Abstract
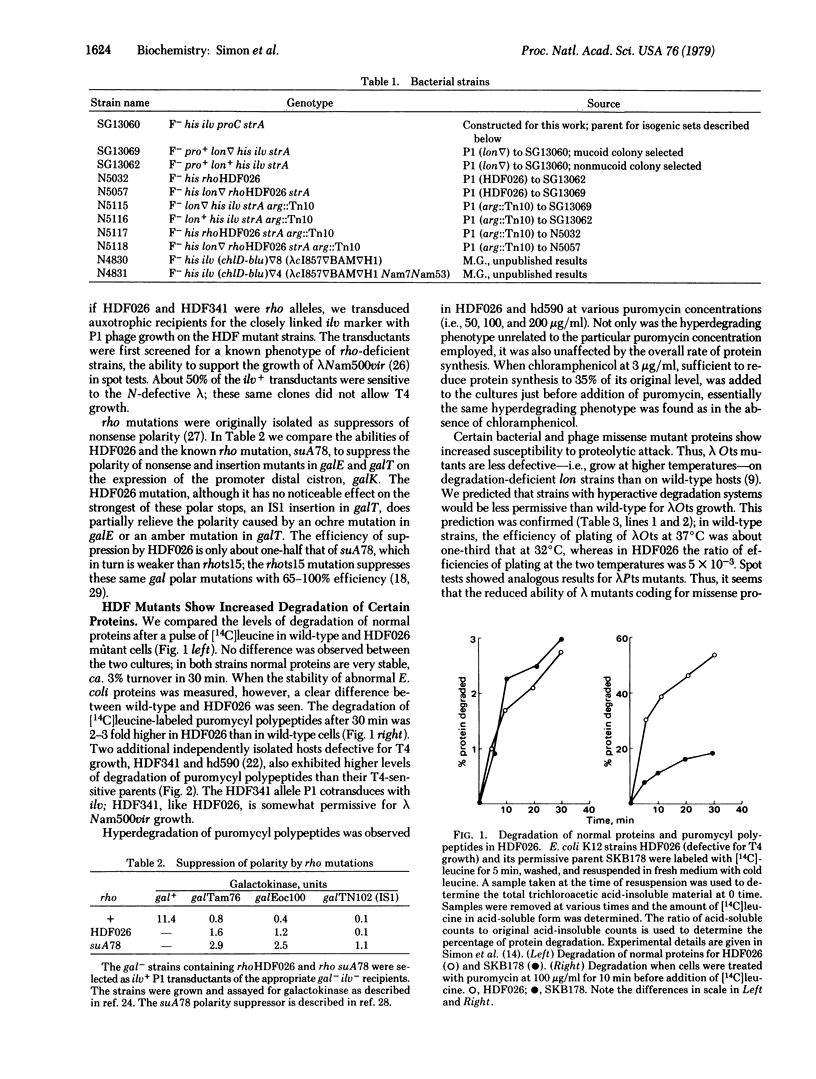
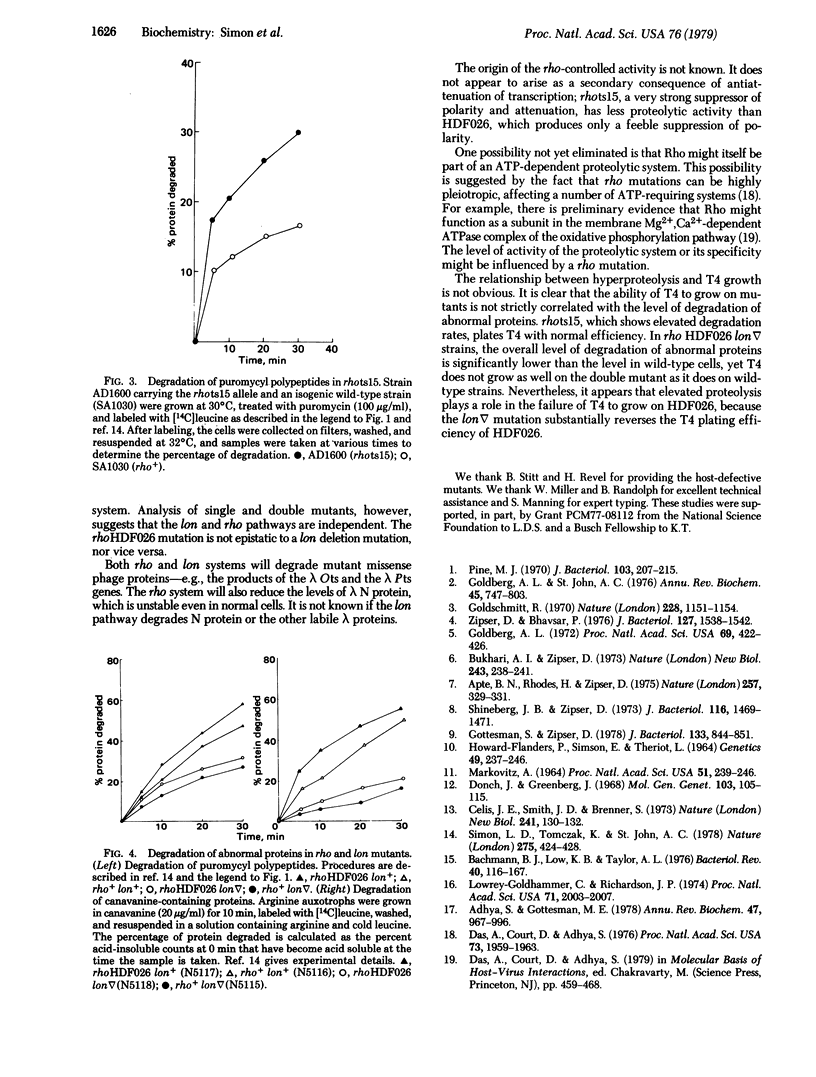
An Escherichia coli mutant, HDF026, defective for growth of phage T4, has been characterized biochemically and genetically. The mutant displays an elevated level of degradation of abnormal proteins, such as puromycyl polypeptides or canavanine-containing polypeptides. Genetically, HDF026 appears to be an allele of rho, which also encodes the transcription termination factor and RNA-dependent ATPase, Rho. The mutation contransduces by phage PI with ilv, weakly suppresses polar mutations in gal, and permits some growth of lambda N- phage. Temperature sensitive lambda mutants in gene O exhibit a reduced efficiency of plating at intermediate temperature on HDF026 mutants; presumably the lambda Ots protein is rapidly degraded in these strains. The ability of wild-type lambda to grow on HDF026 is also reduced, apparently the result of the lambda N product deficiency. gal escape synthesis, which reflects the level of lambda N activity, is decreased 50-66% in the HDF026 mutant. lambda r32, which requires more N function than wild-type phage, does not grow at all in HDF026. A lon mutation, which decreases protein degradation, partially reverses some of these phenotypes, suggesting that they are related to the protein hyperlability of HDF026.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Adhya S., Gottesman M., De Crombrugghe B. Release of polarity in Escherichia coli by gene N of phage lambda: termination and antitermination of transcription. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2534–2538. doi: 10.1073/pnas.71.6.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte B. N., Rhodes H., Zipser D. Mutation blocking the specific degradation of reinitiation polypeptides in E. coli. Nature. 1975 Sep 25;257(5524):329–331. doi: 10.1038/257329a0. [DOI] [PubMed] [Google Scholar]
- BECKWITH J. RESTORATION OF OPERON ACTIVITY BY SUPPRESSORS. Biochim Biophys Acta. 1963 Sep 17;76:162–164. [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachet P., Eisen H., Rambach A. Mutations of coliphage lambda affecting the expression of replicative functions O and P. Mol Gen Genet. 1970;108(3):266–276. doi: 10.1007/BF00283357. [DOI] [PubMed] [Google Scholar]
- Bukhari A. I., Zipser D. Mutants of Escherichia coli with a defect in the degradation of nonsense fragments. Nat New Biol. 1973 Jun 20;243(129):238–241. doi: 10.1038/newbio243238a0. [DOI] [PubMed] [Google Scholar]
- Carter T., Newton A. New polarity suppressors in Escherichia coli: suppression and messenger RNA stability. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2962–2966. doi: 10.1073/pnas.68.12.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis J. E., Smith J. D., Brenner S. Correlation between genetic and translational maps of gene 23 in bacteriophage T4. Nat New Biol. 1973 Jan 31;241(109):130–132. doi: 10.1038/newbio241130a0. [DOI] [PubMed] [Google Scholar]
