Abstract
Rats were administered zearalenone (ZEA) via gavage at dosages of 0, 1, 5, and 30 mg/kg for 36 days. On treatment day 8, inactivated porcine parvovirus vaccine (Vac) was injected intraperitoneally. Antibody production against porcine parvovirus was then measured as a function of ZEA treatment. Compared to the vaccine alone, ZEA treatment, with or without Vac, decreased the serum level of IgG. The level of IgM decreased in all ZEA groups at day 22, but the decrease was sustained only in the medium-dose ZEA group at day 36. The level of IgA was unchanged in the Vac only and ZEA groups at day 22, but was decreased in the 5 mg/kg ZEA plus Vac group compared to the Vac only group at day 36. The level of IgE was decreased by all doses of ZEA at day 22, but was unaffected in ZEA plus Vac groups compared to the Vac only group. The levels of IL-1 in the thymus and spleen; INF-γ in serum; IL-2, IL-6, and IL-10 in the thymus; and IL-10 and IFN-γ in the spleen decreased after ZEA administration. Furthermore, the levels of IL-1β in the spleen and mesenteric lymph node, IL-1β in the thymus, IL-2 in the thymus and spleen, IL-6 in the thymus, IL-10 and IFN-γ in the spleen, and GM-CSF and TNF-α in the thymus decreased after vaccination in rats exposed to ZEA. In conclusion, these results suggest that ZEA exposure via drinking water can cause an immunosuppressive effect by decreasing immunoglobulins in serum and cytokines in lymphoid organs.
Keywords: Zearalenone, Wistar rat, Immune parameters, Cytokines, Immunoglobulin
INTRODUCTION
Zearalenone (hexahydro-14, 16-dihydroxy-3-methyl-1H- 2-deoxacyclotetradecine-1,7(8H)-dione, ZEA) is a metabolite of fungi of the genus Fusarium (Bennett et al., 1994) and commonly found in grains including barley, corn, oats, rice, and wheat, and foods containing these grains (Urraca et al., 2005; Zinedine et al., 2007). ZEA is commonly found on several foods and feeds in the temperate regions of Europe, Africa, Asia, America and Oceania. Although ZEA is considered to have a relatively low oral acute toxicity in mice, rats and guinea pig, it produces endocrine effects by binding to estrogen receptors, most importantly producing disruptions in the reproductive system, in animals (Kuiper-Goodman et al., 1987; Zinedine et al., 2007). ZEA is also hepatotoxic, inducing apoptotic processes, and disturbs enzymatic and hematological parameter in mice (Abbès et al., 2006). Zearalenone can be excreted into milk if fed in high doses to lactating cows. But zearalenone and its metabolites were not found in the milk of three lactating cows fed 0.1 and 0.33 mg/kg body weight (bw) for 21 days (Prelusky et al., 1990). So, many countries have been setting maximum level for ZEA in feed and food.
Many fungal toxins are reported to have immunotoxicity (Bondy and Pestka, 2000). ZEA is frequently implicated in immunological disorders and hyperoestrogenic syndromes, and contributes to the increased risk of cancer and other diseases. Dietary ZEN and deoxynivalenol (DON) depressed the plaque-forming response to sheep red blood cells, the delayed the hypersensitivity response to keyhole limpet haemocyanin and the ability to resist Listeria monocytogenes by increasing NK cell activity in the mouse (Pestka et al., 1987). In vitro, it increased IL-2 and IL-5 by inhibiting of mitogen-stimulated lymphocyte proliferation (Atkinson and Miller, 1984; Forsell and Pestka, 1985). ZEA decreased IgA and IgG concentrations and expression of CD3+ and CD56+ on blood lymphocyte in mice (Abbès et al., 2006). The immunosuppressive effect of ZEA was mediated by depressed T and B cell activity in chickens (Swamy et al., 2004). ZEA and its derivatives showed divergent effects on important parameters of innate immunity in swine, such as cell proliferation, IL-8 and
![]()
synthesis (Marin et al., 2010). High doses of ZEA alone produced significant decreases in total white blood cell counts, immunoglobulin profiles (IgG and IgM), B cells, T-cell sub-types (CD3+, CD4+ and CD8+) and natural killer cells and pro-inflammatory cytokines (Ben Salah-Abbès et al., 2008).
