Abstract
Polychlorinated biphenyls (PCBs) are persistent and bioaccumulative environmental pollutants. Recently, it is suggested that neurotoxic effects such as motor dysfunction and impairment in memory and learning have been associated with PCB exposure. However, structure relationship of PCB congeners with neurotoxic effects remains unknown. Since PKC signaling pathway is implicated in the modulation of motor behavior as well as learning and memory and the role of PKC are subspecies-specific, we attempted to study the effects of structurally distinct PCBs on the total PKC activity as well as subspecies of PKC in cerebellar granule cell culture model. Cells were exposed to 0, 25 and 50 μM of PCB-126, PCB-169, PCB-114, PCB-157, PCB-52 and PCB-4 for 15 min. Cells were subsequently analyzed by [3H] phorbol ester binding assay or immunoblotted against PKC-α and -ε monoclonal antibodies. While non-dioxin-like-PCB (PCB-52 and PCB-4) induced a translocation of PKC-α and -ε from cytosol to membrane fraction, dioxin-like PCBs (PCB-126, -169, -114, -157) had no effects. [3H] Phorbol ester binding assay also revealed structure-dependent increase similar to translocation of PKC isozymes. While PCB-4 induced translocation of PKC-α and -ε was inhibited by ROS inhibitor, the pattern of translocation was not affected in presence of AhR inhibitor. It is suggested that PCB-4-induced PKC activity may not be mediated via AhR-dependent pathway. Taken together, our findings suggest that chlorination of ortho-position in PCB may be a critical structural moiety associated with neurotoxic effects, which may be preferentially mediated via non-AhR-dependent pathway. Therefore, the present study may contribute to understanding the neurotoxic mechanism of PCBs as well as providing a basis for establishing a better neurotoxic assessment.
Keywords: Polychlorinated biphenyls, Protein kinase C, Cerebellar granule cell, ROS, AhR, Structureactivity relationship
INTRODUCTION
Halogenated Aromatic Hydrocarbons (HAH) is a class of widely dispersed, environmentally persistent compounds. Intensive industrial use and improper disposal of these chemicals resulted in a global contamination. One of the major class of HAH is Polychlorinated biphenyls (PCBs) with different structural characteristics of congeners. PCBs are a large class of aromatic chlorinated hydrocarbons comprising 209 congeners that differ in the number and position of chlorine atoms. These chemicals are widely distributed environmental contaminants that have been found at many different levels in the food chain (Carpenter, 1998). They are persistent and bioaccumulative in the body and cause a wide range of tissue- and species-specific toxic effects such as carcinogenicity, teratogenicity, immune suppression, and endocrine disruption (Swanson et al., 1995). Among a variety of toxic responses, neurotoxic effect recently draws a lot of attention. It is of a particular concern that exposure to the relatively low concentrations of PCBs may be associated with subtle behavioral and neurological deficits if exposure occurs during development (Jacobson and Jacobson, 1996). Animal studies also demonstrated the neurotoxic potentials of PCBs including psychomotor dysfunction and cognitive deficits (Schantz et al., 1995). Although mechanism of PCB-induced neurotoxic effects still remains unclear, structure- activity relationship of PCB congeners has been described for a few neuronal activities in neurons including intracellular calcium buffering and tyrosine hydroxylase activity (Carpenter, 1998). It was reported that the ortho-, non-coplanar PCB altered intracellular Ca2+ homeostasis by inhibiting Ca2+ buffering system and caused protein kinase C (PKC) translocation at low micromolar concentrations, while non-ortho, coplanar PCB did not have any effects on these second messenger systems (Kodavanti et al., 1993, 1994, 1996). Alteration of normal Ca2+ buffering system leading to the increase of intracellular Ca2+ may initiate many second messenger systems, which may lead to alteration of neuronal functions. One of the most critical second messenger molecules involved in neuronal function and development is protein kinase C (PKC). PKC signaling pathways have been associated with an important factor in learning and memory processes (Matsushima et al., 1996). While Structural difference of PCBs plays a key role in PKC activation (Yang and Kodavanti, 2001), relationship between structure of PCB and PKC subspecies remains to be elucidated. Since cerebellum is a storage site for the memory traces for discrete motor learning and classical conditioning of eyeblink response (Molchan et al., 1994), it is suggested that alteration of PKC in cerebellum is associated with impaired motor dysfunction (Chen et al., 1995). PKC subspecies are located in different subcellular compartment and have their unique activation profile (Nishizuka, 1988). Thus, it is important to analyze the individual PKC isozymes to understand their biological significance in the cellular system.
