13 and 14 days after dosing. In addition, to reduce the differences from individual body weight changes of animals at treatment, body weight gains during Day 0~Day 7, Day 7~Day 13 and Day 0~Day 14 was also calculated based on measured body weight at each point.
Necropsy. All unscheduled died animals were grossly observed immediately after finding them and all survived animals were subjected to terminal necropsy. Animals were asphyxiated by carbon dioxide and gross necropsy was performed in all animals at Day 14 after overnight fasting (about 18 h, water was not restricted).
Final organ weight measurements and sampling. The final absolute organ weight was measured and then the final relative organ weight (% for body weight) was calculated. The following organs were collected for histopathological observation.
Measured and sampled organs: lung, heart, thymus, left kidney, left adrenal gland, spleen, left testis or ovary, liver, splenic lobe of pancreas, brain, left epididymis or total uterus and left submandibular lymph node.
Histopathology. Samples were fixed in 10% neutral buffered formalin. After 18 hrs of fixation, paraffin embedding was conducted and 4 μm sections were prepared using rotary microtome (M1R rotary microtome, Shandon, UK). Representative sections of each specified organs were stained with hematoxylin-eosin and observed under light microscope (E400, Nikon, Japan), magnification X40- 400.
Statistical analyses. Multiple comparison tests for different dose groups were conducted. Variance homogeneity was examined using the Levene test. If the Levene test indicated no significant deviations from variance homogeneity, the obtain data were analyzed by one way ANOVA test followed by Scheffe test to determine which pairs of group comparison were significantly different. In case of significant deviations from variance homogeneity were observed at Levene test, a non-parametric comparison test, the Mann- Whitney U test was conducted to determine the specific pairs of group comparison, which are significantly different. LD50 and 95% confidence limits were calculated by Probit method. Statistical analyses were conducted using SPSS for Windows (Release 14.0K, SPSS Inc., USA) and a p-value of less than 0.05 was considered to be a significant difference. In addition, degree of clinical signs, gross and histopathological findings were subdivided into 3 degrees: 3+ Severe, 2+ moderate, 1+ slight.
RESULTS
Mortalities. No unscheduled or BR extracts-treatment related mortalities were detected in all dose levels tested in this study. At termination, all of animals (5/5; 100%) were survived in all dose levels tested including vehicle control.
Clinical signs. In this study, no BR extracts treatment related abnormal clinical signs were observed during observation periods regardless of male and female mice, except for soft feces (Fig. 1), which were detected in four (4/5; 80%) male mice treated with 2,000 mg/kg restricted to the treatment day.