Immune function is an important barrier against infectious disease. Immune suppression by chemicals inevitably increases the rate of infection by pathogens in humans and other animals, and decreases vaccination efficacy. Parvovirus can produce a transient aplastic crisis with hemolytic anemia and is associated with the common childhood illness erythematic infectiosum (Brown and Young, 1995; Chisaka et al., 2003) and abortion in swine (Bodine et al., 1981). Consumption of some mycotoxins, at concentrations that do not cause overt clinical mycotoxicosis, can suppress immune functions and decrease resistance to infectious disease.
The aim of the present work is to investigate the effect of ZEA on cytokines in lymphoid organs and serum, and antibody production against parvovirus in female Wistar rats administered with ZEA and inactivated porcine parvovirus vaccine.
MATERIALS AND METHOD
Chemicals and reagents. ZEA and inactivated porcine parvovirus vaccine were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Green Cross Veterinary Products Co., Ltd. (YoungIn, Korea), respectively. Commercial immunoglobulin and cytokine kits were purchased from Bethyl Laboratories Inc. (TX, USA) and Bio-Rad (CA, USA) and hemagglutin inhibition kits were provided by Animal, Plant and Fisheries Quarantine and Inspection Agency (Anyang, Korea).
Animals and treatment. Forty female Wistar rats (6 weeks old) obtained from Charles River Inc. (Yokohama, Japan) were maintained in SPF animal care room artificially conditioned at 22~26℃ and 40~60% humidity, and on a 12-h light/dark cycle. All rats were housed in polycarbonate cages with sterilized softwood bedding and provided ultra-pure water and rodent diet (Purina Laboratory Rat Chow) ad libitum. After 1 week of acclimation, animals were divided into 8 groups (two or three per each cage) by randomization of body weight (bw). Rats in the low, medium and high groups were administered with 1, 5 and 30 mg ZEA/kg bw by gavage once per day from day 0 to day 35, respectively. Rats of vaccine (Vac), ZEA Low with Vac, ZEA Medium with Vac and ZEA High with Vac groups were inoculated with 2.0 ml of PPV per rat once subcutaneously on day 8 of treatment. Blood samples were collected from jugular vein on day 22. Feed and water consumption and body weight were measured daily during treatment. At the end of treatment, all animals were sacrificed and dissected organs (liver, spleen, adrenal gland, kidney, mesenteric lymph node, vagina, uterus, ovary, thymus, lung, thyroid, pituitary, and brain) for the determination of organ weights and cytokines contents in spleen, thymus and mesenteric lymph node, and examination of gross and pathological findings.
Antibody titer against porcine parvovirus. The heatinactivated serum (56℃ for 30 min) was diluted 1 : 4 in PBS (pH 7.2) with 25 μg of 25% kaolin to avoid non-specific reaction, left for 20 min at room temperature, then centrifuged at 700 g for 10 min. The supernatant serum was collected for the hematogglutination inhibition test.
Immunoglobulins content. Serum immunoglobulin (IgG, IgA, IgM and IgE) content was examined by Spectra max plus (Molecular Devices, USA) using ELISA kit (Bethyl Loabroatories Inc., USA) according to the manufacturer’s instructions.
Cytokines content. Quantization of interleukin-1α (IL- 1α), interleukin-1β beta (IL-1β), interleukin-2 (IL-2), interleukin- 4 (IL-4), interleukin-6 (IL-6), interleukin-10 (IL-10), granulocyte macrophage colony-stimulating factor (GMCSF), interferon-gamma (IFN-γ), and tumor necrosis factor- α (TNF-α) in serum and lymphoid organs (spleen, mesenteric lymph node and thymus) was performed using a Bio- Plex Rat cytokine 9-plex panel (Bio-Rad R171-K11070, Bio-Rad, CA, USA) according to the manufacturer’s instructions.
