Abstract
While the ability to develop nanomaterials and incorporate them into products is advancing rapidly worldwide, understanding of the potential health safety effects of nanomaterials has proceeded at a much slower pace. Since 2008, Korea Food and Drug Administration (KFDA) started an investigation to prepare “Strategic Action Plan” to evaluate safety and nano risk management associated with foods, drugs, medical devices and cosmetics using nano-scale materials. Although there are some studies related to potential risk of nanomaterials, physical-chemical characterization of nanomaterials is not clear yet and these do not offer enough information due to their limitations. Their uncertainties make it impossible to determine whether nanomaterials are actually hazardous to human. According to the above mention, we have some problems to conduct the human exposure risk assessment currently. On the other hand, uncertainty about safety may lead to polarized public debate and to businesses unwillingness for further nanotechnology investigation. Therefore, the criteria and methods to assess possible adverse effects of nanomaterials have been vigorously taken into consideration by many international organizations: the World Health Organization, the Organization for Economic and Commercial Development and the European Commission. The object of this study was to develop risk assessment principles for safety management of future nanoproducts and also to identify areas of research to strengthen risk assessment for nanomaterials. The research roadmaps which were proposed in this study will be helpful to fill up the current gaps in knowledge relevant nano risk assessment.
Keywords: Nanomaterials, Nanoproducts, Risk assessment, Research roadmap
INTRODUCTION
In recent years, nanotechnology has advanced rapidly worldwide, and thus nanotechnology has been increasingly applied to a larger number of products (Roco and Boinbridge, 2005; Chaundry et al., 2006). Nanotechnology could potentially provide significant benefits in various areas, including; addressing a number of the current needs in the fields of energy and the environment (Service, 2006; SCENIHR, 2007; US FDA, 2007). On the other hand, rapid increase in the number of nanoproducts worldwide has raised concerns regarding potential adverse effects in consumers (Service, 2003; Lam et al., 2004; Boxall et al., 2008). There is limited evidence which indicates that the use of nanomaterials actually causes harm, but it is commonly accepted that there is a possibility for nanomaterials to do so.
The introduction of nanotechnology applications in the public health-related areas such as food, cosmetics or drug will largely depend on how nanoproducts are regulated in the interest of protecting consumers against potential risks posed by using nanoproducts. Therefore, strategies for managing potential risks of nanoproducts need to ensure that a high level of protection of public health and consumer safety (Silbergeld et al., 2011). Korea Food and Drug Administration (KFDA) started an investigation to prepare “Strategic Action Plan” to evaluate safety and nano risk management associated with foods, drugs, medical devices and cosmetics using nano-scale materials since 2008. We started this study, which aims to develope risk assessment principles for safety management of future nanoproducts, to execute this plan. For this study, we investigated the feasibility associated with conducting a human health risk assessment for nanomaterials based on the open literature and to develop the risk assessment principle utilizing an approach similar to that of a classical regulatory risk assessment (Aschberger et al., 2010; Christensen et al., 2010; Cristensen et al., 2011). We will also present a research road map for the scientific assessment of nanomaterial’s potential risks.
METHODS
Collecting information to identify possibility of exposure induced by nanoproducts. Exposure estimates are essential for assessing risk and it is necessary to investigate information related with nanoproducts to determine real exposure caused by nanoproducts. Information-gathering for conducting a study generally occurs throughout related-area studies, but the study in the initial phase focuses on collecting from available resources. The nanoproductsrelated study was started recently, and related-information can be obtained more easily and quickly on the internet than from traditional sources. So, we carried out a web-based research on several internet resources to investigate nanotechnology- related patents and the current distribution of nano-labeled products in the market. The targeted resources include the World Intellectual Property Organization and Consumer Product Database compiled by Wilson Center Project on Emerging Nanotechnologies (NNPC, 2011; Woodrow wilson center, 2012). But, the US Woodrow Wilson Center relied on data provided by private companies and did not conduct an additional verification, so it is not certain whether nanotechnology was actually applied to products or not. Nevertheless, we used these data to determine consumer’s exposure possibility from nanoproducts because there is no evidence that can identify whether a targeted product is actually a nanoproduct and there is no inventory of nanoproducts at the governmental level as of now.
