Abstract
Flavonoids, which form a major component in Houttuynia cordata Thunb., display a wide range of pharmacological activities. The expression of plant flavonoids is partly regulated by fermentation. Therefore, we studied the effects of fermentation on H. cordata in order to identify the strains present during the fermentation process, and to determine whether fermented H. cordata could be used as a probiotic. Our results showed that all 6 of the bacterial strains isolated from fermented H. cordata (FHC) belonged to the genus Bacillus. As expected, fermenting H cordata also increased the flavonoid content as increases were observed in the levels of rutin, quercitrin, and quercetin. To test the effects of fermentation, we treated LPS-stimulated RAW264.7 cells with non-fermented H. cordata extracts (HCE) or FHC extracts (FHCE). Compared to the HCE-treated cells, the FHCE-treated cells showed increased viability. No cytotoxic effects were detected in the FHCE-treated groups in the 2 cell lines used in the study, namely, RAW264.7 and RBL-2H3. FHCE-treated HepG2 cells showed decreased growth, compared to HCE-treated HepG2 cells. These results indicate that the fermented H. cordata predominantly contained Bacillus strains. Furthermore, FHCE are able to prevent LPS-induced inflammatory effects and inhibit the growth of HepG2 cells.
Keywords: Fermentation, Houttuynia cordata, Flavonoid, Inflammatory, Cytotoxic, Bacillus
INTRODUCTION
The interest in finding natural sources of antioxidants, in particular, of plant origin, has witnessed a recent upsurge. Numerous crude extracts and purified natural compounds from plants have antioxidant and radical-scavenging activities (Fardet et al., 2008; Niki, 2010; Tian et al., 2011). Lowmolecular weight compounds such as vitamins, zeaxanthin, and flavonoids are considered the active ingredients in plants (Beker et al., 2011). Flavonols such as quercetin and morin act as chain-breaking antioxidants in cetyl trimethylammonium bromide (CTAB) micelles by inhibiting linoleic acid autoxidation (He et al., 2012).
Houttuynia cordata possesses pharmacological properties that reduce hypertension, edema, and inflammation since it has anti-pyretic, anti-purulent, anti-viral, and antimutagenic properties (Chen et al., 2011; Lau et al., 2008; Tian et al., 2011). It is also effective in treating pneumonia, severe acute respiratory syndrome, human immunodeficiency virus infection, influenza, and refractory hemoptysis (Nuengchamnong et al., 2009). H. cordata also acts as an antioxidant, thereby promoting detoxification (Tian et al., 2011).
The chemical components of H. cordata comprise 6 major types: volatile oils, flavonoids, alkaloids, fatty acids, sterols, and polyphenolic acids (Wu et al., 2009; Meng et al., 2009). Our group recently demonstrated microbial fermentation as a promising alternative source for many flavonoid molecules, including anthocyanins, flavones, and flavanones (Leonar et al., 2006). Consequently, there is growing interest in the fermented products of herbal plants. Specifically, fermentation can improve the nutraceutical value of a product by breaking down certain undesirable compounds and inducing effective microbial conversion (Oboh et al., 2008). Currently, most industries employ the time-consuming traditional fermentation method, which uses sugar in its native form and requires 2 to 3 years for completion. Therefore, it is necessary to develop methodologies that would allow plants to be fermented using specific microorganisms at a large scale, over shorter durations. To compensate for the shortcomings in the conventional fermentation process and produce homogeneous fermented products, bacterial strains were identified and isolated from traditionally fermented H. cordata extracts (FHCE), produced using the conventional fermentation process. Further, we aimed to compare the effects of FHCE and H. cordata extracts (HCE) by using the aforementioned optimal fermentation conditions on the alleviation of oxidative stress. In this study, 6 Bacillus strains were isolated from FHCE. They were confirmed to belong to the genus Bacillus, and were identified as B. amyloliquefaciens subsp. plantarum FZB42T, B. licheniformis ATCC 14580T, B. aerius 24KT, B. methylotrophicus CBMB205T, B. safensis FO-036bT, B. sonorensis NRRL B-23154T, with ~99% similarity. The use of viable spores of Bacillus as a probiotic supplement raises some concerns, including safety. Several Bacillus species used as animal feed supplements, probiotics, plant protection products, or seed-coating agents are also known to cause food poisoning (Gaggìa et al., 2010). Using fermentation, the flavonoid content can be increased, thereby increasing the antioxidant activity.