Statistical analysis. Data are expressed as mean ± S.D. Statistical significance of differences was determined using Statistica software (ver 6.0; StatSoft, USA) by performing one-way analysis of variance (ANOVA), followed by Duncan test as a post-hoc comparison. A p value < 0.05 was taken as a statistically significant between the groups.
RESULTS
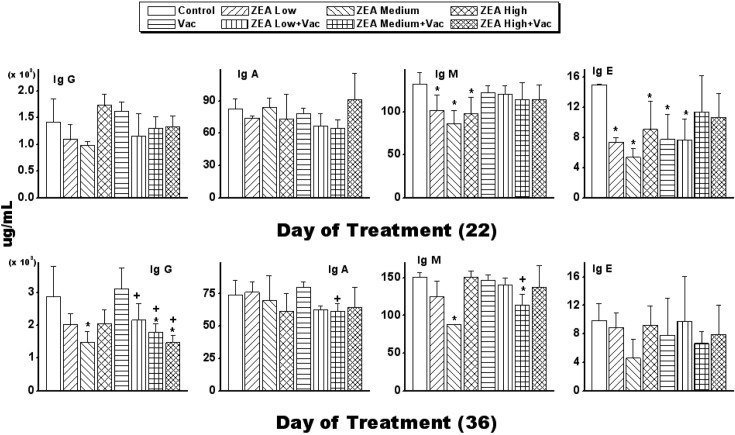
Antibody to porcine parvovirus started to be detected 2 weeks after vaccination and was increased more than 6 fold over the following 2 weeks. Although the ZEA medium with Vac group decreased antibody production at 2 weeks, it was not dose-dependent (Table 1). The IgG concentration in serum was decreased by ZEA treatment with or without Vac, and the concentration was also significantly decreased (p < 0.05) in ZEA with Vac groups compared to the Vac only group. The concentration of IgM was decreased significantly (p < 0.05) by ZEA treatment on day 22 but was decreased significantly (p < 0.05) only in the ZEA medium group at day 36, and the concentration was also significantly decreased (p < 0.05) in the ZEA medium with Vac group compared to the Vac only group. IgA content was not changed in the Vac only group and ZEA with/without Vac groups at day 22, but the concentration was significantly decreased (p < 0.05) in the ZEA medium with Vac group compared to for the Vac only group on day 36. Regarding the concentration of IgE, it was decreased significantly (p < 0.05) by all doses of ZEA alone on day 22, but the concentration was not affected by ZEA with Vac groups compared to the Vac only group (Fig. 1).
Table 1.
Antibody level in serum against porcine parvovirus by the exposure to ZEA in female Wistar rats
| Treatment | Antibody titer | ||
|---|---|---|---|
| Before | 2 weeks | 4 weeks | |
| Vac | ND | 32.0 ± 2.0 | 194.0 ± 2.2 |
| ZEA Low with Vac | ND | 27.9 ± 3.5 | 256.0 ± 2.7 |
| ZEA Medium with Vac | ND | 6.3 ± 1.5 | 168.9 ± 2.2 |
| ZEA High with Vac | ND | 25.4 ± 1.5 | 181.0 ± 2.0 |
Rats in ZEA low, medium and high groups were administered with 1mg, 5mg and 30 mg ZEA/kg bw by gavage once per day for 35 days and Vac groups were vaccinated with 2.0mL of inactivated porcine parvovirus vaccine subcutaneously on day 8 (Vac, ZEA Low with Vac, ZEA Medium with Vac and ZEA High with Vac groups). Values are mean ± S.D. of 5 replicas. ND, not detected
Fig. 1. Changes of immunoglobulins in serum by administration of zearalenone to female Wistar rats. Rats in ZEA low, medium and high groups were administered 1mg, 5mg and 30 mg ZEA/kg bw by gavage once per day for 35 days. Rats in Vac groups were administered and 2.0ml of inactivated porcine parvovirus vaccine subcutaneously on day 8 (Vac, ZEA Low+ Vac, ZEA Medium + Vac and ZEA High + Vac groups). Values are mean ± S.D. of 5 replicas. *: Significantly different from control at p< 0.05. +: Significantly different from Vac at p < 0.05.