General approaches to develop nano risk assessment principles. It is essential to secure a data set that is objective and accurate, to develop principles for assessment of potential health risk posed by nanoproducts. Therefore, we adopted general criteria for data selection concerning risk assessment on hazardous materials in food and drug (WHO, 1999). Sources of information considering primary for nanomaterial safety principles were documents written by government agencies or organizations holding databases of relevant nanotechnology (EFSA, 2009; USFDA, 2007; WHO, 2010). After collecting data, we analyzed how much information is available to conduct assessment of health effects induced by exposure to nanomaterials.
RESULTS AND DISCUSSION
Potential exposure to engineered nanomatials in nanoproducts. Nanomaterials are small-scale substances (< 100 nm); an emerging class of functional materials defined by the size-dependent properties (Mahler et al., 2012). Among all nanomaterials that have been brought to the attention of the public, regulators and scientific researchers will have a profound interest in the engineered nanomateirals (ENM) which can be found in a wide variety of products. Therefore, this study will especially take into account ENM related to KFDA regulated products.
Along with toxicological data, exposure estimates are essential for assessing risk. Therefore, we first investigated feasibility for consumer’s real exposure based on open literature review. We looked into patents disclosed by 93 Patent Offices worldwide and World Intellectual Property Organization (WIPO) for 30 days from 1 July 2011. The examination revealed that the number of patents in the category of nano-related products was 14,117, which accounted for 6.09 percent of 232,665 patents in total. South Korea recorded the third highest number (1,193) of patent applications related to nanotechnology in the world, following Japan (3,878) and USA (3,256) (NNPC, 2011).
According to the status of nanotechnology products compiled by the US Woodrow Wilson Center, 1317 products were registered as nanoproducts in 2010 (Fig. 1), of which 587 came from USA, 367 from Europe and 261 from East Asia in terms of region of origin (Fig. 1). By country, South Korea (126) was ranked third following USA and Germany. The number of nanoproducts registered in US Woodrow Wilson Center has grown by nearly 4.2 fold compared to that of 2006 (Fig. 1). In particular, 42 out of 126 nanoproducts were subject to regulation under the food and drug category, including utensils and containers such as cutting boards, feeding bottles, washers and others (Table 1). However, most of products categorized as food or drug were, in fact, food contact materials instead of foods or drugs.
Fig. 1. Number of total products listed, by date of inventory update, with regression analysis (left) and Numbers of products, according to region (right) (adapted from http://www.nanotechproject.org/inventories/consumer/analysis_draft/).
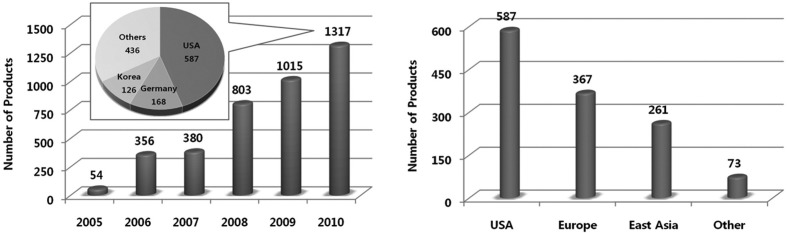
Table 1.