MATERIALS AND METHODS
Reagents, cell lines and cell culture. All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.HepG2 cell and Mouse leukaemic monocyte macrophage (Raw 264.7) cell were acquired from Korean Cell Line Bank (KCLB). The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin and incubated in a humidified 5% CO2 incubator at 37℃.
Bacillus strains of Isolation of the microorganisms from traditionally fermented Houttuynia cordata. Traditionally fermented Houttuynia cordata was purchased from Semyoung Oriental Co., Ltd. in Geochang, Gyeongnam (Republic of Korea). Traditionally fermented Houttuynia cordata was diluted with sterilized water in 10−1~10−4 by 10-fold dilution technique, and then 200 μl of diluted traditionally fermented Houttuynia cordata was inoculated onto the Luria-Bertani (LB) agar medium. Plates incubated at 35℃ for 48 hrs. Colonies representing different morphologies were picked at random and purified by streaking on agar plates of the same medium. The single colony was pure cultured four times. Isolates were stored at −80℃ with 20% sterile glycerol until needed.
Phylogenetic analysis of strain based on 16S rRNA sequences. 16S rRNA gene sequencing of strains were referred to a solgent Co., Ltd. By using EzTaxon server and National Center for Biotechnology Information (NCBI) database, homology was confirmed with type of strain for phylogenic analysis. 16S rRNA gene sequencing of strains were alignmented by Bio-eidt (Hall, 1999) program and Clustal X program. And then, strain evolutionary process was estimated by Kimura two-parameter-model, and phylogenic position was determined by neighbor-joining and maximum parsimony by MEGA 4 program.
Preparation of Houttuynia cordata. Houttuynia cordata was purchased from Semyoung Oriental Co., Ltd. in Geochang, Gyeongnam (Republic of Korea). HC was reflux-condensed by 100 g with 2 l of distilled water at 60℃ for 6 hrs. Then it was concentrated with an evaporator, and sterilized at 121℃ for 20 min.
Bacillus strains isolated from traditionally fermented Houttuynia cordata were inoculated onto the LB medium and shaking cultured for 12 hrs at 35℃ and 180 rpm. The sterilized HC extract was inoculated with 1% (v/v) of Bacillus strains at absorbance of 1.0 at 600 nm, and the inoculated HE was fermented during 38 hrs at 35℃. The fermented HE by Bacillus strains were diluted with the same rate, after which it was concentrated with an evaporator and was freeze-dried. For the non-fermented HE, the purchased dry HE was reflux-condensed by 100 g with 2 l of distilled water at 60℃ for 6 hrs, after which it was concentrated with an evaporator and was freeze-dried.
HPLC analysis. The extracts were analyzed by HPLC systems (Agilent model 1200 series, Hewlett Packard, Palo Alto, CA) with a Symmetry C18-column (Gemini C18, 250 × 4.6 mm) at 30℃. Linear solvent gradient of binary mobile phase (solvent A, 0.1% trifluoacetic acid in HPLC grade water; solvent B, 0.1% trifluoacetic acid in HPLC grade acetonitrile) during HPLC analysis was applied as follows (total 40 min): 75% A/25% B at 0 to 5 min, 65% A/35% B at 5 to 8 min, 62% A/38% B at 8 to 16 min, 20% A/80% B at 16 to 30 min and 75% A/25% B at 30 to 35 min. The flow rate of the solvent was kept constantly at 1.0 ml/min. Sample injction volume was 10 μl and UV absorbance was monitored at 370 nm.
Cell viability assay. Cell viability was determined a MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay provided by Sigma Aldrich (St. Louis, MO, USA). Cells with an initial concentration of 2 × 104/well were seeded in 96-well plate in DMEM supplemented with 10% FBS. After 24 hrs, the culture medium was changed to fresh media, and FSC and NFSC with concentrations of 10 μg/ml, 100 μg/ml and 1000 μg/ml, respectively, were added into the culture. Cells incubated with PBS were used as control. After 48 hrs, respectively, MTT assay was performed to quantitatively evaluate cell activity and ratios of OD 550 nm of each group to the value of control.
