Abstract
Paecilomyces sinclairiis (PS) is known as a functional food or human health supplement. However concerns have been raised about its kidney toxicity. This study was performed to investigate the kidney toxicity of PS by 13 week-oral administration to rats. Blood urea nitrogen (BUN), serum creatinine, and kidney damage biomarkers including beta-2-microglobulin (β2m), glutathione S-transferase alpha (GST-α), kidney injury molecule 1 (KIM-1), tissue inhibitor of matrix metalloproteinase 1 (TIMP-1), vascular endothelial growth factor (VEGF), calbindin, clusterin, cystatin C, neutrophil gelatinase-associated lipocalin (NGAL) and osteopontin were measured during or after the treatment of PS. BUN, creatinine and kidney damage biomarkers in serum were not changed by PS. However, kidney cell karyomegaly and tubular hypertrophy were observed dose-dependently with higher severity in males. KIM-1, TIMP-1 and osteopontin in kidney and urine were increased dose dependently in male or at the highest dose in female rats. Increased urinary osteopontin by PS was not recovered at 2 weeks of post-exposure in both genders. Cystatin C in kidney was decreased at all treatment groups but inversely increased in urine. The changes in kidney damage biomarkers were more remarkable in male than female rats. These data indicate that the PS may provoke renal cell damage and glomerular filtration dysfunction in rats with histopathological lesions and change of kidney damage biomarkers in kidney or urine. Kidney and urinary KIM-1 and cystatin C were the most marked indicators, while kidney weight, BUN and creatinine and kidney damage biomarkers in serum were not influenced.
Keywords: Kidney toxicity, Kidney damage biomarkers, Paecilomyces sinclairiis, Kidney injury molecule 1, Cystatin C
INTRODUCTION
Cordyceps, an entomogenous fungus formed from larvae and pupae of insects, are regarded as human health supplement or potential drugs for their beneficial effects such as anti-tumorigenicity, immunostimulation, hypoglycemic effects and decreased production of peroxisome (Kuo et al., 1994; Kiho et al., 1996; Shim et al., 2000).
Especially, cordyceps formed from silk worms larvae have been used as a traditional folk remedy or food ingredient to strengthen the immune function and regain vitality in Asian countries including China, Japan and Korea (Ji et al., 2011).
Paecilomyces sinclairiis, a new kind of Cordyceps spp., isolated from cicada larvae has been successfully mass produced through artificial cultivation from silk worm larvae after infected with Paecilomyces sinclairiis (Kim et al., 2003; Shin et al., 2003).
Many studies regarding PS toxicity were performed but there are limited numbers of studies for toxicity of PS only. When adult SD rats were administered orally with the complex powder suspension of Paecilomyces sinclairii with its host Bommbyx mori larvae at doses from 0.008 g/kg bw to 5 g/kg bw for 2 weeks, any toxicological effects were not observed excepting reduced weights of thymus in males (Kwack and Lee, 2009). Single oral toxicity study of the complex showed that doses showing 50% lethality (LD50) were above 5~10 g/kg bw in rats and dogs, which means it is practically non-toxic material (Kim et al., 1996). The complex did not induce any genotoxic effects in a battery of tests; bacterial reverse mutation assay, mammalian cell chromosome aberration assay and rodent micronucleus assay (Ahn et al., 2004b). However, histopathological lesions of kidney were manifested when the complex of fruiting body of Paecilomyces sinclairii and silk worm larvae were provided via feed for 13 weeks (Ahn et al., 2004a). The lesions were tubular destruction, tubular edema and tubular cell abnormalities.
In another study, cordyceps mushrooms have been reported to support kidney functions via increase of 17- hydroxy-corticosteroid and 17-ketosteroid levels (Zhu et al., 1998). So, it is required to elucidate whether PS is beneficial or toxic to kidney functions and the dose of effects if it is a nephrotoxic material.
Kidney histopathology and biochemistry markers such as serum creatinine and BUN have been commonly used for the evaluation of nephrotoxicity of chemicals and bio-materials. However, these indicators show changes only when significant degree of renal functions is damaged (Perazella, 2009). So, the indicators have been skeptical as markers of prediction and diagnosis for kidney damage.
Recently, USA FDA and European Medicines Agency have validated seven urinary renal biomarkers, KIM-1, albumin, clusterin, trefoil factor 3 (TFF3), total protein, cystatin C and β2m for monitoring nephrotoxicity in preclinical toxicity test, which biomarkers were proposed by the Predictive Safety Testing Consortium’s Nephrotoxicity Working Group (Dieterle et al., 2010).
Zhou et al. (2008) suggested a number of novel biomarkers including KIM-1, NGAL, α-GST, glutathione S-transferase- yb1 (GST-Yb1) and Pap X Clone 5C10 (RPA-1) for the detection of acute kidney injury, which are more sensitive than traditional biomarkers, BUN and serum creatinine. These biomarkers have been proposed as sensitive and specific markers of site-specific renal tubular injury following ischemia, necrosis or glomerular filtration dysfunction by renal toxicants (Harpur et al., 2011).