- Das A., Court D., Adhya S. Isolation and characterization of conditional lethal mutants of Escherichia coli defective in transcription termination factor rho. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1959–1963. doi: 10.1073/pnas.73.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donch J., Greenberg J. Genetic analysis of lon mutants of strain K-12 of Escherichia coli. Mol Gen Genet. 1968;103(2):105–115. doi: 10.1007/BF00427138. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Degradation of abnormal proteins in Escherichia coli (protein breakdown-protein structure-mistranslation-amino acid analogs-puromycin). Proc Natl Acad Sci U S A. 1972 Feb;69(2):422–426. doi: 10.1073/pnas.69.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Goldschmidt R. In vivo degradation of nonsense fragments in E. coli. Nature. 1970 Dec 19;228(5277):1151–1154. doi: 10.1038/2281151a0. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Zipser D. Deg phenotype of Escherichia coli lon mutants. J Bacteriol. 1978 Feb;133(2):844–851. doi: 10.1128/jb.133.2.844-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoko H., Shigesada K., Imai M. Isolation and characterization of conditional-lethal rho mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1162–1166. doi: 10.1073/pnas.74.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Goldhammer C., Richardson J. P. An RNA-dependent nucleoside triphosphate phosphohydrolase (ATPase) associated with rho termination factor. Proc Natl Acad Sci U S A. 1974 May;71(5):2003–2007. doi: 10.1073/pnas.71.5.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKOVITZ A. REGULATORY MECHANISMS FOR SYNTHESIS OF CAPSULAR POLYSACCHARIDE IN MUCOID MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1964 Feb;51:239–246. doi: 10.1073/pnas.51.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J. Steady-state measurement of the turnover of amino acid in the cellular proteins of growing Escherichia coli: existence of two kinetically distinct reactions. J Bacteriol. 1970 Jul;103(1):207–215. doi: 10.1128/jb.103.1.207-215.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shineberg B., Zipser D. The ion gene and degradation of beta-galactosidase nonsense fragments. J Bacteriol. 1973 Dec;116(3):1469–1471. doi: 10.1128/jb.116.3.1469-1471.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L. D., Snover D., Doermann A. H. Bacterial mutation affecting T4 phage DNA synthesis and tail production. Nature. 1974 Dec 6;252(5483):451–455. doi: 10.1038/252451a0. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Tomczak K., St John A. C. Bacteriophages inhibit degradation of abnormal proteins in E. coli. Nature. 1978 Oct 5;275(5679):424–428. doi: 10.1038/275424a0. [DOI] [PubMed] [Google Scholar]
- Zipser D., Bhavsar P. Missense mutations in the lacZ gene that result in degradation of beta-galactosidase structural protein. J Bacteriol. 1976 Sep;127(3):1538–1542. doi: 10.1128/jb.127.3.1538-1542.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