Protective effect of fermented Houttuynia cordata extract against of LD50 on Raw 264.7. Raw 264.7 cells were seeded into a 96-well microplate at a density of 2 × 104 cells/well. After 24 hrs, cells were then washed twice with PBS and given fresh complete growth medium. The various doses SCE and SCFE were treated, and incubated for 24 hrs. Then 38.65 μg/ml of LPS was treated, and incubated for 4 hrs. MTT was then added (0.5 mg/ml) for 4 hrs, the medium was removed, and the formazan crystals were dissolved in DMSO and isopropanol (1 : 1, v/v). O.D. of solution in each well measured at 540 nm on SynergyHT (Bio-Tek instruments, USA). Cell viability rate was calculated as the percentage of MTT absorbance.
Statistical analysis. Data were expressed as mean S. D. [standard error of the mean (SEM)]. Statistical analysis was performed using multiple analyses of variance (ANOVA) with repeated measures. When significant main effects or an interaction between the main effects was found, specific comparisons were made with Student’s paired t-tests. Statistical significance was represented by p < 0.05, p < 0.01, and p < 0.001. Statistical analysis was performed with SPSS version 12.0 statistical software (SPSS Inc., Chicago, IL, USA).
RESULTS AND DISCUSSION
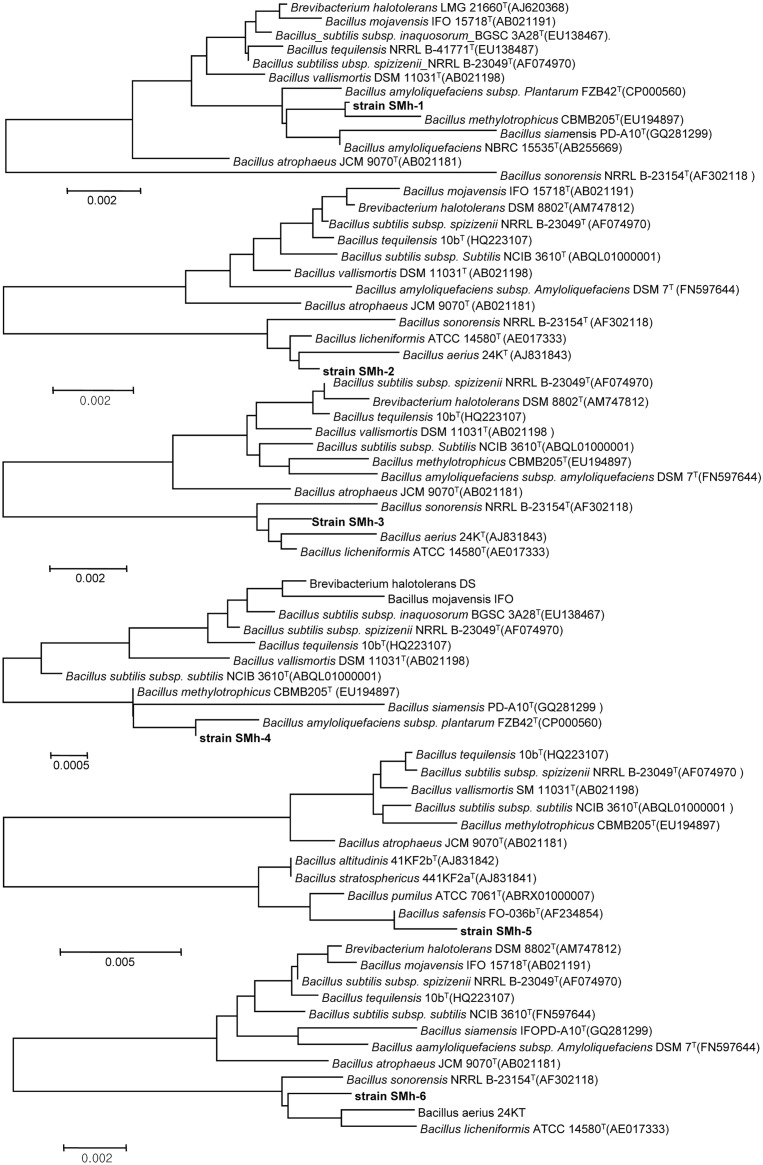
To standardize the fermented H. cordata (FHC) manufacturing process, 6 Bacillus strains were isolated and identified using 16S rDNA sequencing. The results of BLAST analyses showed that the sequences were most closely related to various strains of the Bacillus genus, with a maximal identity of 99%. The phylogenetic tree, which was constructed on the basis of 16S rDNA sequences, is shown in Fig. 1. The 16S rRNA gene domains of the strains 1, 2, 3, 4, 5, and 6 consisted of 1388 bp, 564 bp, 556 bp, 1441 bp, 556 bp, and 522 bp, respectively (Fig. 2). They were confirmed to belong to the Bacillus genus, and identified as B. amyloliquefaciens subsp. plantarum FZB42T, B. licheniformis ATCC 14580T, B. aerius 24KT, B. methylotrophicus CBMB205T, B. safensis FO-036bT, and B. sonorensis NRRL B-23154T strains (Fig. 3). The different strains were designated as SMh-1 to SMh-6. Thus, all the species isolated from these extracts belonged to the Bacillus genus. Notably, majority of Bacillus species are assigned the “generally regarded as safe” (GRAS) status (Patel et al., 2010).