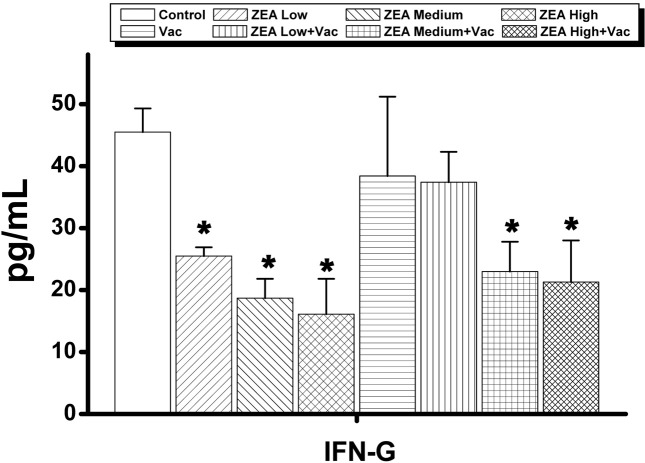
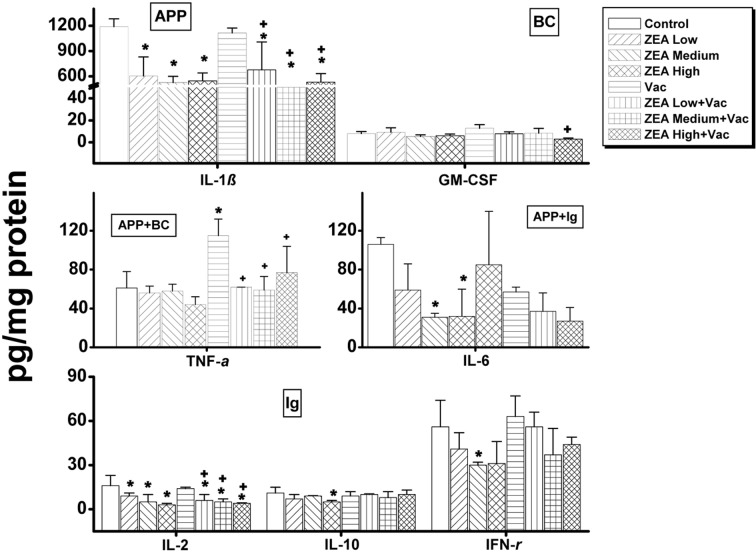
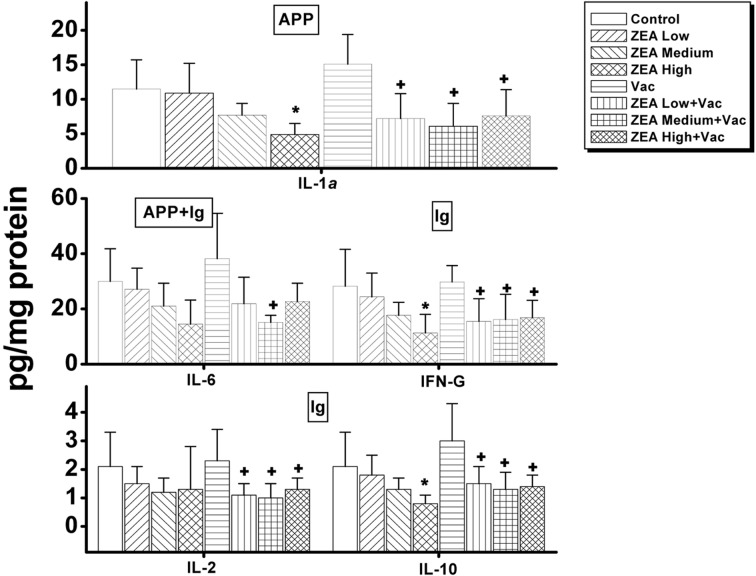
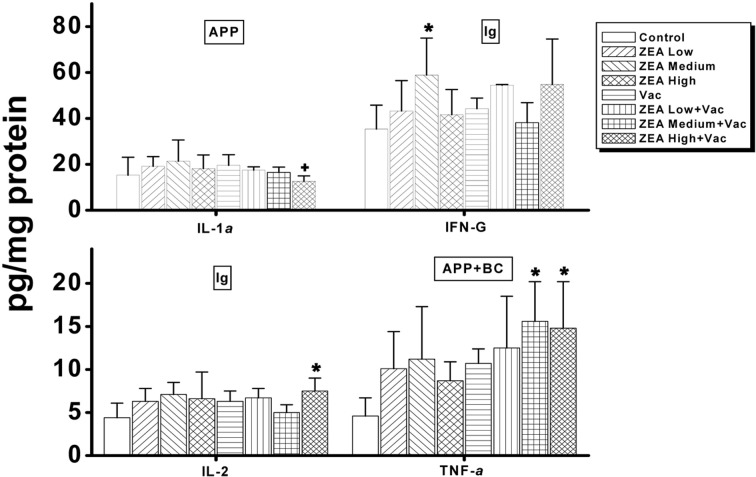
In serum, the only cytokine that was decreased dosedependently was interferon (IFN)-γ, which was decreased in ZEA medium with Vac and ZEA high with Vac groups compared to the Vac only group (Supp Table 1 and Fig. 2). In thymus, the concentrations of IL-1β, IL-2, IL-6, IL-10 and INF-γ were significantly decreased (p < 0.05) by the treatment of ZEA alone while of IL-1β, IL-2, Granulocytemacrophage colony stimulating factor (GM-CSF) and tumor necrosis factor (TNF)-α were significantly decreased (p <0 .05) by the treatment of ZEA in vaccinated rats compared to that of vaccine alone treatment (Supp Table 2 and Fig. 3). In spleen, the amounts of IL-1α, IL-10 and INF-γ were decreased significantly (p < 0.05) by the treatment of high dose of ZEA alone, and IL-1α , IL-2, IL-10 and IFN-γ were decreased by the treatment of ZEA in vaccinated rats compared to that of vaccine alone treatment (Supp Table 3 and Fig. 4). In mesenteric lymph node, only the concentration of INF-γ was decreased significantly (p < 0.05) by the treatment of medium dose of ZEA alone while IL-1α was decreased by the treatment of ZEA in vaccinated rats compared to that of group treated vaccine alone (Supp Table 4 and Fig. 5). A summary of these finding can be found in Table 2.