The objective of the present study was to determine whether the PS induce kidney toxicity or not, by using sensitive novel biomarkers of kidney damage.
MATERIALS AND METHODS
Animals. Specific pathogen-free 5-week-old male and female Sprague Dawley (SD) rats were purchased from a commercial animal breeder (Hanrim Lab animal Institute, Gyeongido, Korea). Each fifty male and female rats were acclimated for 1 week in environmentally controlled room at 22 ± 2℃, 50 ± 10% humidity, air ventilation of 10~15 times/hr and a 12 h light/dark cycle, and were provided commercial pellets (Purina Co., Korea) and UV-sterilized and filtered tap water ad libitum.
The animal experiments were conducted in accordance with the Ethics for the Care and Use of Laboratory Animals prepared by Hoseo Toxicological Research Center of Hoseo University.
Materials. Paecilomyces sinclairii endophytically parasitizing dead or living Cicadae subspecies was isolated from conidiospores and cultured in potato dextrose agar medium and then inoculated onto silkworms (Rural Development Administration, KOREA). The fruiting bodies of Paecilomyces sinclairii was collected and then dried and homogenized to be a powder-form. Each amount of the powder of the complex was mixed with feed powder (Rat and Mouse 18% 5L79, PMI Nutrition International, MO, USA) at 5,000 ppm, 10,000 ppm and 50,000 ppm. The mixture was knead by adding distilled water and then made into pellet feed by drying at temperature 57℃.
Treatments. Forty SD male and female rats each were randomly divided into four groups (Control, 5,000, 10,000 and 50,000 ppm) of ten animals each dose group and sex. Further 10 males and females each were allocated into control and the highest group to see recovery of any toxic effects at 2 weeks post-exposure. Control group was provided with normal feed without addition of the powder complex of PS. Male and female rats were administered with PS at 0, 5,000, 10,000 and 50,000 ppm via feeding for 13 weeks.
After 13 weeks of treatment or further 2 weeks postexposure, animals were anesthetized and blood was collected via abdominal artery. Serum samples were collected by centrifugation at 13,000 ×g for 15 min and supernatants were stored at −80℃ until analysis.
Kidneys were taken out and weighed. Half of vertical and horizontal sections of each kidney were fixed in formalin for histopathological examination. Remained parts were weighed on a milligram scale and then transferred to 25 μl of lysis buffer 500 mM NaH2PO4/Na2HPO4 buffer and 0.1% tween 20 in H2O with protease inhibitor cocktail (Roche, Germany) per mg of wet weight tissue. The tissue was ground using a motor glass-teflon homogenizer (DaiHan Scientific Co., Ltd., Korea) with 7 strokes on ice and centrifuged at 16,000 ×g for 15 min at 4℃. The supernatant was collected and stored at ?20℃ until analysis.
On days 13, 55, 90 of treatment and day 13 of recovery, the rats were transferred to individual metabolic cages and 24-hour urine was collected on ice in 50 ml polypropylene tubes containing 1 ml 1% sodium azide. The urine was measured in volume and stored at −80℃ until analysis.
Serum biochemistry analysis. BUN and creatinine in serum were measured using clinical chemistry analyzer (HITACHI 7020) using each assay kit.
Kidney damage biomarkers determination. Serum, kidney tissue homogenates and urine samples were analyzed for KIM-1, TIMP-1, VEGF, osteopontin, clusterin, NGAL, β2m, GST-α, calbindin, and cystatin C using commercially available rat multiplex immunoassay kits (Merck KGaA Darmstadt, Germany) on the multiplex flow cytometry (Luminex xMAP system).
Statistical analysis. Data are expressed as mean ± SD. Statistical significance between the control and treated groups were determined by one-way analysis of variance (ANOVA) followed by Duncan’s multiple tests using the STATISTICA program. A difference in the mean values of p < 0.05 was considered to be statistically significant.
RESULTS
The changes of kidney weight. The absolute and relative kidney weights were not different between control group and treatment groups after 13 weeks of treatment or further 2 weeks post exposure in male and female rats (Table 1).
Table 1.