Fig. 1. Soft feces detected as clinical signs after single oral treatment of BR extracts. Note that slight soft feces were detected as BR extract treatment related clinical signs in four (4/5; 80%) male mice treated with 2,000mg/kg at treatment day; they recovered to normal from 2 days after end of oral treatment.

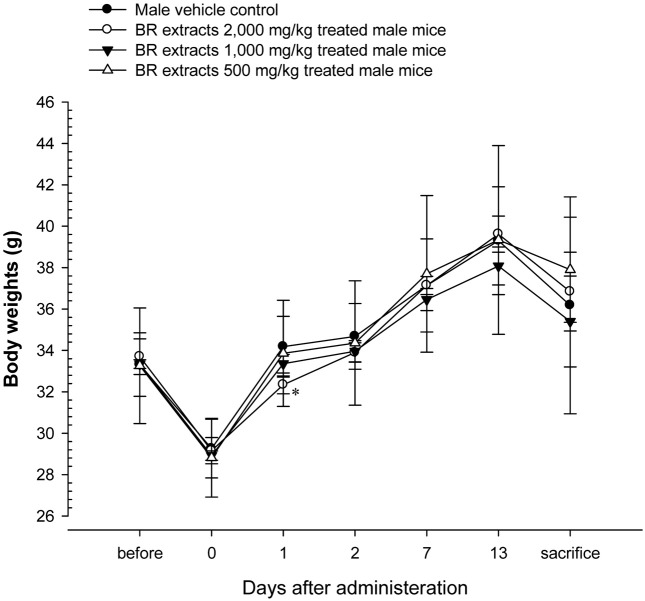
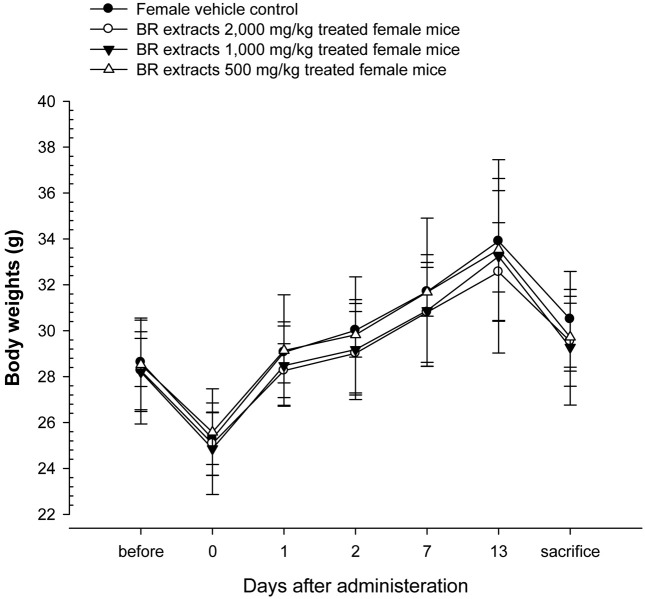
Changes on body weights and gains. No significant changes in body weight were detected compared to that of vehicle control in all dose levels tested except for significant (p < 0.05) decrease of body weights detected in BR extracts 2,000 mg/kg treated male mice at 1 day after treatment as compared with male vehicle control mice (Table 1, Fig. 2 and 3).
Table 1.
Body weight gains after oral treatment of BR extracts
| Group | Intervals | ||
|---|---|---|---|
| Day 0a~Day 7 | Day 7~Day 13 | Day 0~Day 14b | |
| Male | |||
| Vehicle control | 7.88 ± 1.11 | 2.16 ± 0.40 | 6.92 ± 5.87 |
| 2,000 mg/kg | 7.98 ± 0.80 | 2.48 ± 0.73 | 7.68 ± 2.40 |
| 1,000 mg/kg | 7.54 ± 0.38 | 1.62 ± 0.56 | 6.48 ± 2.17 |
| 500 mg/kg | 8.88 ± 1.98 | 1.64 ± 0.87 | 9.08 ± 2.31 |
| Female | |||
| Vehicle control | 6.40 ± 0.48 | 2.20 ± 1.48 | 5.20 ± 2.74 |
| 2,000 mg/kg | 5.72 ± 1.69 | 1.76 ± 1.68 | 4.46 ± 2.84 |
| 1,000 mg/kg | 6.02 ± 0.75 | 2.36 ± 2.25 | 4.42 ± 2.08 |
| 500 mg/kg | 6.10 ± 1.57 | 1.86 ± 0.54 | 4.14 ± 2.10 |
Values are expressed as mean ± SD, g of five mice.
aDay of treatment after overnight fasted.
bDay of sacrifice after overnight fasted.
Fig. 2. Body weight changes in male mice after once oral administration of BR extracts. No meaningful changes were detected in all BR extracts treated groups as compared with vehicle control, except for significant decreases of body weights detected in 2,000mg/kg at 1 day after treatment. Before means 1 day before administration; Day 0 means at administration; All animals at sacrifice and Day 0 overnight fasted; *p< 0.05 as compared with vehicle control by MW test.
Fig. 3. Body weight changes in female mice after once oral administration of BR extracts. No meaningful changes were detected in all BR extracts treated groups as compared with vehicle control. Before means 1 day before administration; Day 0 means at administration; All animals at sacrifice and Day 0 overnight fasted.
Changes on the final organ weight. No meaningful changes on the final absolute and relative organ weight of principal organs were observed in all BR extracts treated female and male mice as compared with each equal gender of vehicle control, except for significant (p < 0.01) increases of absolute testis weights restricted to 1,000 mg/kg of BR extracts treated male mice as compared with equal genders of vehicle control mice in the present study (Table 2, 3).
Table 2.