The present study attempted to assess structure-activity relationship of coplanar and non-coplanar PCBs with activation of PKC isozymes and to identify structural moiety responding to PKC activation in cerebellar granule cells in culture.
MATERIALS AND METHODS
Materials. All reagents were purchased from Sigma- Aldrich (St. Louis, MO, USA), but otherwise it has been described.
Cerebellar granule cell culture. Cerebellar granule cell cultures were prepared from the cerebella of 7-day old SD rat pups as described previously (Kodavanti et al., 1993). Cells were plated at 3 × 106 cells/well in 6-well plates. After plating, cells were incubated at 37℃ in a humidified incubator with 5% CO2 atmosphere. Cytosine arabinoside (5 μM) was added after 24 hr to prevent growth of non-neuronal cells. Cells were used for the experiments after 7 days in culture. Cultures typically contained > 95% neurons.
Exposure. Cerebellar granule cells grown on 6-well culture plates were exposed to 0, 25, and 50 μM 3, 3' , 4, 4', 5-penta-CB (PCB-126), 3, 3' , 4, 4' , 5, 5'-hexa-CB (PCB- 169), 2, 3, 4, 4', 5-penta-CB (PCB114), 2, 3, 3', 4, 4', 5- hexa-CB (PCB-157), 2,2',5,5'-tetra-CB (PCB-52), 2, 2'-di-CB (PCB-4) (> 99% purity; Accu-Standard, New Haven, CT, USA) for 15 min, respectively. In order to get enough protein for immunoblots, 4 culture plates were used for each concentration. After the exposure, cultures were washed twice with locke’s buffer (154 mM NaCl, 5.6 mM KCl, 3.6 mM NaHCO3, 2.3 mM CaCl2, 5.6 mM D-glucose, 5 mM HEPES, pH 7.4) and the cells were harvested in a final volume of 2 ml buffer A (20 mM Tris-HCl. pH 7.5, containing 0.25 M sucrose, 2 mM EDTA, 2 mM EDTA and cocktail of protease inhibitors including 0.5 mM phenylmehylsulfonylfluoride (PMSF), 10 μg/ml leupeptin, and 10 μg/ ml pepstatin). For the inhibition study, cells were treated with α-naphthoflavone (α-NF) (10 μM) or N-acetyl cysteine (NAC) (10 mM) for 1 hr prior to the exposure of PCB-4 (50 μM).
[3H]Phorbol Ester Binding assay. Cerebellar granule cells grown on 12-well culture plates (Costar) were tested at 7 days in culture for [3H] phorbol ester binding assay following the method outlined by Vaccarino et al. (1991). Briefly, the monolayers were washed with Locke’s buffer containing 0.1% fatty acid free bovine serum albumin. Following washing, the cells were incubated in Locke’s buffer containing 1 nM 4-β-[3H] phorbol 12,13-dibutyrate ([3H] PDBu; 0.1 μCi/ml; Dupont, New England Nuclear Co. Boston, MA, USA) for 15 min at room temperature with the test chemicals (0~50 μM). An aliquot of the sample (0.7 ml) was added to 9 ml Ultima Gold™ (Packard, Meriden, CT) and the radioactivity was determined using scintillation spectroscopy (Beckman LS6500, Fullerton, CA).
Measurement of reactive oxygen species (ROS). Formation of intracellular ROS was measured using a fluorescent probe, 2',7'-dichlorofluorescin diacetate (DCFH-DA) (Invitrogen, Carlsbad, CA, USA) as described by Mariussen et al. (2002). Cells were treated with DMSO (0.1%) or 50 μM PCBs for 15 min at 37℃ in 5% CO2 incubator. Fluorescence was recorded (excitation wavelength 485 nm, emission wavelength 530 nm) at 37℃ for 1 h. Results are calculated as AUC and presented as values relative to control (% of control).