Fig. 1. Phyogenetic tree analysis of the isolated 16S rDNA with different Bacillus strains from the NCBR database by MEGA4.
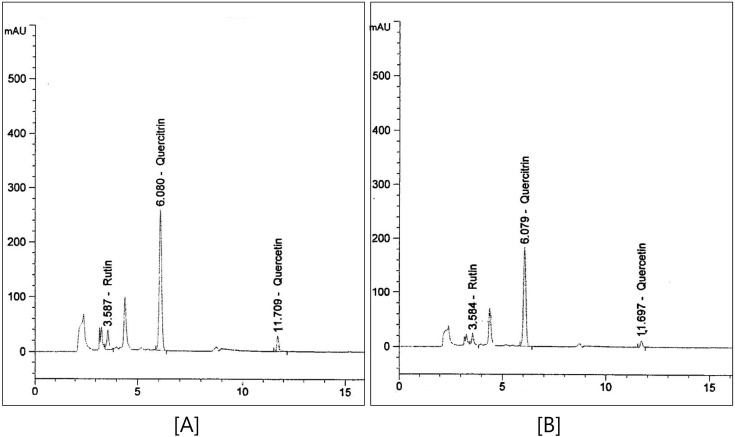
Fig. 2. Typical HPLC chromatograms of flavonoids. [A] FHCE, fermented Houttuynia cordata extract, [B] HCE, Houttuynia cordata extract.
Fig. 3. Viability activity of non-fermented Houttuynia cordata extract and fermented Houttuynia cordata extract on the raw 264.7 cell and RBL2H3 cells by MTT assay. concentrations of 1000~10 μg (p < 0.05) do not significantly affect cell viability.
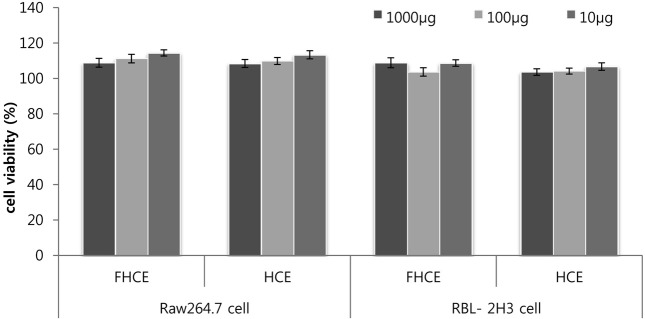
Philip et al. (2009) have showed that fermentation increases the flavonoid content (Philip et al., 2009). FHCE and HCE were examined for their flavonoid composition by using HPLC. Individual constituents were identified by comparing their peaks, UV spectra, and retention times, with those of the corresponding reference standards (Fig. 2). The percentage of each flavonoid was estimated using a calibration curve. The flavonoids present in the FHCE and HCE were rutin (2.1 mg/100 g, 1.1 mg/100 ml), quercitrin (16.6 mg/ 100 g, 11.8 mg/100 g), and quercetin (0.5 mg/100 g, 0.2 mg/ 100 g). The contents of rutin, quercitrin, and quercetin increased in the FHCE by 1.9-fold, 1.4-fold, and 2.5-fold, respectively, compared to the HCE. The increase in the flavonoid content of FHCE was attributed to the change in the organic content, which was due to fermentation carried out by the Bacillus strains identified in the FHCE. This broad spectrum of nutraceuticals with different characteristics allowed a comprehensive assessment of the cytotoxicity associated with these flavonoids as well as a comparison between the different cytotoxicity assays and the different fish cell lines, namely, RBL-2H3 and RAW264.7. To determine the cell viability of RAW264.7 macrophages and RBL-2H3 cells that had been treated with HCE and FHCE, we used an MTT assay. In this assay, a mitochondrial enzyme in living cells, succinate dehydrogenase, cleaves the tetrazolium ring, converting the MTT to an insoluble purple formazan. Therefore, the amount of formazan produced is directly proportional to the number of viable cells. The cytotoxic effects of FHCE and HCE on the 2 kinds of cells, namely, RAW264.7 and RBL-2H3, were studied at different concentrations, i.e., 1000 μg/ml, 100 μg/ml, and 10 μg/ ml. Cell viability was not affected by FHCE and HCE at any of these concentrations. Thus, FHCE and HCE are not cytotoxic.