Changes on the final absolute organ weights after oral treatment of BR extracts
| Dose (mg/kg) | Organs: Male | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung | Heart | Thymus | Kidney L | Adrenal gland L | Spleen | Testis L | Liver | Pancreas S | Brain | Epididymis L | Lymph node La | |
| 0 | 0.177 ± 0.017 | 0.164 ± 0.010 | 0.048 ± 0.009 | 0.272 ± 0.022 | 0.004 ± 0.002 | 0.107 ± 0.016 | 0.108 ± 0.008 | 1.447 ± 0.113 | 0.161 ± 0.017 | 0.469 ± 0.024 | 0.045 ± 0.003 | 0.003 ± 0.001 |
| 2,000 | 0.186 ± 0.009 | 0.161 ± 0.009 | 0.064 ± 0.017 | 0.271 ± 0.021 | 0.005 ± 0.002 | 0.116 ± 0.022 | 0.120 ± 0.018 | 1.436 ± 0.076 | 0.147 ± 0.012 | 0.477 ± 0.030 | 0.042 ± 0.004 | 0.004 ± 0.003 |
| 1,000 | 0.188 ± 0.008 | 0.159 ± 0.016 | 0.059 ± 0.011 | 0.254 ± 0.036 | 0.003 ± 0.001 | 0.117 ± 0.016 | 0.123 ± 0.005* | 1.391 ± 0.068 | 0.161 ± 0.019 | 0.467 ± 0.015 | 0.047 ± 0.004 | 0.003 ± 0.001 |
| 500 | 0.171 ± 0.021 | 0.154 ± 0.020 | 0.064 ± 0.021 | 0.267 ± 0.038 | 0.005 ± 0.002 | 0.098 ± 0.022 | 0.118 ± 0.025 | 1.414 ± 0.215 | 0.155 ± 0.027 | 0.467 ± 0.034 | 0.039 ± 0.006 | 0.004 ± 0.003 |
| Dose (mg/kg) | Organs: Female | |||||||||||
| Lung | Heart | Thymus | Kidney L | Adrenal gland L | Spleen | Testis L | Liver | Pancreas S | Brain | Epididymis L | Lymph node La | |
| 0 | 0.172 ± 0.009 | 0.133 ± 0.009 | 0.078 ± 0.027 | 0.191 ± 0.009 | 0.008 ± 0.004 | 0.134 ± 0.030 | 0.016 ± 0.002 | 1.267 ± 0.022 | 0.155 ± 0.016 | 0.469 ± 0.023 | 0.143 ± 0.038 | 0.005 ± 0.004 |
| 2,000 | 0.173 ± 0.013 | 0.136 ± 0.020 | 0.070 ± 0.023 | 0.184 ± 0.039 | 0.006 ± 0.002 | 0.139 ± 0.029 | 0.016 ± 0.002 | 1.176 ± 0.164 | 0.161 ± 0.016 | 0.477 ± 0.020 | 0.164 ± 0.062 | 0.005 ± 0.001 |
| 1,000 | 0.172 ± 0.023 | 0.136 ± 0.011 | 0.066 ± 0.024 | 0.186 ± 0.033 | 0.006 ± 0.001 | 0.122 ± 0.040 | 0.020 ± 0.004 | 1.171 ± 0.190 | 0.163 ± 0.031 | 0.474 ± 0.026 | 0.129 ± 0.036 | 0.007 ± 0.007 |
| 500 | 0.175 ± 0.015 | 0.141 ± 0.015 | 0.073 ± 0.023 | 0.177 ± 0.020 | 0.005 ± 0.003 | 0.121 ± 0.031 | 0.015 ± 0.006 | 1.172 ± 0.194 | 0.153 ± 0.017 | 0.477 ± 0.033 | 0.151 ± 0.027 | 0.005 ± 0.004 |
Values are expressed as mean ± SD, g of five mice.
L, left sides; S, splenic lobes; aSubmandibular lymph node.
*p < 0.01 compared with male vehicle control by MW test.
Table 3.