Cell fractionation. Cells were scraped off into buffer A. The cells were briefly sonicated and centrifuged at 100,000 g for 1 h. The supernatants were designated as cytosolic fraction. The membrane proteins in the pellets were extracted with buffer B (20 mM Tris-HCl, pH 7.5, containing 1% Nonidet P-40, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA and protease inhibitors) on ice for 30 min followed by centrifugation at 15,000 g, and the supernatants were saved as detergent-soluble-membrane fraction.
Immunoblotting. Immunoblot analysis was performed as described previously (Yang et al., 1999). Proteins (10 μg) from cytosolic and membrane fractions were separated by 7.5% SDS-PAGE and transferred to nitrocellulose membrane by Semi-Dry Transfer Cell (Bio-Rad, Hercules, CA). The nitrocellulose membrane was blocked with 5% non-fat dry milk in Tris-buffered saline. PKC isoforms were detected with monoclonal antibodies for PKC-α, and -ε, (Transduction Lab, Lexington, KY, USA). The blots were reacted with a peroxidase-conjugated anti-mouse IgG and detected by the Super Signal (Pierce, Rockford, IL). The membrane was reprobed with anti-GAPDH antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), which was used as loading control. The density of respective bands was analyzed by the Chemi-Doc XRS imaging system (Bio-Rad, Hercules, CA). The data was represented as % controls.
Statistics. The data was analyzed by one way analysis of variance followed by Tukey’s multiple comparison test. The significance was set at p < 0.05.
RESULTS
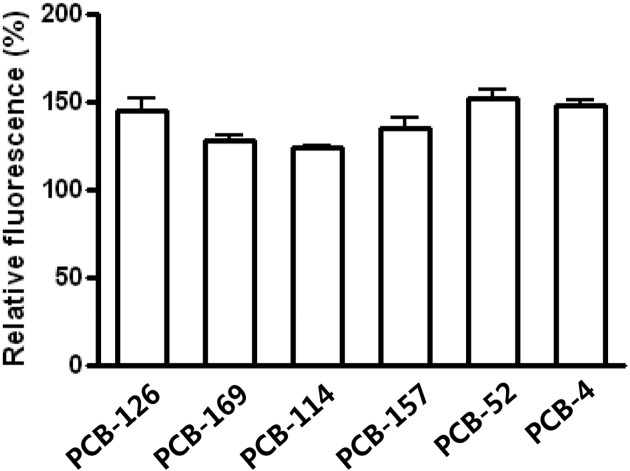
ROS generation. ROS generation following PCB exposure was measured. At 50 μM level, all of PCB congeners induced ROS generation. There was no significant difference on ROS generation among PCB structural moieties. However, it is interesting to note that PCB-4 showed a level of ROS generation similar to PCB-126, while general toxicity of PCB-126 is much potent than that of PCB-4, based on Toxic Equivalent Factor (TEF) values (Fig. 1).
Fig. 1. The effects of PCBs on ROS generation. ROS production in cerebellar granule cells treated with 0.1% DMSO as a control or 50 μM of PCBs (PCB-126, -169, -114, -157, -52, -4). All values are relative to the control cells (the response of cells with DMSO; 100%). Values represent mean ± SD of three independent experiments.
Structural relationship with the [3H]PDBu binding. [3H] PDBu binding assay has been used as a surrogate measure to determine the activity of PKC because it measures the total activity of PKCs bound to the membrane diacyl glycerol (DAG). Measuring percent increase of [3H]PDBu binding at 50 μM, non-ortho-substituted PCBs (PCB-126 and 169) and mono-ortho-substituted PCBs (PCB-114 and 157) showed 24% and 47% increase, respectively. Di-ortho PCBs (PCB-52 and PCB-4) showed 101% increase from untreated DMSO control cells (Table 1).
Table 1.