Supp Table 1.
Changes in serum cytokine concentrations by administration of ZEA to female Wistar rats (pg/mL)
| Parameter | Control | ZEA Low | ZEA Medium | ZEA High With Vac | ZEA Low With Vac | ZEA Medium With Vac | ZEA High With Vac | |
|---|---|---|---|---|---|---|---|---|
| IL-1β | 52.1 ± 6.1 | 46.2 ± 22.3 | 66.9 ± 7.4 | 37.8 ± 6.9 | 45.3 ± 2.4 | 44.7 ± 7.9 | 48.3 ± 11.2 | 51.9 ± 11.9 |
| IL-2 | 41.1 ± 6.3 | 47.8 ± 10.6 | 40.1 ± 5.2 | 38.5 ± 11.2 | 45.9 ± 7.6 | 34.0 ± 10.5 | 71.2 ± 25.7 | 73.2 ± 58.5 |
| IL-4 | 6.3 ± 11.7 | 6.5 ± 1.6 | 72.3 ± 21.5 | 41.8 ± 4.5 | 53.4 ± 10.0 | 61.1 ± 3.9 | 71.2 ± 25.7 | 73.2 ± 58.5 |
| IL-6 | 67.4 ± 16.2 | 40.2 ± 10.0 | 56.9 ± 10.1 | 50.5 ± 13.2 | 60.7 ± 26.4 | 69.5 ± 10.1 | 80.6 ± 20.2 | 57.4 ± 24.6 |
| IL-10 | 21.7 ± 6.5 | 25.4 ± 10.1 | 13.0 ± 13.2 | 18.3 ± 4.3 | 16.8 ± 10.8 | 24.8 ± 16.7 | 22.1 ± 0.0 | 25.8 ± 3.4 |
| IFN-γ | 45.5 ± 3.8 | 25.5 ± 1.4* | 18.7 ± 3.1* | 16.1 ± 5.7* | 38.4 ± 12.8 | 37.4 ± 4.9 | 23.0 ± 4.8* | 21.3 ± 6.7* |
Rats in ZEA low, medium and high groups were administered with 1mg, 5mg and 30mg ZEA/kg bw by gavage once per day for 35 days.