Category of relevant products with KFDA (* is marked when overlapped with other category’s products)
| Main category | Sub category | Product(type) | Etc |
|---|---|---|---|
| Food and Beverage | Cooking | 1 | Nano silver cutting board |
| Storage | 4* | (Overlapped with feeding bottle(1 type) of Goods for Children) | |
| - | 1 | Washer | |
| Generic | 4 | ||
| Goods for Children | Bascics | 7* | (Overlapped 2 type products of Personal Care) |
| Health and Fitness | Cosmetics | 9 | |
| Personal Care | 19* | (Overlapped 2 type products of Goods for Children) | |
| Total | 42 | ||
According to literature search in the field of nanotechnology application in foods and agriculture, nanotechnology is being applied to develop products such as manufactured nanomaterials, nanoemulsion, nanocapsules that contain flavor enhancers, nutrient enhancers, processed foods that enable more freshness and longer storage, packaging materials for food, and ingredients that come into contact with food (EFSA, 2009; WHO, 2010). On the other hand, results from some surveys which were performed on nano-labeled products in Korean marketplaces, demonstrated that it was impossible to confirm whether they are actual nanoproducts that contain nanoscale materials.
The aforementioned result illustrates that research on nanotechnology is well underway in Korea, with rapid responses to the latest trend in cutting-edge, next generation technologies. Moreover, it appears that exposure of consumer to the wide variety of nanoproducts in health and fitness, food and beverages, and many other product categories is likely to become both frequent and extensive in the near future.
Necessity to set principles of risk assessment for nanoproducts. The introduction of new technologies often creates new challenges for regulatory agency if the associated consumer products raise concerns about health risks. General safety management is based on scientific risk assessment principle. In this study, we surveyed literature compiled by other governments concerning policy development of risk assessment to assist safety management of nanomaterials. The OECD established a Working Party on Manufactured Nanomaterials (WPMN) in 2006. Sub-committees (Steering Groups, SG) of the WPMN are looking at different issues such as databases, test guidelines and risk assessment. Particularly, issues related to risk assessment and exposure measurement are being handled by SG6 and SG8. In regulatory aspects of nanomaterials within the EU, it can be concluded that the risks in relation to nanomaterials can be dealt with under the current legislative framework, but it may have to be modified in the light of newly available information (Bengt et al., 2012). Also, EFSA has published “Guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain” (EFSA, 2009). The report provides guidance on nano-specific considerations that need to be assessed in addition to conventional aspects and concluded that the risk assessment paradigm is appropriate for nanomateirals as well. In USA, there are currently no regulations that specifically target nanomaterials related to food or drug safety. In Korea, nanotechnology has been supported as part of a national project, which was established under the framework of nanotechnology development since 2001. Since 2011, specific regulation systems in food and cosmetic sectors have been considered to manage nanoproducts effectively. At present, many regulatory agencies recognize the need to establish a risk assessment policy for nanomaterials safety management. Yet no international standardized principles have been developed to assess potential risk of nanomaterials.
Based on the aforementioned results, we identified the following reasons behind why it is necessary to develop risk assessment principles for ENM. First, there is a high possibility that users of nanoproducts become exposed to nanomaterials; nanomaterials with a relatively small size may exhibit significant toxicity because nanomaterials have a much larger surface or interface area than those of counterpart materials with the same mass of the same chemical structure. Second, the inference caused by biological activity of nanomaterials based on their constituents does not adequately describe the hazards of engineered nanomaterials, which have distinct characteristics designed into them through advanced method. Therefore, we cannot entirely depend on the risk assessment inferences which derived from studies of conventional chemical. Third, the general public has a low level of awareness concerning safety of nanomaterials. Last, over 60 percent of experts said comprehensive measures need to be established and safety assessment needs to be performed to ensure safe management of nanomaterials (Silbergeld et al., 2011; Yoon, 2007; Zero Waste Citizen Center, 2010; Ministry of Environment, 2011). Consequently, this study suggests that the risk of exposure to nanomaterials cannot be overlooked and it is urgent to set up risk assessment principles to address the safety issue concerning exposure to nanomaterials and then regulatory agency should establish precautionary safety management policy based on risk assessment approach to protect public health.
On the other hand, we recognized that there are significant limitations regarding developing the risk assessment principles for safety management of nanoproducts. The first is the absence of unified definition for regulatory targets, as well as the lack of appropriate information to assess potential risk to consumer health. The second is that most of nanotechnology- applied products are currently at the R&D stage. The last is a significant knowledge gap in our current understanding of the risk assessment of nanomaterials in nanoproducts. We incorporated these limitations into the research roadmap introduced in the last section, since these make ultimately difficult to assess the potential risk of nanomaterials.