[3H] PDBu binding following the exposure of substances (% of control)
| Non-ortho-substituted PCBs (μM) | 0.1 | 1 | 25 | 50 |
|---|---|---|---|---|
| 3,3',4,4',5-penta-CB (PCB-126) | 103 ± 09 | 110 ± 04 | 118 ± 12 | 126 ± 16 |
| 3,3',4,4',5,5'-hexa-CB (PCB-169) | 108 ± 08 | 105 ± 11 | 127 ± 21 | 121 ± 12 |
| Mono-ortho-substituted PCBs (μM) | 0.1 | 1 | 25 | 50 |
| 2,3,4,4',5-penta-CB (PCB-114) | 96 ± 11 | 105 ± 04 | 130 ± 18 | 145 ± 16* |
| 2,3,3',4,4',5-hexa-CB (PCB-157) | 92 ± 15 | 108 ± 18 | 135 ± 11* | 148 ± 13* |
| Non-dioxin-like PCBs (μM) | 0.1 | 1 | 25 | 50 |
| 2,2',5,5'-tetra-CB (PCB-52) | 102 ± 06 | 105 ± 12 | 155 ± 16* | 194 ± 11* |
| 2,2'-di-CB (PCB-4) | 105 ± 07 | 112 ± 05 | 168 ± 15* | 208 ± 18* |
The total activity of PKC was measured by [3H] phorbol ester binding assay in the presence of various concentrations (0, 0.1, 1, 25, 50 μM) of PCBs. All values are relative to the control cells treated with DMSO. Values represent the mean ± SD of three independent experiments. * p < 0.05 compared with the control.
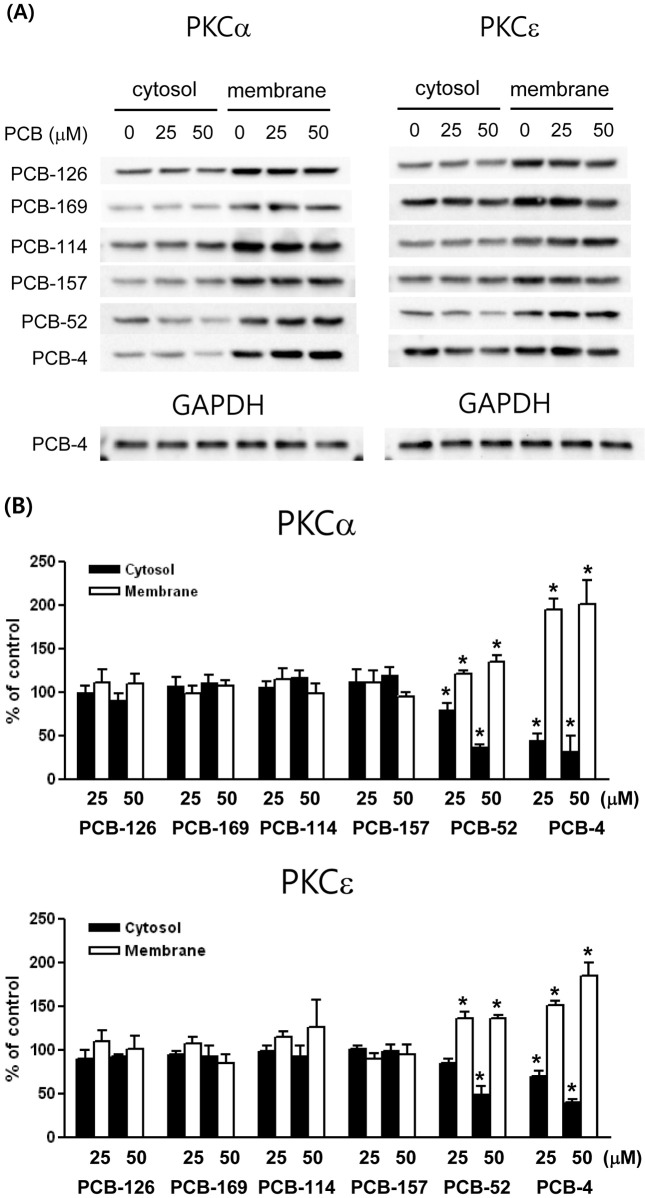
Subcellular changes in PKC isoforms. [3H] PDBu binding assay is to measure the total activity of PKC only. To understand the biological significance of PKC system, it is necessary to assess the effects of specific PKC isozymes following the exposure of various PCB congeners. Because the translocation of PKC-α and -ε were previously observed upon PCBs exposure, structure-activity relationship on the translocation of these PKC isozymes was analyzed. PCB-4, di-ortho-substituted, non-coplanar PCB congener, induced a slight decrease of PKC-α in cytosol (% of control; 45 ± 12.6 at 25 μM and 33 ± 30.7 at 50 μM) and a significant increase of membrane (% of control; 195 ± 21.6 at 25 μM and 201 ± 61.9 at 50 μM) fractions. PCB-4 also induced a significant decrease of PKC-ε in cytosol (% of control; 70 ± 12.1 at 25 μM and 40 ± 6.3 at 50 μM) and a significant increase of membrane (% of control; 152 ± 9.2 at 25 μM and 186 ± 24.8 at 50 μM) fractions. PCB-52 also showed translocation of PKC-α and -ε in a pattern similar to PCB- 4. However, non-ortho-substituted PCBs (PCB-126 and 169) or mono-ortho-substituted PCBs (PCB-114 and 157) did not show a significant translocation of these PKC isozymes (Fig. 2A and 2B).