Rats in Vac groups were administered 2.0mL of inactivated porcine parvovirus vaccine subcutaneously on day 8 (Vac, ZEA Low with Vac, ZEA Medium with Vac and ZEA High with Vac groups). Values are mean ± SD of 5 replicas.
*: Significantly different from control at p<0.05.
Fig. 2. Changes in serum cytokines involved in primarily immunoglobulin actions by administration of zearalenone to female Wistar rats. Rats in ZEA low, medium and high groups were administered with 1mg, 5mg and 30 mg ZEA/kg bw by gavage once per day for 35 days. Rats in Vac groups were administered 2.0ml of inactivated porcine parvovirus vaccine subcutaneously on day 8 (Vac, ZEA Low+Vac , ZEA Medium+ Vac and ZEA High + Vac groups). Values are mean ± S.D. of 5 replicas. *: Significantly different from control at p<0.05.
Supp Table 2.
Changes in cytokines in the thymus by administration of ZEA to female Wistar rats (pg/mg protein)
| Parameter | Control | ZEA Low | ZEA Medium | ZEA High | Vac | ZEA Low With Vac | ZEA Medium With Vac | ZEA High With Vac |
|---|---|---|---|---|---|---|---|---|
| IL-1α | 12.4 ± 3.5 | 7.1 ± 1.7 | 8.8 ± 3.7 | 6.3 ± 2.6 | 16.7 ± 4.7 | 11.1 ± 6.6 | 9.4 ± 5.0 | 7.6 ± 2.3 |
| IL-1β | 1188 ± 93 | 602 ± 226* | 526 ± 74* | 545 ± 94* | 1114 ± 59 | 676+ ± 330* | 477+ ± 26* | 530+ ± 101* |
| IL-2 | 16.0 ± 6.7 | 8.6 ± 1.6* | 4.6 ± 4.8* | 2.5 ± 1.4* | 13.6 ± 1.1 | 5.5+ ± 4.0** | 4.5++ ± 2.3** | 3.9++ ± 0.3* |
| IL-6 | 106.5 ± 6.5 | 59.2 ± 27.2 | 30.9 ± 3.6* | 31.7 ± 28.7* | 84.9 ± 55.1 | 57.3 ± 5.1 | 36.8 ± 18.8* | 27.4 ± 13.5* |
| IL-10 | 11.5 ± 4.0 | 7.0 ± 2.6 | 9.4 ± 0.3 | 4.9 ± 1.0* | 9.3 ± 3.2 | 9.9 ± 0.5 | 7.8 ± 4.3 | 9.5 ± 3.4 |
| IFN-γ | 56.4 ± 18.4 | 41.2 ± 10.7 | 29.6 ± 2.4* | 31.4±15.0 | 63.1 ± 14.4 | 55.9 ± 10.3 | 37.1 ± 18.3 | 44.5 ± 4.7 |
| GM-CSF | 8.0 ± 1.8 | 9.2 ± 4.0 | 5.4 ± 1.4 | 6.0 ± 1.6 | 12.8 ± 3.3 | 7.8 ± 1.7 | 8.4 ± 4.3 | 2.9++ ± 1.1 |
| TNF-α | 61.1 ± 16.5 | 55.9 ± 6.9 | 57.5 ± 7.3 | 44.2 ± 7.6 | 115.0 ± 16.5* | 62.0+ ± 0.0 | 59.0+ ± 14.0 | 76.8+ ± 27.0 |
Rats in ZEA low, medium and high groups were administered 1mg, 5mg and 30mg ZEA/kg bw by gavage once per day for 35 days. Rats in the Vac groups were administered 2.0mL of inactivated porcine parvovirus vaccine subcutaneously on day 8 (Vac, ZEA Low with Vac, ZEA Medium with Vac and ZEA High with Vac groups). Values are mean ± SD of 5 replicas.