Proposal of risk assessment principles for safety management of nanoproduts. In this study, we suggested the effective principles and steps for risk assessment to strengthen good science-based decision-making for nanofood and nanodrug products.
Principles of nano risk assessment: The principles for science-based risk assessment of nanomaterials, based on the current approaches to chemical risk assessment framework (hazard identification, hazard characterization, exposure assessment and risk characterization), are as follows; First, risk assessment of nanomaterials should be taken into account in precautionary management strategies until exposure limits are established to avoid risks that might be caused by nanotechnology-applied products. Second, for the risk assessment of nanomaterials, the current toxicity test methods, their limitations, and other factors should be considered according to the 4-phased risk assessment of chemicals. Third, the targets for risk assessment are products in the regulation area of KFDA such as food, drug and cosmetics. Naturally-occurring and incidental nanoparitcles were not included the targets, which only deal with engineered nanomateials (ENMs) related with nanotechnology-enabled products in public health. Fourth, integrated risk assessment of nanomateirals throughout their lifecycles from production to disposal to recycling has to be applied as basic principles during the process of risk assessment. Fifth, upon gathering information about toxicity and safety, it will be possible to yield a critical point of departure (such as NOAEL, BMDL etc.) based on dose-response modeling. These are useful to set up health-based guidance value for nanomaterials. However, if no health-based guidance value is derived, we determine a risk characterization of nanomaterials by performing assessment throughout the application of margin of exposure (MOE) method. Finally, we have to manage relevant information such as data on monitoring, toxicity and exposure in an effective and systemic framework.
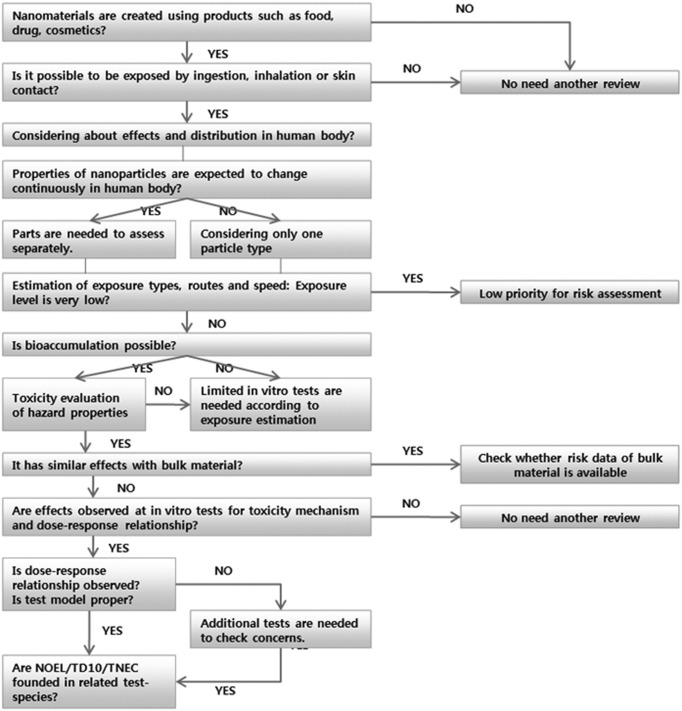
Step by step procedure of nano risk assessment: We suggested the step-by-step procedure to determine risk assessment concerning human exposure to risk from nanomaterial in nanoproducts (Fig. 2). According to the flowsheet, it was concluded that 1) there is little or no exposure, no additional risk assessment is needed, and 2) it is possible to use conventional dose-response modelling for chemicals at the last phase of the flow-sheet. This procedure represents only the current state of knowledge in nanotechnology, and it will be revised in the future. For the full implementation of this flow sheet, substantial methodological developments will be required. It is also required to further develop appropriate dose-response relationship methods for nanomaterials.