Fig. 2. The effects of PCBs on the translocation of PKC isozymes. (A) Representative PKC-α and -ε immunoblot profiles of cytosol and membrane fractions from cells treated with PCBs (0, 25, 50 μM). (B) Each band was quantified by densitometric analysis and presented in a bar graph. The values are mean ± SD from three independent experiments and presented as % of the respective controls. * p< 0.05 compared with the control.
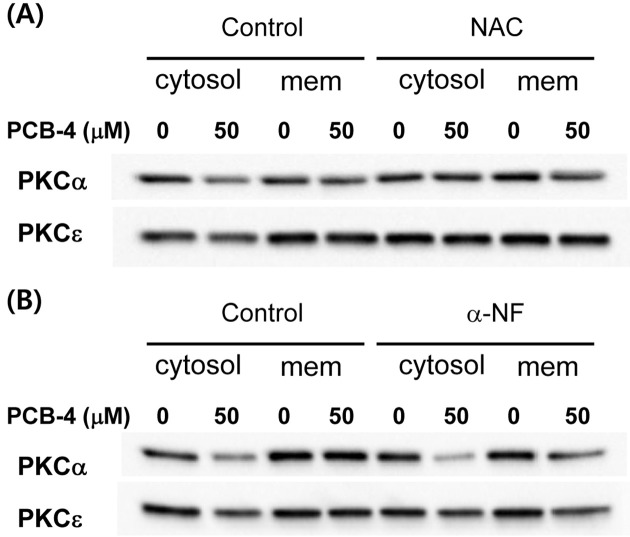
Effects of AhR and ROS on PKC translocation. To assess whether PKC translocation is regulated via AhR or ROS-mediated pathway, PCB-4 induced translocations of PKC-α and -ε were measured in presence of AhR inhibitor, α-NF and ROS blocker, NAC. Prior treatment of NAC showed inhibition of translocations, but AhR inhibitor did not affect the translocation patterns of PKC-α and -ε (Fig. 3).
Fig. 3. The effects of AhR and ROS on the translocation of PKC isozymes induced by PCB-4. Representative PKC-α and -ε immunoblot profiles of cytosol and membrane fractions from cells treated with PCB-4 (0 and 50 μM) in the presence or absence of 10 mM NAC (A) and 10 μM α-NF (B). (mem, membrane).
DISCUSSION
It is reported that the ortho-substituted PCB caused the perturbation of calcium homeostasis and PKC translocation in cerebellar granule cells, while the non-ortho-substituted PCBs did not show such effects (Kodavanti et al., 1993, 1994, 1996). Since PKC translocation as measured by the [3H]PDBu binding was observed only in the presence of the extracellular calcium (Kodavanti et al., 1994), it was assumed that the classical PKCs, Ca2+-dependent isozymes, may be involved in this translocation process.
PKC-α is selectively associated with lithium-induced memory impairments (Manji et al., 1993) and translocation of PKC-α has been associated with long-term potentiation in a hippocampus region (Son et al., 1996). PKC-ε has been suggested to be a candidate isoform associated with this presynaptic mechanism of long-term potentiation. This isozyme also plays a role in expression of GAP-43 in neuronal cells, which is the neuron-specific phosphoprotein associated with axonal development and regeneration (Meiri et al., 1988). Our results are consistent with previous report that a significant translocation of PKC-α and PKC-ε from cytosol to membrane fraction plays a key role in the PCB-altered signal transduction pathway. However, it remains unknown whether structural characteristic of PCBs determine PKC translocation.