*: Significantly different from control at p<0.05.
+: Significantly different from Vac at p<0.05.
Fig. 3. Changes in cytokines involved in immunoglobulin actions, acute phase proteins and primary blood cell actions in thymus by administration of zearalenone to female Wistar rats. Rats in ZEA low, medium and high groups were administered with 1mg, 5mg and 30 mg ZEA/kg bw by gavage once per day for 35 days. Rats in Vac groups were administered 2.0ml of inactivated porcine parvovirus vaccine subcutaneously on day 8 (Vac, ZEA Low+ Vac, ZEA Medium + Vac and ZEA High + Vac groups). Values are mean ± S.D. of 5 replicas. *: Significantly different from control at p< 0.05. +: Significantly different from Vac at p< 0.05. Ig: cytokines involved in primarily immunoglobulin actions; BC: cytokines involved in primarily blood cell actions; APP: cytokines involved in primarily acute phase protein actions.
Supp Table 3.
Changes in cytokines in spleen by administration of ZEA to female Wistar rats (pg/mg protein)
| Parameter | Control | ZEA Low | ZEA Medium | ZEA High | Vac | ZEA Low With Vac | ZEA Medium With Vac | ZEA High With Vac |
|---|---|---|---|---|---|---|---|---|
| IL-1α | 11.5 ± 4.2 | 10.9 ± 4.3 | 7.7 ± 1.7 | 4.9 ± 1.6* | 15.1 ± 4.3 | 7.2+ ± 3.6 | 6.1+ ± 3.3 | 7.6+ ± 3.3 |
| IL-2 | 2.1 ± 1.2 | 1.5 ± 0.6 | 1.2 ± 0.5 | 1.3 ± 1.5 | 2.3 ± 1.1 | 1.1+ ± 0.4 | 1.0+ ± 0.5 | 1.3+ ± 0.4 |
| IL-6 | 30.0 ± 11.8 | 27.1 ± 7.7 | 21.0 ± 8.3 | 14.5 ± 8.7 | 38.2 ± 16.4 | 21.9 ± 9.6 | 15.1+ ± 2.6 | 22.7 ± 6.6 |
| IL-10 | 2.1 ± 1.2 | 1.8 ± 0.7 | 1.3 ± 0.4 | 0.8 ± 0.3* | 3.0 ± 1.3 | 1.5+ ± 0.6 | 1.3+ ± 0.6 | 1.4+ ± 0.4 |
| IFN-γ | 28.2 ± 13.4 | 24.4 ± 8.6 | 17.7 ± 4.7 | 11.3 ± 6.7* | 29.8 ± 5.9 | 15.5+ ± 8.2 | 16.2+ ± 9.1 | 16.8+ ± 6.3 |
| GM-CSF | 0.4 ± 0.3 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.5 ± 0.3 | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.2 ± 0.1 |
Rats in ZEA Low, Medium and High groups were administered 1mg, 5mg and 30mg ZEA/kg bw by gavage once per day from Day 0 to Day 35 of treatment, respectively. Rats of Vac, ZEA Low with Vac, ZEA Medium with Vac and ZEA High with Vac groups were inoculated with 2.0mL of Parvo virus inactivated vaccine once subcutaneously on Day 8 of treatment. Values are mean ± SD of 5 replicas.