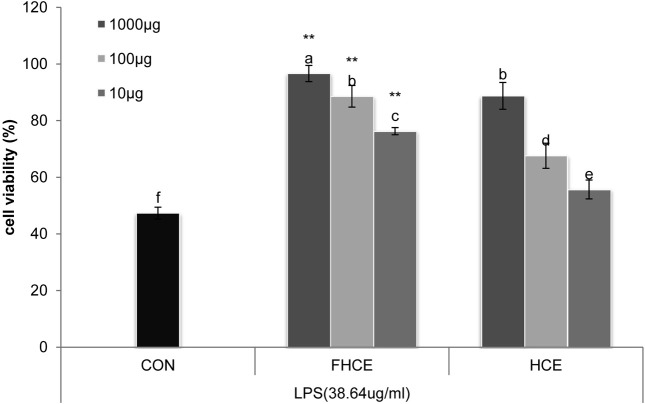
Macrophages are the first line of host defense against bacterial infection and cancer growth (Higgins et al., 2007). Lipopolysaccharide (LPS) from gram-negative bacteria can quantitatively induce iNOS-mediated cellular responses and cause responses that result in inflammation, sepsis, and stroke (Taira et al., 2009). In our study, LPS treatment decreased cell viability with increasing doses, indicating that LPS has dose-dependent effects on cytotoxicity. We evaluated the protective effects of FHCE and HCE by testing the in vitro cell viability of RAW264.7 cells treated with LPS (LD50, 38.64 μg/ml LPS). Protective effects of FHCE and HCE on LPS-treated RAW264.7 cells at 1000 μg/ml, 100 μg/ml, and 10 μg/ml were 94.14%, 88.61%, and 76.28%, respectively, and 88.76%, 67.58%, and 55.70%, respectively. These results showed that fermentation of H. cordata increased the viability of LPS-stimulated RAW264.7 cells. The expression of flavonoids in LPS-induced inflammatory cells is shown in Table 1. As expected, FHCE induced higher levels of rutin, quercetin, and quercitrin compared to HCE. The flavonoid levels in LPS-stimulated FHC-treated cells were similar to those in FHCE-treated cells. The effects of different concentrations of FHCE and HCE on HepG2 cell viability are shown in Fig. 5. The percentages of inhibition induced by 1000 μg/ml, 100 μg/ml, and 10 μg/ml FHCE and HCE on HepG2 cell viability were 38.31%, 47.40%, and 50.54%, respectively, and 47.72%, 62.66%, and 68.66%, respectively. In addition, the detection of rutin, quercetin, and quercitrin at approximately 20 μM, 100 μM, and 20 μM correlated with the effects observed in the MTT assay (Table 1). Viability and activity of the HepG2 cells treated with FHCE were lower than those of the HepG2 cells treated with HCE at various concentrations. These results showed that traditionally fermented H. cordata products contain Bacillus strains. Treating cells with extracts derived from fermented H. cordata inhibited LPS-induced inflammatory cell death and inhibited the growth of HepG2 cells. FHCE also have an increased content of flavonoids, including rutin, quercetin, and quercitrin. Our results also indicated that the flavonoid constituents of FHCE exhibit excellent anti-inflammatory effects in LPS-stimulated cells. These results suggest that the effects of fermentation on H. cordata warrant further investigation.
Table 1.