In an attempt to analyze the pattern of PKC activity following exposure with a variety of PCB congeners in the cerebellar granule cells, we performed [3H]PDBu binding assay. Compared to non-ortho- or mono-ortho PCBs, di-ortho PCBs, which are also known as non-dioxin-like PCBs, showed a significantly higher level of [3H]PDBu binding. The results suggest that chlorination of ortho-position may be important in neuronal cell function and provide PCB congeners with more neuroactive properties. It is interesting to note that general toxicities of dioxin-like PCBs (mono- and non-ortho PCBs) are much higher than non-dioxin-like PCBs with respect to toxic potency, which is classified with Toxic Equivalent Factor (TEF).
PKC consists of 11 isoforms and their function is species- and isoform-specific. PKCs are abundant in neuronal tissue and are involved in neuronal survival and functions of neuronal trophic factors, suggesting a crucial role for PKC in the signal transduction between neurons and the etiology of the neuronal diseases (Matsushima et al., 1996; Hama et al., 1986). Since PKC isoforms are located in different subcellular compartment and have their unique activation profile (Nishizuka, 1988), it is important to analyze the individual PKC isoforms to understand their biological significance in the cellular system. Immunoblot analysis with the monoclonal antibodies revealed that translocation patterns of PKC-α, -and -ε, were similar to those of the total activity measured by [3H]PDBu binding assay. While PKC translocations of non-dioxin-like PCBs are profound, those of dioxin-like PCBs do not show such a pattern. The results suggest that there may exist specific PKC isozymes more susceptible to ortho position of PCBs and structural difference of PCBs may play an important role in modulating a pivotal signal transduction in neurons.
PKC translocation was blocked by antioxidant, suggesting that PKC activation may be mediated via ROS-dependent pathway. The results also demonstrate that oxidative stress may be an important biological event in understanding PCB-mediated alteration of signal transduction pathways. While PKC translocation was blocked by antioxidant, AhR blocker, α-NF, did not affect the translocation pattern of PKC-α, and -ε, following PCB-4 exposure. It is suggested that PCB-4-induced PKC activity may not be mediated via AhR-dependent pathway. Our finding is consistent with other studies which demonstrated more neuroactive responses among non-coplanar PCBs than coplanar PCBs. Most of toxicological assessments of dioxins and PCBs are based on TEF values, which are generated under assumption of AhR-mediated pathways. Thus, AhR-independent responses are not included in the risk assessment process. Our findings confirm that the current risk assessment concept on halogenated aromatic hydrocarbons does not accommodate the neuroactive events that are mediated via non- AhR pathways. Therefore, the present study may contribute to understanding the neurotoxic mechanism of PCBs as well as providing a basis for establishing a better neurotoxic assessment, which has been neglected from the current risk assessment system.
Acknowledgments
This work was supported by the Marine Biomaterials Research Center grant from Marine Biotechnology Program funded by the Ministry of Land, Transport and Maritime Affairs, Republic of Korea.
References
- 1.Carpenter D.O. Polychlorinated biphenyls and human health. Int. J. Occup. Med. Environ. (1998);11:291–303. [PubMed] [Google Scholar]
- 2.Chen C., Kano M., Abeliovich A., Chen L., Bao S., Kim J.J., Hashimoto K., Thompson R.F., Tonegawa S. Impaired motor coordination correlates with persistent multiple climbing fiber innervation in PKC gamma mutant mice. Cell. (1995);83:1233–1242. doi: 10.1016/0092-8674(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 3.Hama T., Huang K.P., Guroff G. Protein kinase C as a component of a nerve growth factor-sensitive phosphorylation system in PC12 cells. Proc. Natl. Acad. Sci. USA. (1986);83:2353–2357. doi: 10.1073/pnas.83.8.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson J.L., Jacobson S.W. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N. Engl. J. Med. (1996);335:783–789. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- 5.Kodavanti P.R., Shafer T.J., Ward T.R., Mundy W.R., Freundrich T., Harry G.J., Tilson H.A. Differential effects of polychlorinated biphenyl congeners on phosphoinositide hydrolysis and protein kinase C translocation in rat cerebellar granule cells. Brain Res. (1994);662:75–82. doi: 10.1016/0006-8993(94)90797-8. [DOI] [PubMed] [Google Scholar]