Fig. 2. Step-by-step flow sheet for exposure estimation and risk assessment of nanomaterials such as nano-related food and drug.
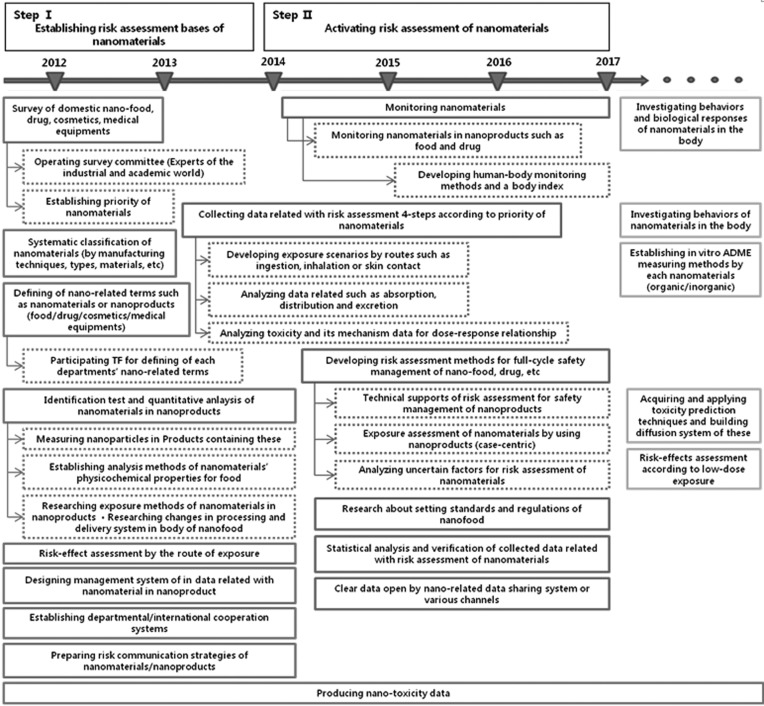
Research roadmap for filling up of knowledge gaps in risk assessment. Risk assessment has been used to provide a decision making-relevant scientific basis for safety management of food and drug. However, available scientific data and information are needed as a tool for decision making related to risk assessment because the scientific underpinnings of risk assessment are increasingly complex. We first identified the knowledge gaps and limitation of information to understand the potential human health effects by ENMs, and then suggested new research fields that will be secured to strengthen nano risk assessment (Table 2 and Fig. 3). KFDA’s Nano Scientific Committees reviewed a number of knowledge gaps in current risk assessment of ENM, and their comments and recommendations were incorporated into the research roadmaps.
Table 2.
Research fields that will be secured to risk assessment nanomaterials by nano-food and drug
| Consistent with various fields experts and comprehensively well-designed research plan are needed | |||
|---|---|---|---|
| Research planning & information management | - Establishing sampling strategies for collecting exposure data and guidelines for collecting standardized data | ||
| - Conducting survey periodically at the national level about the current state of customer exposure by using nanoproducts | |||
| - Statistical analysis, data quality assurance and database development of collecting exposure information | |||
| Exposure assessment (monitoring methods) | - Developing and standardizing measurement methods (establishing guidelines) of nanomaterials (nanomaterials in products, low-dose analysis, etc) | ||
| - Developing exposure by routes such as ingestion, inhalation or skin contact monitoring methods and human-body monitoring methods | |||
| - Developing biomarkers for identifying early exposure | |||
| - Developing techniques and model for quantitative, half-quantitative, or qualitative exposure prediction | |||
| - Developing exposure scenarios of nanomaterials (exposue period attributes: acute/chronic, persistent/ intermittent, cumulative/complex, etc) | |||
| Dose-response (toxicity mechanism) | - Toxicity mechanism studies for analyzing co-relationship between exposure and internal dose, such as dose in target vessel, toxicologically | ||
| - Data related preclinical effects (animal toxicity tests such as immune toxicity, neurotoxicity, reproductive toxicity) | |||
| - Studies related high-dose, low-dose, or extrapolation and human body effects by risks identified in animal toxicity tests | |||
| - Studies related the prediction model of toxicokinetic data or behaviors in the body | |||
| Education and communication | - Training for making experts in each field | ||
| - Continuous exchange of information with the industrial and academic world, consumer group, etc) | |||
| - Establishing system for information exchange through International Symposium or international cooperation research | |||
Fig. 3. Research and development roadmap for the risk assessment of nanomaterials.