The cytotoxicity inhibitory effects of flavonoid in Raw 264.7 macrophages
| Group | Treated LPS (38.64 μg/ml) of Raw 264.7 cell | ||
|---|---|---|---|
| Rutin | Quercetin | Quercitrin | |
| 100 μM | 97.66±2.57a | 96.23 ± 2.75a | 93.96 ± 2.64ab |
| 80 μM | 95.33±1.70ab | 85.06 ± 3.58c | 76.07 ± 2.06d |
| 60 μM | 89.81±1.75ac | 76.18 ± 3.14d | 75.59 ± 1.94d |
| 40 μM | 75.87±3.30d | 62.06 ± 2.95f | 71.59 ± 1.58de |
| 10 μM | 64.07±2.47e | 46.30 ± 3.80g | 67.31 ± 1.74e |
abcdefeMeans in the same row not sharing a common superscript are significantly different between groups at p < 0.05.
Fig. 5. Inhibitory effects of Viability activity of HepG2 cells. abcdefMeans in the same row not sharing a common superscript are significantly different between groups at p<0.05. *p<0.05 in two-sided student’s t-test on FHCE and HCE.
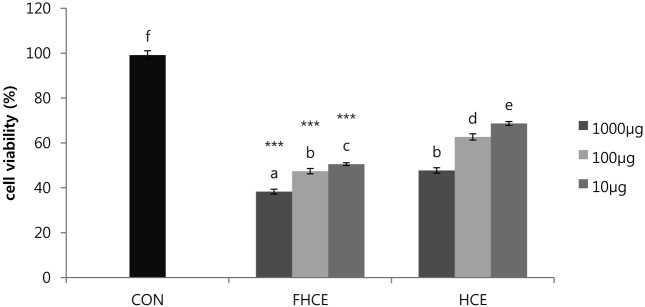
Fig. 4. The cytotoxicity of LPS and determination of LD50 value in Raw 264.7 macrophages. abcdef Means in the same row not sharing a common superscript are significantly different between groups at p< 0.05. *p< 0.05 in two-sided student’s t-test on FHCE and HCE.
Table 2.
Inhibitory effects of viability activity in HepG2 cell of flavonoid
| Group | HepG 2 cell | ||
|---|---|---|---|
| Rutin | Quercetin | Quercitrin | |
| 100 μM | 29.16 ± 1.48bc | 46.10 ± 2.80e | 21.64 ± 3.38a |
| 80 μM | 31.70 ± 1.98cd | 54.04 ± 1.78f | 26.83 ± 2.20b |
| 60 μM | 32.34 ± 1.54cd | 52.27 ± 3.22f | 31.92 ± 3.56cd |
| 40 μM | 34.76 ± 2.40de | 53.67 ± 2.23f | 34.90 ± 3.90de |
| 10 μM | 37.19 ± 0.80e | 60.71 ± 3.14g | 38.14 ± 2.42e |
abcdefMeans in the same row not sharing a common superscript are significantly different between groups at p<0.05.
Acknowledgments
This work was supported by in part by grants (No. A101836) from the Korea Health Technology R&D Project, Ministry of Health, Welfare & Family Affairs, Republic of Korea and the KRIBB Research Initiative Program.