Absolute and relative kidney weight after 13-week exposure or 2-week recovery after the exposure to diet containing 0 (control group), 5,000, 10,000, 50,000 ppm of Paecilomyces sinclairii in rats
| Dose group (ppm) | 13-Week exposure | 2-Week recovery after exposure | |||||
|---|---|---|---|---|---|---|---|
| 0 | 5000 | 10,000 | 50,000 | 0 | 50,000 | ||
| Male | Right kidney | 1.52 ± 0.24a | 1.51 ± 0.27 | 1.44 ± 0.15 | 1.44 ± 0.08 | 1.59 ± 0.16 | 1.53 ± 0.13 |
| (0.28 ± 0.03)b | (0.29 ± 0.04) | (0.28 ± 0.03) | (0.29 ± 0.02) | (0.28 ± 0.04) | (0.29 ± 0.02) | ||
| Left kidney | 1.49 ± 0.25 | 1.49 ± 0.26 | 1.42 ± 0.15 | 1.41 ± 0.09 | 1.59 ± 0.15 | 1.50 ± 0.09 | |
| (0.28 ± 0.03) | (0.28 ± 0.04) | (0.28 ± 0.03) | (0.29 ± 0.02) | (0.28 ± 0.04) | (0.29 ± 0.02) | ||
| Female | Right kidney | 0.97 ± 0.11 | 0.93 ± 0.12 | 0.87 ± 0.09 | 0.91 ± 0.10 | 0.94 ± 0.07 | 0.87 ± 0.13 |
| (0.32 ± 0.03) | (0.28 ± 0.03) | (0.29 ± 0.02) | (0.31 ± 0.04) | (0.29 ± 0.03) | (0.28 ± 0.02) | ||
| Left kidney | 0.96 ± 0.12 | 0.90 ± 0.11 | 0.86 ± 0.09 | 0.90 ± 0.10 | 1.94 ± 0.05 | 0.86 ± 0.14 | |
| (0.32 ± 0.03) | (0.27 ± 0.03) | (0.29 ± 0.02) | (0.30 ± 0.03) | (0.29 ± 0.03) | (0.28 ± 0.02) | ||
aAbsolute kidney weight (g); bRelative kidney weight (%); Data are mean ± SD (n = 10).
Renal histopathological lesions. At the end of the administration period of 13 weeks, histopathological findings related to the treatment were elucidated in kidney tissues of male and female rats. Kidney cell karyomegaly and tubular cell hypertrophy were observed in almost animals at all doses of treatment with severity was increased with dose dependency. After 2 weeks of withdrawal, the incidence and severity of karyomegaly and tubular cell hypertrophy were decreased but still the pathological lesions were observed in 50% of animals treated. Male rats were affected more severely than female rats (Table 2).
Table 2.
Summary of incidence and severity of rat kidney histopathological lesions after 13-week exposure or 2-week recovery after the exposure to diet containing 0 (control group), 5,000, 10,000, 50,000 ppm of Paecilomyces sinclairii in rats
| Dose group (ppm) | 13-Week exposure | 2-Week recovery after exposure | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5000 | 10,000 | 50,000 | 0 | 50,000 | ||||||||
| Sex | M | F | M | F | M | F | M | F | M | F | M | F | |
| Karyomegaly | Minimal | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 |
| Mild | 0 | 0 | 1 | 1 | 0 | 10 | 0 | 5 | 0 | 0 | 1 | 2 | |
| Moderate | 0 | 0 | 8 | 0 | 10 | 0 | 8 | 3 | 0 | 0 | 4 | 2 | |
| Marked | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | |
| Severity of karyomegaly* | 0 | 0 | 26 | 11 | 30 | 20 | 32 | 21 | 0 | 0 | 14 | 11 | |
| Tubular hypertrophy | 0 | 0 | 8 | 8 | 10 | 10 | 5 | 9 | 0 | 0 | 5 | 5 | |
| Tubular dilatation | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | |
Data represent number of animals showing the lesions among 10 rats of each dose group.
*Severity of karyomegaly: Sum of incidence multiply by severity (0 = not observed, 1 = minimal, 2 = mild, 3 = moderate, 4 =marked).
M, male; F, female.
Serum biochemistry. No significant treatment-related changes were observed in the levels of BUN and serum creatinine after 13 weeks treatment or 2 weeks withdrawal in male and female rats (Data not shown).
Kidney toxicity biomarkers. None of kidney damage biomarkers in serum of treatment groups were significantly different from control group both in male and female rats after 13 weeks of treatment or 2 weeks post exposure (data not shown).