Changes on the final relative organ weights after oral treatment of BR extracts
| Dose (mg/kg) | Organs: Male | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung | Heart | Thymus | Kidney L | Adrenal gland L | Spleen | Testis L | Liver | Pancreas S | Brain | Epididymis L | Lymph node La | |
| 0 | 0.497 ± 0.089 | 0.462 ± 0.091 | 0.136 ± 0.034 | 0.768 ± 0.141 | 0.010 ± 0.006 | 0.301 ± 0.068 | 0.304 ± 0.059 | 4.075 ± 0.739 | 0.456 ± 0.095 | 1.320 ± 0.212 | 0.125 ± 0.015 | 0.009 ± 0.003 |
| 2,000 | 0.506 ± 0.030 | 0.437 ± 0.019 | 0.176 ± 0.054 | 0.736 ± 0.059 | 0.013 ± 0.006 | 0.316 ± 0.062 | 0.326 ± 0.057 | 3.903 ± 0.262 | 0.402 ± 0.051 | 1.298 ± 0.092 | 0.113 ± 0.016 | 0.011 ± 0.009 |
| 1,000 | 0.534 ± 0.045 | 0.448 ± 0.039 | 0.167 ± 0.027 | 0.717 ± 0.092 | 0.009 ± 0.004 | 0.334 ± 0.063 | 0.349 ± 0.034 | 3.942 ± 0.337 | 0.453 ± 0.038 | 1.322 ± 0.080 | 0.134 ± 0.016 | 0.009 ± 0.004 |
| 500 | 0.454 ± 0.063 | 0.407 ± 0.045 | 0.169 ± 0.052 | 0.706 ± 0.092 | 0.012 ± 0.004 | 0.256 ± 0.049 | 0.310 ± 0.057 | 3.732 ± 0.501 | 0.409 ± 0.070 | 1.236 ± 0.123 | 0.103 ± 0.014 | 0.011 ± 0.007 |
| Dose (mg/kg) | Organs: Female | |||||||||||
| Lung | Heart | Thymus | Kidney L | Adrenal gland L | Spleen | Ovary L | Liver | Pancreas S | Brain | Uterus | Lymph node La | |
| 0 | 0.565 ± 0.042 | 0.437 ± 0.038 | 0.257 ± 0.087 | 0.630 ± 0.056 | 0.026 ± 0.013 | 0.441 ± 0.100 | 0.054 ± 0.008 | 4.171 ± 0.325 | 0.513 ± 0.087 | 1.544 ± 0.126 | 0.475 ± 0.158 | 0.018 ± 0.012 |
| 2,000 | 0.590 ± 0.076 | 0.463 ± 0.093 | 0.236 ± 0.074 | 0.628 ± 0.146 | 0.019 ± 0.007 | 0.471 ± 0.092 | 0.056 ± 0.010 | 4.006 ± 0.704 | 0.548 ± 0.083 | 1.623 ± 0.161 | 0.555 ± 0.205 | 0.016 ± 0.002 |
| 1,000 | 0.589 ± 0.083 | 0.466 ± 0.059 | 0.225 ± 0.076 | 0.639 ± 0.124 | 0.022 ± 0.004 | 0.419 ± 0.132 | 0.069 ± 0.018 | 4.016 ± 0.669 | 0.563 ± 0.130 | 1.631 ± 0.205 | 0.449 ± 0.158 | 0.022 ± 0.021 |
| 500 | 0.591 ± 0.058 | 0.475 ± 0.052 | 0.243 ± 0.068 | 0.597 ± 0.080 | 0.017 ± 0.010 | 0.410 ± 0.116 | 0.052 ± 0.019 | 3.955 ± 0.724 | 0.514 ± 0.061 | 1.612 ± 0.173 | 0.510 ± 0.105 | 0.017 ± 0.013 |
Values are expressed as mean ± SD, % of body weight at sacrifice of five mice.
L, left sides; S, splenic lobes; aSubmandibular lymph node.
Necropsy findings. No BR extracts-treatment related changes on the gross findings were observed as compared with equal gender of vehicle control except for some sporadic findings such as slight (1+) congestion spots of lung, atrophy of thymus, cyst in kidney, spleen atrophy or hypertrophy, hypertrophy of submandibular lymph node and edematous changes of uterus, which were sporadically detected throughout all experimental groups tested in the present including both gender of vehicle control (Table 4).
Table 4.
Necropsy findings after oral treatment of BR extracts
| Dose (mg/kg) | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2,000 | 1,000 | 500 | 0 | 2,000 | 1,000 | 500 | |
| Lung | ||||||||
| Normal | 3/5 | 4/5 | 3/5 | 4/5 | 3/5 | 4/5 | 5/5 | 5/5 |
| Congestion | 2/5 | 1/5 | 2/5 | 1/5 | 2/5 | 1/5 | 0/5 | 0/5 |
| Thymus | ||||||||
| Normal | 4/5 | 5/5 | 5/5 | 5/5 | 5/5 | 4/5 | 5/5 | 5/5 |
| Atrophy | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 |
| Spleen | ||||||||
| Normal | 4/5 | 5/5 | 3/5 | 3/5 | 3/5 | 4/5 | 4/5 | 4/5 |
| Atrophy | 1/5 | 0/5 | 2/5 | 2/5 | 1/5 | 1/5 | 1/5 | 1/5 |
| Hypertrophy | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 | 0/5 |
| LNa | ||||||||
| Normal | 3/5 | 3/5 | 4/5 | 3/5 | 2/5 | 4/5 | 4/5 | 3/5 |
| Hypertrophy | 2/5 | 2/5 | 1/5 | 2/5 | 2/5 | 1/5 | 1/5 | 2/5 |
| Congestion | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 | 0/5 |
| Uterus | ||||||||
| Normal | 3/5 | 3/5 | 5/5 | 2/5 | ||||
| Edema | 2/5 | 2/5 | 0/5 | 3/5 | ||||
Observed animals/total observed animals (five mice).