One of the limitations is that there is no internationally harmonized definition regarding nano related terms. Generally, targets for risk assessment have to be clearly determined. Therefore, a regulatory agency has to determine what needs to be regulated, and a risk assessor has to determine what needs to be assessed. Recently, national regulatory agencies will be working to define adequate nanorelated terms (Stamm, 2011). As of now, KFDA is contemplating unifying the definition of nanotechnology with other regulatory agencies. The other limitation for nano risk assessment is that there are no approved methods to identify the real exposure from nano-products. The Korean government has implemented the research projects to address human health safety aspects of ENM, but these are still at the initial stage. As shown in Fig. 3, first, it is necessary to obtain information on suitable measuring methods, monitoring of products on the market and validated test methods to establish risk assessment.
KFDA is implementing the interagency cooperation activities to develop “National Nano-safety Strategic Plan (2011~ 2015)” on nanomaterials, nanotechnology, nanoproducts and occupational safety collaborating with Ministry of Education, Science and Technology; Ministry of Knowledge and Economy; Ministry of Employment and Labour; and Ministry of Environment. The Korean government has well recognized the important of potential risk of ENMs, and several projects are on progress, regarding on the human health and environmental safety issues of ENM. This study suggested the risk assessment principles designed to address consumer’s health risk of ENM from nanoproducts in accordance with KFDA’s “Strategic Action Plan”. Also, further research roadmap formulated by Nano Scientific Committees will be helpful to enhance knowledge on and confidence in the scientific assessments carried out in KFDA. More information about the risk assessment principles for nanomaterials is available on the KFDA’s Research Management System (http://rnd.kfda.go.kr).
Acknowledgments
This work was financed by KFDA under the “08181Nanodok547 & 11161Yuhaemool757” research budget.
References
- 1.Aschberger K., Johnston H.J., Stone V., Aitken R.J., Tran C.L., Hankin S.M., Peters S.A., Cristensen F.M. Review of fullerene toxicity and exposure-appraisal of a human health risk assessment, based on open literature. Regul. Toxicol. Pharmacol. (2010);58:455–473. doi: 10.1016/j.yrtph.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Bengt F., Antonio P., Anna A.S. Adverse effects of engineered nanomaterials; exposure, toxicology, and impact on human health. Firth edition. Elsevier Inc.; (2012). pp. 97–117. ISBN 978-0-12-386940-1. [Google Scholar]
- 3.Boxall A.B.A., Chaudhry Q., Sinclair C., Jones A., Aitken R., Jefferson B., Watts C. Current and future predicted environmental exposure to engineered nanoparticles. Central Science Laboratory; York: (2008). p. 89. [Google Scholar]
- 4.Chaundhry Q., Blackburn J., Floyd P., George C., Nwaogu T., Boxall A., Aitken R. A scoping study to identify gaps in environmental regulation for the products and applications of nanotechnologies. Department for Environment, Food and Rural Affairs.; London: (2006). [Google Scholar]
- 5.Cristensen F.M., Johnston H.J., Stone V., Aitken R.J., Hankin S., Peters S., Aschberger K. Nono-silver-feasibility and challenges for human health risk assessment based on open literature. Nanotoxicology. (2010);4:284–295. doi: 10.3109/17435391003690549. [DOI] [PubMed] [Google Scholar]
- 6.Cristensen F.M., Johnston H.J., Stone V., Aitken R.J., Hankin S., Peters S., Aschberger K. Nono-TiO2-feasibility and challenges for human health risk assessment based on open literature. Nanotoxicology. (2011);5:110–124. doi: 10.3109/17435390.2010.504899. [DOI] [PubMed] [Google Scholar]