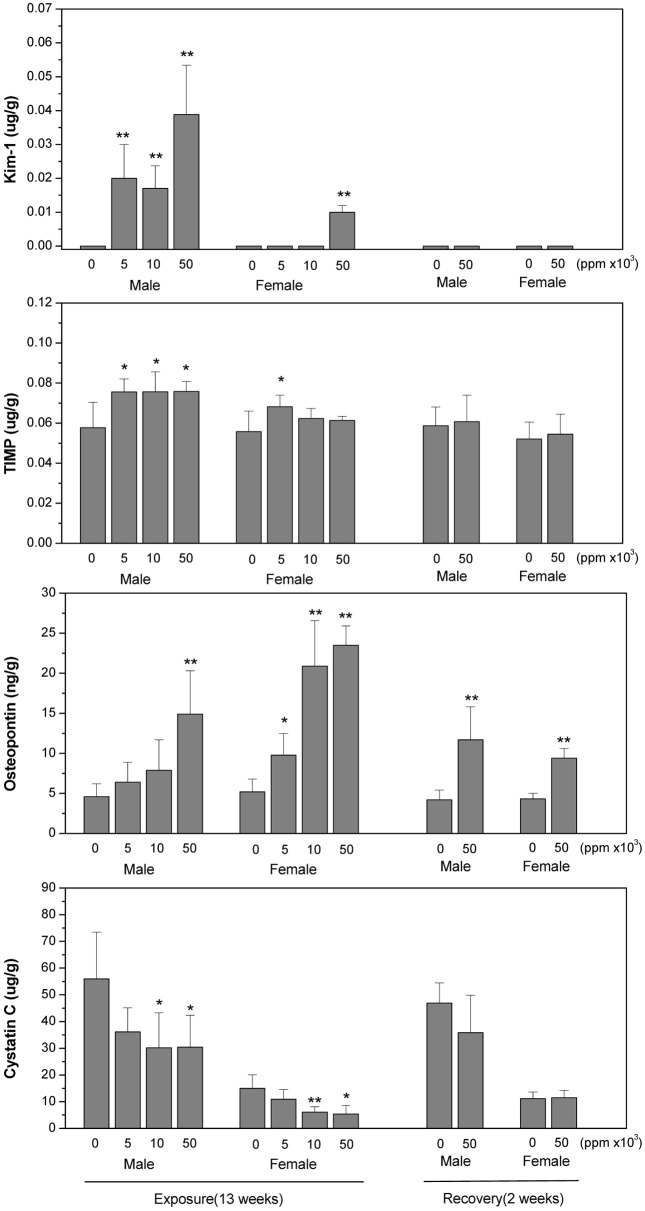
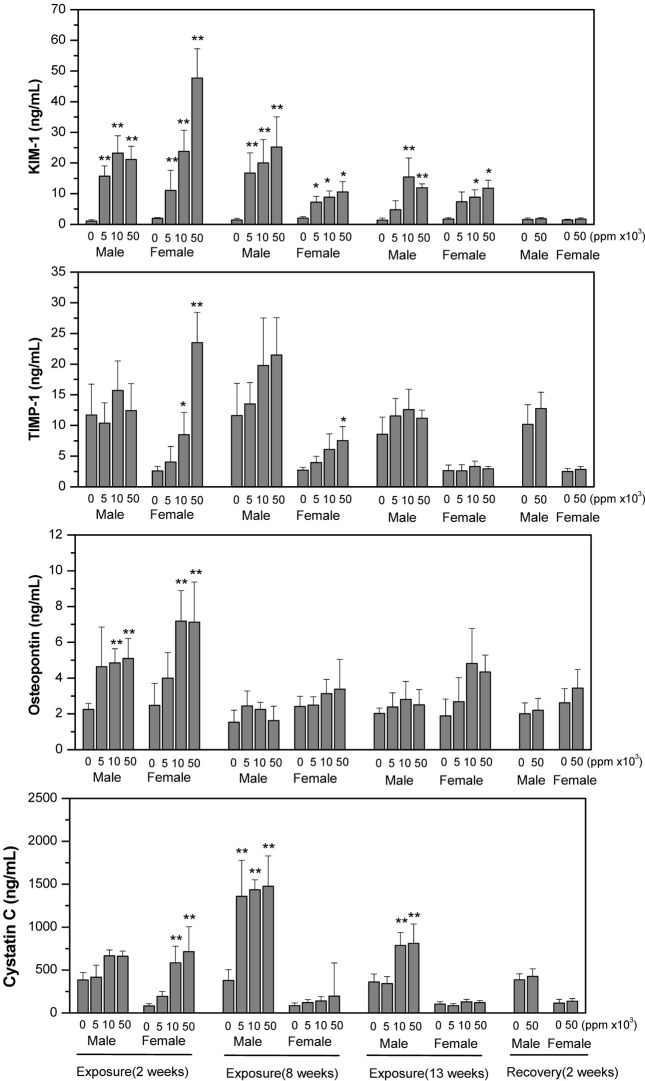
However, the level of KIM-1, TIMP-1 and osteopontin in kidney and urine were significantly increased at all doses of treatment in males and at the highest dose in females after or during 13 weeks of exposure. The increment of osteopontin in kidney of both genders was not recovered at 2 weeks post-exposure. Cystatin C in kidney was significantly decreased at all doses of treatment in both male and female rats but urinary cystatin C was significantly increased at 2, 8 and 13 weeks of exposure and then became normal at 2 weeks post-exposure. The changes in kidney damage biomarkers were more remarkable in male than in female rats (Fig. 1 and 2).
Fig. 1. Changes of KIM-1, TIMP-1, osteopontin and cystatin C in kidney of SD rats at 13 weeks of exposure and 2 weeks of postexposure to the diet containing 0 (control group), 5000, 10000 and 50000 ppm Paecilomyces sinclairii. At the end of exposure and recovery period kidney was collected for analysis. The data are expressed as means ± SD for each dose group (n = 10). * p < 0.05 and ** p < 0.01 between control and treatment group.