aBilateral submandibular lymph node.
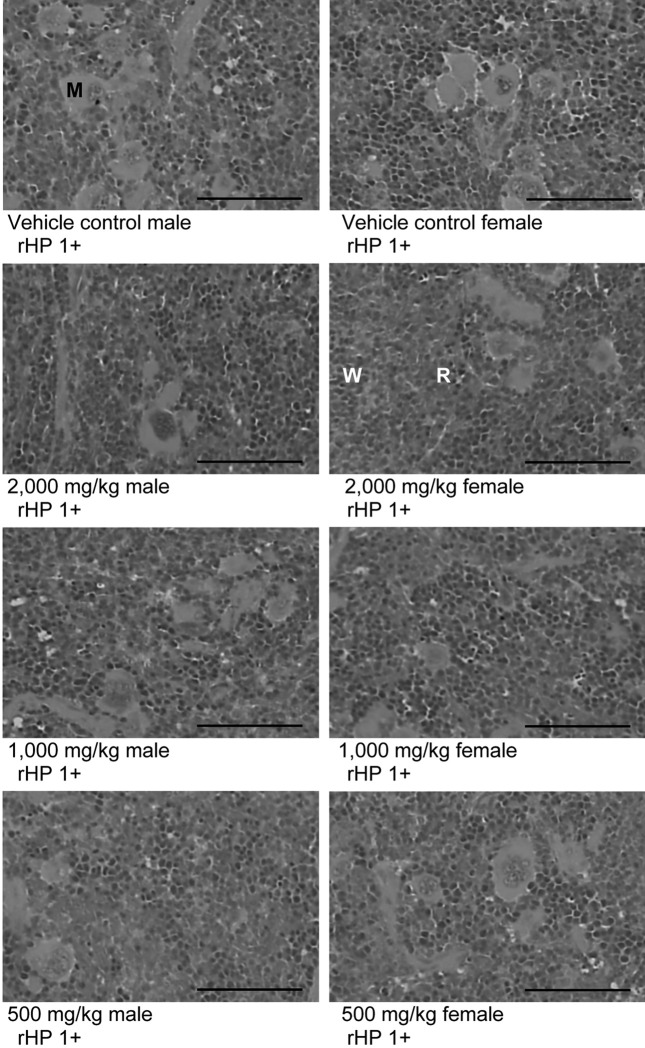
Histopathological findings. No meaningful changes on the histopathological findings of 14 principal organs were observed in BR extracts treated groups as compared with equal gender of vehicle control except for some sporadic findings such as slight (1+) hypertrophy of lung alveolus wall with focal hemorrhage, decreases of lymphoid cells in the cortex of thymus, cyst formation in the kidney, hyperplasia of lymphoid cells in the red pulp of spleen (Fig. 4), focal inflammatory cell infiltration in the liver, edematous changes on the uterus, and cyst and diffused hyperplasia of lymphoid cells in the submandibular lymph node, which were sporadically detected throughout all experimental groups tested in the present study including both gender vehicle controls (Table 5).
Fig. 4. Histopathological changes detected on the spleen after single oral treatment of BR aqueous extracts. Note that slight (1+) hyperplasia of lymphoid cells in the red pulp (rHP) of spleen was randomly detected with/without megakaryocytic hyperplasia throughout the whole experimental groups including both genders of vehicle control as sporadic finings not BR treatment related toxicological signs. M, megakaryocyte; W, white pulp; R, red pulp; BR, Bupleuri Radix; All Hematoxylin & Eosin stain; Scale bars = 80 μm.
Table 5.