- 7.EFSA; European Food Safety Authority. The potential risks arising from nanoscience and nanotechnologies on food and feed safety (EFSA-Q-2007-124a). The EFSA Journal. (2009);958:1–39. [Google Scholar]
- 8.Lam C.W., James J.T., McCluskey R., Hunter R.L. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci. (2004);77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 9.Mahler G.J., Esch M.B., Tako E., Southard T.L., Archer S.D., Glahn R.P., Shuler M.L. Oral exposure to polystyrene nanoparticles affects iron absorption. Nat. Nanotechnol. (2012);7:246–271. doi: 10.1038/nnano.2012.3. [DOI] [PubMed] [Google Scholar]
- 10.Ministry of Environment, Republic of Korea. Research report. Public perception about nanomaterials; (2011). [Google Scholar]
- 11.National nanotechnology policy center (NNPC) Available from URL: http://www.nnpc.re.kr Nanotechnology patent (No. 4). (2011)
- 12.Roco M.C., Bainbridge W.S. Societal implications of nanoscience and nanotechnology: Maximizing human benefit. J. Nanopart. Res. (2005);7:1–13. doi: 10.1007/s11051-004-2336-5. [DOI] [Google Scholar]
- 13.Scientific committee on emerging and newly-identified health risks (SCENIHR) Available from URL: http://ec.europa.eu/health/ph_risk/risk_en.htm Opinion on the appropriateness of the risk assessment methodology in accordance with the technical guidance documents for new and existing substances for assessing the risks of nanomaterials. (2007)
- 14.Service R.F. American chemical society meeting. Nanomaterials show signs of toxicity. Science. (2003);300:243. doi: 10.1126/science.300.5617.243a. [DOI] [PubMed] [Google Scholar]
- 15.Service R.F. Priorities needed for nano-risk research and development. Science. (2006);314:45. doi: 10.1126/science.314.5796.45. [DOI] [PubMed] [Google Scholar]
- 16.Silbergeld E.K., Contreras E.Q., Hartung T., Hirsch C., Honberg H., Jachak A.C., Jordan W., Landsiedel R., Morris J., Patri A., Pounds J.G., de Vizcaya Ruiz A., Shvedova A., Tanguay R., Tatarazako N., van Vliet E., Walker N.J., Wiesner M., Wilcox N., Zurlo J. t4 workshop report. Nanotoxicology: “The end of the beginning” - Signs on the roadmap to a strategy for assuring the safe application and use of nanomaterials. ALTEX. (2011);28:236–241. doi: 10.14573/altex.2011.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stamm H. Risk factors: Nanomaterials should be defined. Nature. (2011);476:399. doi: 10.1038/476399c. [DOI] [PubMed] [Google Scholar]
- 18.US FDA. Available from URL: http://www.fda. gov/ScienceResearch/SpecialTopics/Nanotechnology/nanotechnologyTaskTorceReport2007. Nanotechnology: A report of the US Food and Drug Administration Nanotechnology task force. (2007)
- 19.WHO. IPCS environmental health criteria 210: Principles for the toxicological assessment of risks to human health from exposure to chemicals. World health organization; Geneva: (1999). [Google Scholar]
- 20.WHO. FAO/WHO Expert meeting on the application of nanotechnologies in the food and agriculture sectors: potential food safety implications meeting report. (2010)
- 21.Woodrow wilson center. Available from URL: http://www.nanotechproject.org/inventories/consumer/ [Accessed by 2012 01.];The project on emerging nanotechnologies. (2012)
- 22.Yoon C.S. Potential health risks and issues of nanoparticles. Biochem News. (2007);27:7–19. [Google Scholar]
- 23.Zero waste citizen center. Available from URL: http://zerowaste21.org/mkBoard/mkboard.php Provision of information on nanomaterial-containing household products to consumers & survey on consumer’s perception of related risks. (2010)