Fig. 2. Change of urinary Kim-1, TIMP-1, osteopontin and cystatin C in SD rats at 2, 8 and 13 weeks of exposure and 2 weeks of post-exposure to diet containing 0 (control group), 5000, 10000 and 50000 ppm Paecilomyces sinclairii. During exposure and recovery period, 24-hour urine samples were collected for analysis. The data are expressed as means ± SD for each dose group (n = 5). * p < 0.05 and ** p < 0.01 between control and treatment group.
DISCUSSION
Paecilomyces sinclairii and Isaria sinclairii are entomopathogenic fungi formed from larvae and pupae of cicada and silk worms, respectively. Recently, a technique of mass production of Paecilomyces sinclairii has been successfully developed by isolation of Paecilomyces PS from cicada larvae and then artificial infection and cultivation from silk worm larvae (Kim et al., 2003; Shin et al., 2003).
In the present study, toxicity of the fruiting body of Paecilomyces sinclairii was investigated in the viewpoint of kidney damage.
There have been several studies of the safety of PS; those are acute oral toxicity studies in rats and dogs, sub-acute oral toxicity study in rats and a series of genotoxicity studies (Ahn et al., 2003; Kim et al., 2003, Ahn et al., 2004).
The LD50 of both fungi was above 5 g/kg bw in rats and dogs, which means that they are practically non toxic materials. Isaria sinclairii presented no evidences of genotoxicity in Ames’ test, in vitro chromosome aberration assay and rodent micronucleus test. However, the treatment of feed containing above 1.25% Isaria sinclarii (equivalent to 1 g/kg bw/day) resulted in increased numbers of tubular epithelial cells showing karyomegaly and edematous change with dose dependency in both sexes; however serum biochemical indicators (BUN and creatinine) remained in normal ranges (Ahn et al., 2004; Ahn et al., 2007).
Systemic karyomegaly associated with chronic interstitial nephritis could be the result of the action of some antimitotic agent such as chemical toxins or virus infections (Mihatsch et al., 1979). Ochratoxin A, a well-known nephrotoxic mycotoxin produced by Aspergillus ochraceus, induced karyomegaly with alteration of the tubular tissue and abnormal mitosis together with apoptotic-like kidney cells (Maaroufi et al., 1999). Ochratoxin A suppresses protein synthesis via inhibition of RNA synthesis and also lowers glucose level by the inhibition of phosphoenolpyruvate carboxykinase, a key enzyme in gluconeogenesis (Dirheimer and Creppy, 1991).
In the present study, histopathological lesions of kidney cells; karyomegaly and tubular cell hypertrophy were elucidated with dose-dependency and not recovered at 2 weeks post exposure. The reasons of the histopathological lesions of kidney cells are not clear, but it can be assumed that tubular cell mitosis is interrupted and renal dysfunction may be produced by the complex of fruiting body of Paecilomyces sinclairii and silk worm larvae.
Renal function is routinely monitored by serum creatinine and BUN levels. However, these biochemical indicators often only show elevation when a significant level of kidney functions is damaged. Early detection and diagnosis of kidney injury is critical for the prevention of severe kidney damage and failure.
In recent years, a number of urinary proteins have been proposed as early and sensitive biomarkers of renal injury and indicators of the localization of kidney lesions. These new biomarkers are KIM-1, NGAL, clusterin, GST-α, osteopontin, TIMP-1 and cystatin C (Dieterle et al., 2010; Khan et al., 2010; Lock, 2010; Cruz et al., 2011; Harpur et al., 2011).
The goal of this study was to determine if these new biomarkers are able to detect kidney injury at early stages of nephropathy in rats following a dietary exposure to the powder of PS.
In the present study, a suite of novel nephrotoxicity biomarkers was investigated using a multiplex assay system. Among kidney damage biomarkers investigated, KIM-1 and cystatin C were sensitively influenced by the treatment.
KIM-1 was increased but cystatin C was decreased in kidney with dose-dependency; while these markers in urine samples were all increased in male and female rats during exposure period. Furthermore the increment of KIM-1 in kidney of male rats was not recovered at 2 weeks postexposure.
KIM-1 is a type 1 membrane protein having extracellular immunoglobulin and mucin domains (Ichimura et al., 2004). It is increased dramatically in kidney tissues and urine after injury in proximal tubule epithelial cells in postischemic rodent kidney. KIM-1 is suggested as a sensitive biomarker early-detected when cells undergo regeneration under oxidative stress and inflammation (Ichimura et al., 1998; Swain et al., 2011). When folic acid is treated, urinary KIM-1 is significantly increased despite no change in serum creatinine. Also, cisplatin, a well known nephrotoxicant, results in early increment of urinary KIM-1 and high expression in proximal tubule cells, which occurs before the increment of serum creatinine (Ichimura et al., 2004). When hexachloro-1:3-butadiene (HCBD), a nephrotoxic solvent, was administered intraperitoneally to rats, KIM-1 was increased 4.4 times in the urine comparing control, while 69 times increment in kidney KIM-1 mRNA expression (Swain et al., 2011). Our study also presented that urinary KIM-1 reached peak with 9 times increment after 8 weeks of treatment and when 5 times increment was found in urine, there was 30 times rise in kidney at 13 weeks of exposure.