Histopathological findings after oral treatment of BR extracts
| Dose (mg/kg) | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2,000 | 1,000 | 500 | 0 | 2,000 | 1,000 | 500 | |
| Lung | ||||||||
| Normal | 3/5 | 4/5 | 3/5 | 4/5 | 4/5 | 4/5 | 5/5 | 5/5 |
| Congestion | 2/5 | 1/5 | 2/5 | 1/5 | 1/5 | 1/5 | 0/5 | 0/5 |
| Thymus | ||||||||
| Normal | 4/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 |
| cDE* | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Kidney | ||||||||
| Normal | 4/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 |
| Cyst formation | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Spleen | ||||||||
| Normal | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 |
| rHP* | 1/5 | 1/5 | 1/5 | 1/5 | 1/5 | 1/5 | 1/5 | 1/5 |
| Liver | ||||||||
| Normal | 5/5 | 5/5 | 4/5 | 5/5 | 3/5 | 4/5 | 4/5 | 5/5 |
| IF* | 0/5 | 0/5 | 1/5 | 0/5 | 2/5 | 1/5 | 1/5 | 0/5 |
| LNa | ||||||||
| Normal | 3/5 | 3/5 | 4/5 | 4/5 | 2/5 | 4/5 | 4/5 | 3/5 |
| dHP* | 2/5 | 2/5 | 1/5 | 1/5 | 2/5 | 1/5 | 1/5 | 2/5 |
| Congestion | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 | 0/5 |
| Uterus | ||||||||
| Normal | 4/5 | 5/5 | 5/5 | 4/5 | ||||
| DM* | 1/5 | 0/5 | 0/5 | 1/5 | ||||
Observed animals/total observed animals (five mice).
aLeft submandibular lymph node.
*Abbreviations: cDE, decreases of cortex lymphoid cells; rHP, hyperplasia of lymphoid cells in the red pulp; IF, focal inflammatory cell infiltration; dHP, diffused lymphoid cell hyperplasia; DM, desquamation of the mucosa.
DISCUSSION
In the present study, we investigated the single oral dose toxicity of BR extracts on the mice as a part of the safety test. In order to observe LD50 and ALD, test substances were administered orally to female and male ICR mice at dose levels of 2,000, 1,000 and 500 mg/kg. We could not find any BR extracts treatment related mortalities, clinical signs, changes on the body and organ weights, gross and histopathological observations against 14 principal organs up to 2,000 mg/kg in both female and male mice, except for soft feces and related body weight decrease detected in male mice treated with 2,000 mg/kg, significant (p < 0.01) increase of the absolute testis weights in 1,000 mg/kg treated male mice, and some sporadic accidental gross and histopathological findings.
In KFDA Guidelines (2009-116, 2009), the recommended highest dose of test materials were 2,000 mg/kg or the maximum solubility, and they also recommended that in case of single dose toxicity in mouse, the dosage volume were below 20 ml/kg (Flecknell, 1996). In the present study, the highest dosage was selected as 2,000 mg/kg in a volume of 20 ml/kg, the recommended oral dose volume in mice (Flecknell, 1996) and the limited highest dosages recommended by KFDA Guidelines (2009-116, 2009), and 1,000 and 500 mg/kg are selected using common ratio 2. In addition, each female and male vehicle control groups were added. Test material was orally administered using distilled water as vehicle in the present study.
Soft feces detected in 4 (4/5; 80%) mice treated with BR extracts 2,000 mg/kg were considered as treatment related toxicological signs, and BR extracts may be induced the digestive disorders, like soft feces when administered over 2,000 mg/kg of BR extracts. However, these possibilities of digestive disorders can be disregard in clinical use because they are transient in the highest dosages male only. Slight but significant (p < 0.05) decreases of body weights detected in male 2,000 mg/kg treated mice restricted to 1 day after administration were considered as secondary changes from soft feces detected as clinical signs. Anyway all animals including 2,000 mg/kg treated male mice showed body weight increases ranged in normal age-matched mice (Plata and Murphy, 1972; Yamaguchi et al., 1983).
Increases of the final absolute testis weights restricted to the BR extracts 1,000 mg/kg treated male mice as compared with equal genders of vehicle control, are not considered as BR extracts treatment related toxicological signs because they did not showed any dosage-dependent changes and no meaningful gross and histopathological changes were detected in the testis in the present study.