References
- 1.Beker B.Y., Bakır T., Sönmezoğlu İ., İmer F., Apak R. Antioxidant protective effect of flavonoids on linoleic acid peroxidation induced by copper(II)/ascorbic acid system. Chem. Phys. Lipids. (2011);164:732–739. doi: 10.1016/j.chemphyslip.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Chen X., Wang Z., Yang Z., Wang J., Xu Y., Tan R.X., Li E. Houttuynia cordata blocks HSV infection through inhibition of NF-κB activation. Antiviral Res. (2011);92:341–345. doi: 10.1016/j.antiviral.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fardet A., Rock E., Rémésy C. Is the in vitro antioxidant potential of whole-grain cereals and cereal products well reflected in vivo?. J. Cereal Sci. (2008);48:258–276. doi: 10.1016/j.jcs.2008.01.002. [DOI] [Google Scholar]
- 4.Gaggìa F., Mattarelli P., Biavati B. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. (2010);141:S15–S28. doi: 10.1016/j.ijfoodmicro.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 5.Hall T.A. Bio-Edit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. (1999);41:95–98. [Google Scholar]
- 6.He N., Yang X., Jiao Y., Tian L., Zhao Y. Characterisation of antioxidant and antiproliferative acidic polysaccharides from Chinese wolfberry fruits. Food Chem. (2012);133:978–989. doi: 10.1016/j.foodchem.2012.02.018. [DOI] [Google Scholar]
- 7.Higgins S.E., Erf G.F., Higgins J.P., Henderson S.N., Wolfenden A.D., Gaona-Ramirez G., Hargis B.M. Effect of probiotic treatment in broiler chicks on intestinal macrophage numbers and phagocytosis of Salmonella enteritidis by abdominal exudate cells. Poult. Sci. (2007);86:2315–2321. doi: 10.3382/ps.2007-00123. [DOI] [PubMed] [Google Scholar]
- 8.Lau K.M., Lee K.M., Koon C.M., Cheung C.S., Lau C.P., Ho H.M., Lee M.Y., Au S.W., Cheng C.H., Lau C.B., Tsui S.K., Wan D.C., Waye M.M., Wong K.B., Wong C.K., Lam C.W., Leung P.C., Fung K.P. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J. Ethnopharmacol. (2008);118:79–85. doi: 10.1016/j.jep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leonard E., Yan Y., Koffas M.A. Functional expression of a P450 flavonoid hydroxylase for the biosynthesis of plant-specific hydroxylated flavonols in Escherichia coli. Metab. Eng. (2006);8:172–181. doi: 10.1016/j.ymben.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Meng J., Leung K.S., Dong X.P., Zhou Y.S., Jiang Z.H., Zhao Z.Z. Simultaneous quantification of eight bioactive components of Houttuynia cordata and related Saururaceae medicinal plants by on-line high performance liquid chromatography- diode array detector-electrospray mass spectrometry. Fitoterapia. (2009);80:468–474. doi: 10.1016/j.fitote.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Niki E. Assessment of Antioxidant Capacity in vitro and in vivo. Free Radic. Biol. Med. (2010);49:503–515. doi: 10.1016/j.freeradbiomed.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Nuengchamnong N., Krittasilp K., Ingkaninan K. Rapid screening and identification of antioxidants in aqueous extracts of Houttuynia cordata using LC-ESI-MS coupled with DPPH assay. Food Chem. (2009);117:750–756. doi: 10.1016/j.foodchem.2009.04.071. [DOI] [Google Scholar]
- 13.Oboh G., Alabi K.B., Akindahunsi A.A. Fermentation changes the nutritive values, polyphenol distribution, and antioxidant properties of Parkia giglobosa seeds (African locust beans). Food Biotechnol. (2008);22:363–376. doi: 10.1080/08905430802463404. [DOI] [Google Scholar]
- 14.Patel A.K., Ahire J.J., Pawar S.P., Chaudhari B.L., Shouche Y.S., Chincholkar S.B. Evaluation of Probiotic Characteristics of Siderophoregenic Bacillus spp. Isolated from Dairy Waste. Appl. Biochem. Biotechnol. (2010);160:140–155. doi: 10.1007/s12010-009-8583-2. [DOI] [PubMed] [Google Scholar]
- 15.Philip K., Sinniah S.K., Muniandy S. Antimicrobial peptides in aqueous and ethanolic extracts from microbial, plant and fermented sources. Biotechnology. (2009);8:248–253. doi: 10.3923/biotech.2009.248.253. [DOI] [Google Scholar]
- 16.Taira J., Nanbu H., Ueda K. Nitric oxide-scavenging compounds in Agrimonia pilosa Ledeb on LPS-induced RAW264.7 macrophages. Food Chem. (2009);115:1221–1227. doi: 10.1016/j.foodchem.2009.01.030. [DOI] [Google Scholar]
- 17.Tian L., Zhao Y., Guo C., Yang X. A comparative study on the antioxidant activities of an acidic polysaccharide and various solvent extracts derived from herbal Houttuynia cordata. Carbohydr. Polymer. (2011);83:537–544. doi: 10.1016/j.carbpol.2010.08.023. [DOI] [Google Scholar]
- 18.Wu L.S., Si J.P., Yuan X.Q., Shi X.R. Quantitive Variation of Flavonoids in Houttuynia cordata from Different Geographic Origins in China. Chinese Journal of Natural Medicines. (2009);7:40–46. doi: 10.3724/SP.J.1009.2009.00040. [DOI] [Google Scholar]