Cystatin C is a nonglycosylated 13 kDA protein and an extracellular inhibitor of cysteine proteases. It is produced by all nucleated cells at a constant rate, which makes the level of it unaffected by muscle mass unlike creatinin (Koyner et al., 2008). It is reabsorbed at proximal tubule so the level in urine correlates inversely with glomerular filtration rate or tubular damage (Tenstad et al., 1996; Madero and Sarnak, 2009). Togashi and Miyamoto (2012) presented that urinary cystatin C was detected highly in diabetic nephropathy, which was prior to histopathological changes. In our study, urinary cystatin C was higher at 2, 8 and 13 weeks of treatment and then recovered at 2 weeks post exposure. The ratio of decrement of cystatin C in kidney was almost similar to that of increment in male urine at 13 weeks of treatment. However, serum cystatin C was not changed comparing control. These results indicated that cystatin C is an early and sensitive biomarker for nephropathy and the complex of Paecilomyces sinclairii and silk worm larvae may induce proximal tubule damage or filtration dysfunction.
Of the other biomarkers examined in our study, kidney level of TIMP-1 was found to be higher in male rats at 13 weeks of treatment. Urinary osteopontin was higher at 2 weeks of treatment but recovered thereafter even at exposure time. TIMP-1 and osteopontin are all suggested as biomarkers for nephropathy. TIMP-1 and osteopontin are upregulated following ischemia or cellular regeneration, which reveals kidney epithelial cell damage.
In addition, we also observed more pronounced response in male than female rats both in the changes of levels of biomarkers and histopathological lesions. Female rodents were reported to resist to ischemic acute renal failure more effectively than male animals (Wei et al., 2005). Muller et al. (2002) provided that the estrogen shows protective roles in ischemic acute renal failure in females. Acute renal failure is defined as a sudden loss of kidney functions to excrete metabolic wastes, reabsorb electrolytes and maintain body fluid balance (Schrier et al., 2004).
Overall, a panel of new urinary biomarkers has been used to detect kidney damage following dietary exposure to the powder of PS in rats. The results suggest that KIM-1, cystatin C, TIMP-1 and osteopontin may serve as a promising noninvasive urinary biomarkers for earlier detection and monitoring of renal injury associated with damaged kidney cell and filtration failure.
We also demonstrated that levels of urinary and kidney KIM-1 and cystatin C were the most sensitively changed by the treatment with histopathological lesions of kidney.
Our study reveals that PS exposed orally may induce kidney cell damage and filtration dysfunction with more sensitivity in male rats. The mechanism underlying the nephrotoxicity of PS is unclear. A possible explanation can be obtained by further study focusing nephropathy with mechanistic and specific biomarkers of kidney toxicity.
Acknowledgments
This work was supported by the research grant of the Rural Development Administration Korea in 2011.
References
- 1.Ahn M.Y., Han J.W., Jee S.D., Hwang J.S., Hwang S.J., Hong Y.N., Kim S.N. A thirteen-week oral dose subchronic toxicity study of Isaria sinclairii in rats. J. Toxicol. Pub. Health. (2007);23:363–371. [Google Scholar]
- 2.Ahn M.Y., Kang S.C., Jung N.J., Koo H.J., Kwack S.J., Yoo E.J., Jung J.A., Ko J.K., Ryu K.S., Jee S.D., Lee Y.W., Lee B.M. Acute oral toxicity of Paecilomyces sinclairii in beagle dogs. J. Toxicol. Pub. Health. (2003);19:241–245. [Google Scholar]
- 3.Ahn M.Y., Jee S.D., Kim J.Y., Han J.W., Lee Y.K., Lee Y.W., Ryu K.S., Lee B.M., Jung N.J., Kim S.N. Thirteen- week repeated oral toxicity study of Paecilomyces sinclairii in Sprague-Dawley rats. J. Toxicol. Pub. Health. (2004a);20:339–348. [Google Scholar]
- 4.Ahn M.Y., Ryu K.S., Jee S.D., Kim I., Kim J.W., Kim Y.S., Kim H.S., Kim I.S., Kang S.C., Koo H.J., Park Y.A., Choi S.M., Yoo E.J., Kwack S.J., Yoo S.D., Lee B.M. Genotoxicity evaluation of Isaria sinclarii (ISE) extract. J. Toxicol. Environ. Health Part A. (2004b);67:2037–2044. doi: 10.1080/15287390490514796. [DOI] [PubMed] [Google Scholar]