The slight congestion spots of lung, atrophy of thymus, cyst in kidney, spleen atrophy or hypertrophy, hypertrophy of submandibular lymph node and edematous changes of uterus detected in the present study as gross findings, and hypertrophy of lung alveolus wall with focal hemorrhage, decreases of lymphoid cells in the cortex of thymus, cyst formation in the kidney, hyperplasia of lymphoid cells in the red pulp of spleen, focal inflammatory cell infiltration in the liver, edematous changes on the uterus, and cyst and diffused hyperplasia of lymphoid cells in the submandibular lymph node detected as histopathological findings were considered as accidental findings not toxicological signs related to the BR extracts treatment because they were sporadically detected throughout experimental groups tested in the present study including both genders of vehicle control. Especially, the edematous changes in uterus were considered as secondary changes from different physiological estrus cycles (Banks, 1986; Pineda, 1989), and the hyperplasia of lymphoid cells in the red pulp of spleen were also difficult to considered as BR extracts treatment related toxicological signs because they were also demonstrated in the both genders of vehicle control with similar frequency and severity as observed in BR extract treated female and male rats in the present study. In addition, most of them were also generally observed in normal mice (Roh and Ku, 2010; Lee et al., 2011).
Because no BR extracts treatment related mortalities were detected up to 2000 mg/kg in both male and female mice in the present study, the LD50 and ALD of BR extracts after single oral treatment in female and male mice were considered over 2000 mg/kg, respectively. However, it also observed that the possibilities of digestive disorders, like soft feces when administered over 2,000 mg/kg of BR extracts in the present study, but these possibilities of digestive disorders can be disregard in clinical use because they are transient in the highest dosages male only.
Acknowledgments
This research was supported by a grant from Daegu Haany University Ky-rin Fund, 2011 (2011-901-40).
References
- 1.Abe H., Sakaguchi M., Yamada M., Arichi S., Odashima S. Pharmacological actions of saikosaponins isolated from Bupleurum falcatum. 1. Effects of saikosaponins on liver function. Planta Med. (1980);40:366–372. doi: 10.1055/s-2008-1074987. [DOI] [PubMed] [Google Scholar]
- 2.Abe H., Sakaguchi M., Odashima S., Arichi S. Protective effect of saikosaponin-d isolated from Bupleurum falcatum L. on CCl4-induced liver injury in the rat. Naunyn Schmiedebergs Arch. Pharmacol. (1982);320:266–271. doi: 10.1007/BF00510139. [DOI] [PubMed] [Google Scholar]
- 3.Banks W.J., Banks W.J. Female reproductive system in Applied veterinary histology (Banks, W.J., Ed.) Williams & Wilkins; Baltimore: (1986). pp. 506–526. [Google Scholar]
- 4.Chang W.C., Hsu F.L. Inhibition of platelet activation and endothelial cell injury by flavan-3-ol and saikosaponin compounds. Prostaglandins Leukot. Essent. Fatty Acids. (1991);44:51–56. doi: 10.1016/0952-3278(91)90144-T. [DOI] [PubMed] [Google Scholar]
- 5.Cho B.S., Kim S.D., Park J.K., Chung J.H., Hong M.S., Lee B.C., Ihm C.G. Effects of Bupleurum falcatum and its combination with an angiotensin II receptor blocker on cytokine and chemokine expression in human mesangial cells. Phytother. Res. (2010);24:339–343. doi: 10.1002/ptr.2936. [DOI] [PubMed] [Google Scholar]
- 6.Dourish C.T., Greenshaw A.J., Dourish C.T. Effects of drugs on spontaneous motor activity in Experimental psychopharmacology. Humana Press; Clifton: (1987). pp. 325–334. [Google Scholar]
- 7.Flecknell P. Laboratory Animal Anesthesia. 2nd Ed. Harcourt & Company; New York: (1996). p. 269. [Google Scholar]
- 8.Guo Y., Matsumoto T., Kikuchi Y., Ikejima T., Wang B., Yamada H. Effects of a pectic polysaccharide from a medicinal herb, the roots of Bupleurum falcatum L. on interleukin 6 production of murine B cells and B cell lines. Immunopharmacology. (2000);49:307–316. doi: 10.1016/S0162-3109(00)00245-9. [DOI] [PubMed] [Google Scholar]