- 6.Kodavanti P.R., Ward T.R., McKinney J.D., Waller C.L., Tilson H.A. Increased [3H]phorbol ester binding in rat cerebellar granule cells and inhibition of 45Ca2+ sequestration in rat cerebellum by polychlorinated diphenyl ether congeners and analogs: structure-activity relationships. Toxicol. Appl. Pharmacol. (1996);138:251–261. doi: 10.1006/taap.1996.0123. [DOI] [PubMed] [Google Scholar]
- 7.Kodavanti P.R., Shin D.S., Tilson H.A., Harry G.J. Comparative effects of two polychlorinated biphenyl congeners on calcium homeostasis in rat cerebellar granule cells. Toxicol. Appl. Pharmacol. (1993);123:97–106. doi: 10.1006/taap.1993.1226. [DOI] [PubMed] [Google Scholar]
- 8.Manji H.K., Etcheberrigaray R., Chen G., Olds J.L. Lithium decreases membrane-associated protein kinase C in hippocampus: Selectivity for the α isozyme. J. Neurochem. (1993);61:2303–2310. doi: 10.1111/j.1471-4159.1993.tb07474.x. [DOI] [PubMed] [Google Scholar]
- 9.Mariussen E., Myhre O., Reistad T., Fonnum F. The polychlorinated biphenyl mixture aroclor 1254 induces death of rat cerebellar granule cells: The involvement of the N-methyl-D-aspartate receptor and reactive oxygen species. Toxicol. Appl. Pharmacol. (2002);179:137–144. doi: 10.1006/taap.2002.9353. [DOI] [PubMed] [Google Scholar]
- 10.Matsushima H., Shimohama S., Chachin M., Taniguchi T., Kimura J. Ca2+-dependent and Ca2+-independent protein kinase C changes in the brain of patients with Alzheimer’s disease. J. Neurochem. (1996);67:317–323. doi: 10.1046/j.1471-4159.1996.67010317.x. [DOI] [PubMed] [Google Scholar]
- 11.Meiri K.F., Willard M., Johnson M.I. Distribution and phosphorylation of the growth-associated protein GAP-43 in regenerating sympathetic neurons in culture. J. Neurosci. (1988);8:2571–2581. doi: 10.1523/JNEUROSCI.08-07-02571.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molchan S.E., Sunderland T., McIntosh A.R., Schreurs B.G. A functional anatomical study of associative learning in humans. Proc. Natl. Acad. Sci. USA. (1994);91:8122–8126. doi: 10.1073/pnas.91.17.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. (1988);334:661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- 14.Schantz S.L., Moshtaghian J., Ness D.K. Spatial learning deficits in adult rats exposed to ortho-substituted PCB congeners during gestation and lactation Fundam. Appl. Toxicol. (1995);26:117–126. doi: 10.1006/faat.1995.1081. [DOI] [PubMed] [Google Scholar]
- 15.Son H., Madelian V., Carpenter D.O. The translocation and involvement of protein kinase C in mossy fiber-CA3 long-term potentiation in hippocampus of the rat brain. Brain Res. (1996);739:282–292. doi: 10.1016/S0006-8993(96)00836-0. [DOI] [PubMed] [Google Scholar]
- 16.Swanson G.M., Ratcliffe H.E., Fischer L.J. Human exposure to polychlorinated biphenyls (PCBs): a critical assessment of the evidence for adverse health effects. Regul. Toxicol. Pharmacol. (1995);21:136–150. doi: 10.1006/rtph.1995.1018. [DOI] [PubMed] [Google Scholar]
- 17.Vaccarino F.M., Liljequist S., Tallman J.F. Modulation of protein kinase C translocation by excitatory and inhibitory amino acids in primary cultures of neurons. J. Neurochem. (1991);57:391–396. doi: 10.1111/j.1471-4159.1991.tb03765.x. [DOI] [PubMed] [Google Scholar]
- 18.Yang J.H., Kodavanti P.R. Possible molecular targets of halogenated aromatic hydrocarbons in neuronal cells. Biochem. Biophys. Res. Commun. (2001);280:1372–1377. doi: 10.1006/bbrc.2001.4283. [DOI] [PubMed] [Google Scholar]
- 19.Yang J.H., Vogel C., Abel J. A malignant transformation of human cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin exhibits altered expressions of growth regulatory factors. Carcinogenesis. (1999);20:13–18. doi: 10.1093/carcin/20.1.13. [DOI] [PubMed] [Google Scholar]