- 5.Cruz D.N., de Geus H.R., Bagshaw S.M. Biomarker strategies to predict need for renal replacement therapy in acute kidney injury. Semin. Dial. (2011);24:124–131. doi: 10.1111/j.1525-139X.2011.00830.x. [DOI] [PubMed] [Google Scholar]
- 6.Dieterle F., Sistare F., Goodsaid F., Papaluca M., Ozer J.S., Webb C.P., Baer W., Senagore A., Schipper M.J., Vonderscher J., Sultana S., Gerhold D.L., Phillips J.A., Maurer G., Carl K., Laurie D., Harpur E., Sonee M., Ennulat D., Holder D., Andrews-Cleavenger D., Gu Y.Z., Thompson K.L., Goering P.L., Vidal J.M., Abadie E., Maciulaitis R., Jacobson-Kram D., Defelice A.F., Hausner E.A., Blank M., Thompson A., Harlow P., Throckmorton D., Xiao S., Xu N., Taylor W., Vamvakas S., Flamion B., Lima B.S., Kasper P., Pasanen M., Prasad K., Troth S., Bounous D., Robinson-Gravatt D., Betton G., Davis M.A., Akunda J., McDuffie J.E., Suter L., Obert L., Guffroy M., Pinches M., Jayadev S., Blomme E.A., Beushausen S.A., Barlow V.G., Collins N., Waring J., Honor D., Snook S., Lee J., Rossi P., Walker E., Mattes W. Renal biomarker qualification submission: a dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat. Biotechnol. (2010);28:455–462. doi: 10.1038/nbt.1625. [DOI] [PubMed] [Google Scholar]
- 7.Dirheimer G., Creppy E.E. Mechanism of action of ochratoxin A. IARC Sci. Publ. (1991);115:171–186. [PubMed] [Google Scholar]
- 8.Guan Y.J., Hu Z., Hou M. [Effect of Cordyceps sinensis on T-lymphocyte subsets in chronic renal failure. Chin]. Zhongguo. Zhong. Xi. Yi. Jie. He. Za. Zhi. (1992);12:338–339. [PubMed] [Google Scholar]
- 9.Harpur E., Ennulat D., Hoffman D., Betton G., Gautier J.C., Riefke B., Bounous D., Schuster K., Beushausen S., Guffroy M., Shaw M., Lock E., Pettit S. Biological qualification of biomarkers of chemical-induced renal toxicity in two strains of male rat. Toxocol. Sci. (2011);122:235–252. doi: 10.1093/toxsci/kfr112. [DOI] [PubMed] [Google Scholar]
- 10.Ichimura T., Bonventre J.V., Bailly V., Wei H., Hession C.A., Cate R.L., Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. (1998);273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 11.Ichimura T., Hung C.C., Yang S.A., Stevens J.L., Bonventre J.V. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am. J. Renal. Renal Physiol. (2004);286:F552–F563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 12.Ji S.D., Sung G.B., Kang P.D., Kim K.Y., Choi Y.S., Kim N.S., Woo S.O., Han S.M., Hong I.P., Ha N.G. Synnemata production using silkworm variety, female Yangwonjam by Isaria tenuipes. Mycobiology. (2011);39:158–163. doi: 10.5941/MYCO.2011.39.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan E., Batuman V., Lertora J.J. Emergence of biomarkers in nephropharmacology. Biomark. Med. (2010);4:805–814. doi: 10.2217/bmm.10.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiho T., Yamane A., Hui J., Ukai S., Ukai S. Polysaccharides in fungi. XXXVI. Hypoglycemic activity of a polysaccharide (CS-F30) from the cultural mycelium of Cordyceps sinensis and its effect on glucose metabolism in mouse liver. Biol. Pharm. Bull. (1996);19:294–296. doi: 10.1248/bpb.19.294. [DOI] [PubMed] [Google Scholar]
- 15.Kim H.S., Lee S.K., Kim K.B., Kwack S.J., Ahn M.Y., Choi B.C., Lee B.M. Acute oral toxicity of KDRD-002 in rats. J. Appl. Pharmacol. (1996);4:310–313. [Google Scholar]
- 16.Kim S.W., Hwang H.J., Xu C.P., Choi J.W., Yun J.W. Effect of aeration and agitation on the production of mycelial biomass and exopolysaccharides in an enthomopathogenic fungus Paecilomyces sinclairii. Lett. Appl. Microbiol. (2003);36:321–326. doi: 10.1046/j.1472-765X.2003.01318.x. [DOI] [PubMed] [Google Scholar]
- 17.Koyner J.L., Bennett M.R., Worcester E.M., Ma Q., Raman J., Jeevanandam V., Kasza K.E., O’Connor M.F., Konczal D.J., Trevino S., Devarajan P., Murray P.T. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. (2008);74:1059–1069. doi: 10.1038/ki.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo Y.C., Lin C.Y., Tsai W.J., Wu C.L., Chen C.F., Shiao M.S. Growth inhibitors against tumor cells in Cordyceps sinensis other than cordycepin and polysaccharides. Cancer Invest. (1994);12:611–615. doi: 10.3109/07357909409023046. [DOI] [PubMed] [Google Scholar]