- 9.Hattori T., Ito M., Suzuki Y. Studies on antinephritic effects of plant components in rats (1). Effects of saikosaponins original-type anti-GBM nephritis in rats and its mechanisms. Nippon Yakurigaku Zasshi. (1991);97:13–21. doi: 10.1254/fpj.97.1_13. [DOI] [PubMed] [Google Scholar]
- 10.Irwin S. Comprehensive observational assessment: Ia. A systemic, quantitative procedure for assessing the behavioral and physiological state of the mouse. Psychopharmacologia. (1968);13:222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- 11.Korea Food and Drug Administration. Testing Guidelines for Safety Evaluation of Drugs (Notification No. 2009-116, issued by the Korea Food and Drug Administration on August 24, 2009). (2009)
- 12.Lee B., Shim I., Lee H., Hahm D.H. Effect of Bupleurum falcatum on the stress-induced impairment of spatial working memory in rats. Biol. Pharm. Bull. (2009);32:1392–1398. doi: 10.1248/bpb.32.1392. [DOI] [PubMed] [Google Scholar]
- 13.Lee B.J., Ahn B.W., Kang H.G., Kim Y.B. Fourweek repeated-dose toxicity study on Pinellia Extract. Korean J. Lab. Anim. Sci. (2003);19:127–141. [Google Scholar]
- 14.Lee W.H., Gam C.O., Ku S.K., Choi S.H. Single oral dose toxicity test of platycodin D, a saponin from platycodin radix in mice. Toxicol. Res. (2011);27:217–224. doi: 10.5487/TR.2011.27.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin C.C., Chiu H.F., Yen M.H., Wu C.C., Chen M.F. The pharmacological and pathological studies on Taiwan folk medicine (III): The effects of Bupleurum kaoi and cultivated Bupleurum falcatum var. Am. J. Chin. Med. (1990);18:105–112. doi: 10.1142/S0192415X90000149. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto T., Sun X.B., Hanawa T., Kodaira H., Ishii K., Yamada H. Effect of the antiulcer polysaccharide fraction from Bupleurum falcatum L. on the healing of gastric ulcer induced by acetic acid in rats. Phytother. Res. (2002);16:91–93. doi: 10.1002/ptr.986. [DOI] [PubMed] [Google Scholar]
- 17.Niikawa M., Sakai Y., Ose Y., Sato T., Nagase H., Kito H., Sato M., Mizuno M. Enhancement of the mutagenicity of Trp-P-1, Trp-P-2 and benzo[a]pyrene by bupleuri radix extract. Chem. Pharm. Bull. (Tokyo) (1990);38:2035–2039. doi: 10.1248/cpb.38.2035. [DOI] [PubMed] [Google Scholar]
- 18.Nose M., Amagaya S., Ogihara Y. Corticosterone secretion-inducing activity of saikosaponin metabolites formed in the alimentary tract. Chem. Pharm. Bull. (Tokyo) (1989);37:2736–2740. doi: 10.1248/cpb.37.2736. [DOI] [PubMed] [Google Scholar]
- 19.Pineda M.H., McDonald L.E., Pineda M.H. Female reproductive system in Veterinary endocrinology and reproduction. Lea & Febiger; Philadelphia: (1989). pp. 303–354. [Google Scholar]
- 20.Plata E.J., Murphy W.H. Growth and haematologic properties of the BALB/wm strain of inbred mice. Lab. Anim. Sci. (1972);22:712–720. [PubMed] [Google Scholar]
- 21.Roh S.S., Ku S.K. Mouse single oral dose toxicity study of DHU001, a polyherbal formula. Toxicol. Res. (2010);26:53–59. doi: 10.5487/TR.2010.26.1.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakurai M.H., Matsumoto T., Kiyohara H., Yamada H. B-cell proliferation activity of pectic polysaccharide from a medicinal herb, the roots of Bupleurum falcatum L. and its structural requirement. Immunology. (1999);97:540–547. doi: 10.1046/j.1365-2567.1999.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi C., Fujita S., Obara T., Ueda T. Effects of room temperature on reproduction, body weight and organ weights, food and water intakes, and hematology in mice. Jikken, Dobutsu. (1983);32:1–11. doi: 10.1538/expanim1978.32.1_1. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto M., Kumagai A., Yamamura Y. Structure and actions of saikosaponins isolated from Bupleurum falcatum L. I. Anti-inflammatory action of saikosaponins. Arzneimittelforschung. (1975a);25:1021–1023. [PubMed] [Google Scholar]
- 25.Yamamoto M., Kumagai A., Yamamura Y. Structure and action of saikosaponins isolated from Bupleurum falcatum L. II. Metabolic actions of saikosaponins, especially a plasma cholesterol-lowering action. Arzneimittelforschung. (1975b);25:1240–1243. [PubMed] [Google Scholar]