- 19.Kwack S.J., Lee B.M. Subacute oral toxicity study of a new type of Cordyceps, Paecilomyces sinclairii, in Sprague- Dawley rats. Toxicol. Res. (2009);25:101–106. doi: 10.5487/TR.2009.25.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lock E.A. Sensitive and early markers of renal injury: Where are we and what is the way forward? Toxicol. Sci. (2010);116:1–4. doi: 10.1093/toxsci/kfq128. [DOI] [PubMed] [Google Scholar]
- 21.Maaroufi K., Zakhama A., Baudrimont I., Achour A., Abid S., Ellouz F., Dhouib S., Creppy E.E., Bacha H. Karyomegaly of tubular cells as early stage marker of the nephrotoxicity induced by ochratoxin A in rats. Hum. Exp. Toxicol. (1999);18:410–415. doi: 10.1191/096032799678840192. [DOI] [PubMed] [Google Scholar]
- 22.Madero M., Sarnak M. J. Association of cystatin C with adverse outcomes. Curr. Opin. Nephrol. Hypertens. (2009);18:258–263. doi: 10.1097/MNH.0b013e328326f3dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mihatsch M.J., Gudat F., Zollinger H.U., Heierli C., Thölen H., Reutter F.W. Systemic karyomegaly associated with chronic interstitial nephritis. A new disease entity? Clin. Nephrol. (1979);12:54–62. [PubMed] [Google Scholar]
- 24.Muller V., Losonczy G., Heemann U., Vannay A., Fekete A., Reusz G., Tulassay T., Szabó A.J. Sexual dimorphism in renal ischemia-reperfusion injury in rats: Possible role of endothelin. Kidney Int. (2002);62:1364–1371. doi: 10.1111/j.1523-1755.2002.kid590.x. [DOI] [PubMed] [Google Scholar]
- 25.Perazella M.A. Renal vulnerability to drug toxicity. Clin. J. Am. Soc. Nephrol. (2009);4:1275–1283. doi: 10.2215/CJN.02050309. [DOI] [PubMed] [Google Scholar]
- 26.Schrier R.W., Wang W., Poole B., Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J. Clin. Invest. (2004);114:5–14. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shim J.Y., Lee Y.S., Lim S.S., Shin K.H., Hyun J.E., Kim S.Y., Lee E.B. Pharmacological activities of Paecilomyces japonica, a new type Cordyceps sp. Kor. J. Pharmacogn. (2000);31:163–167. [Google Scholar]
- 28.Shin K.H., Lim S.S., Lee S., Lee Y.S., Jung S.H., Cho S.Y. Anti-tumor and immuno-stimulating activities of the fruiting bodies of Paecilomyces japonica, a new type of Cordyceps spp. Phytother. Res. (2003);17:830–833. doi: 10.1002/ptr.1253. [DOI] [PubMed] [Google Scholar]
- 29.Swain A., Turton J., Scudamore C.L., Pereira I., Viswanathan N., Smyth R., Munday M., McClure F., Gandhi M., Sondha S., Yorka M. Urinary biomarkers in hexachloro-1:3-butadiene-induced acute kidney injury in the female Hanover Wistar rat; correlation of α-glutathione S-transferase, albumin and kidney injury molecule-1 with histopathology and gene expression. J. Appl. Toxicol. (2011);31:366–377. doi: 10.1002/jat.1624. [DOI] [PubMed] [Google Scholar]
- 30.Tenstad O., Roald A.B., Grubb A., Aukland K. Renal handling of radiolabelled human cystatin C in the rat. Scand. J. Clin. Lab. Invest. (1996);56:409–414. doi: 10.3109/00365519609088795. [DOI] [PubMed] [Google Scholar]
- 31.Togashi Y., Miyamoto Y. Urinary cystatin C as a biomarker for diabetic nephropathy and its immunohistochemical localization in kidney in Zucker diabetic fatty (ZDF) rats. Exp. Toxicol. Pathol. (2012):8. doi: 10.1016/j.etp.2012.06.005. Epub. Ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Wei Q., Wang M.H., Dong Z. Differential gender differences in ischemic and nephrotoxic acute renal failure. Am. J. Nephrol. (2005);25:491–499. doi: 10.1159/000088171. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y., Vaidya V.S., Brown R.P., Zhang J., Rosenzweig B.A., Thompson K.L., Miller T.J., Bonventre J.V., Goering P.L. Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury, and chromium. Toxicol. Sci. (2008);101:159–170. doi: 10.1093/toxsci/kfm260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu J.S., Halpern G.M., Jones K. The scientific rediscovery of an ancient chinese herbal medicine: Cordyceps sinensis: part I. J. Altern. Complement Med. (1998);4:289–303. doi: 10.1089/acm.1998.4.3-289. [DOI] [PubMed] [Google Scholar]