*: Significantly different from control at p<0.05.
with: Significantly different from Vac at p<0.05.
Fig. 4. Changes in cytokines in the spleen by the administration of zearalenone to female Wistar rats. Rats in ZEA low, medium and high groups were administered with 1mg, 5mg and 30 mg ZEA/kg bw by gavage once per day for 35 days. Rats in Vac groups were administered 2.0ml of inactivated porcine parvovirus vaccine subcutaneously on day 8 (Vac, ZEA Low+ Vac, ZEA Medium + Vac and ZEA High + Vac groups). Values are mean ± S.D. of 5 replicas. *: Significantly different from control at p< 0.05. +: Significantly different from Vac at p< 0.05. Ig: cytokines involved in primarily immunoglobulin actions; BC: cytokines involved in primarily blood cell actions; APP: cytokines involved in primarily acute phase protein actions.
Supp Table 4.
Changes of cytokines in mesenteric lymph node after administration of ZEA to female Wistar rats (pg/mg protein)
| Parameter | Control | ZEA Low | ZEA Medium | ZEA High | Vac | ZEA Low With Vac | ZEA Medium With Vac | ZEA High With Vac |
|---|---|---|---|---|---|---|---|---|
| IL-1α | 15.3 ± 7.8 | 19.2 ± 4.2 | 21.4 ± 9.2 | 18.2 ± 5.9 | 19.6 ± 4.6 | 17.5 ± 1.4 | 16.5 ± 2.3 | 12.6 ± 2.4 |
| IL-2 | 4.4 ± 1.7 | 6.3 ± 1.5 | 7.1 ± 1.4 | 6.6 ± 3.1 | 6.3 ± 1.2 | 6.7 ± 1.1 | 5.0 ± 0.9 | 7.5 ± 1.5* |
| IL-4 | 0.5 ± 0.1 | 0.4 ± 0.2 | 0.9 ± 0.1 | 0.4 ± 0.4 | 0.4 ± 0.3 | 0.9 ± 0.7 | 0.4 ± 0.2 | 0.7 ± 0.4 |
| IL-6 | 53.5 ± 15.2 | 67.2 ± 21.7 | 84.2 ± 19.6 | 56.7 ± 21.9 | 67.8 ± 7.5 | 78.5 ± 18.9 | 63.7 ± 18.6 | 81.1 ± 22.3 |
| IL-10 | 4.5 ± 2.4 | 5.3 ± 1.3 | 6.7 ± 0.9 | 5.7 ± 2.4 | 5.4 ± 1.4 | 5.2 ± 1.0 | 5.2 ± 1.2 | 4.3 ± 0.8 |
| IFN-γ | 35.4 ± 10.4 | 43.3 ± 13.2 | 58.9 ± 16.1* | 41.6 ± 11.0 | 44.3 ± 4.6 | 54.5 ± 0.3 | 38.2 ± 8.7 | 54.8 ± 19.8 |
| GM-CSF | 0.8 ± 0.2 | 0.9 ± 0.1 | 1.0 ± 0.2 | 0.7 ± 0.1 | 1.0 ± 0.2 | 0.9 ± 0.1 | 0.8 ± 0.2 | 0.9 ± 0.2 |
| TNF-α | 4.6 ± 2.1 | 10.1 ± 4.3 | 11.2 ± 6.1 | 8.7 ± 2.2 | 10.7 ± 1.7 | 12.5 ± 6.0 | 15.6 ± 4.6* | 14.8 ± 5.4* |
Rats in ZEA low, medium and high groups were administered with 1mg, 5mg and 30mg ZEA/kg bw by gavage once per day for 35 days.
Vac groups were administered 2.0mL of inactivated porcine parvovirus vaccine subcutaneously on day 8 (Vac, ZEA Low with Vac, ZEA Medium with Vac and ZEA High with Vac groups). Values are mean ± SD of 5 replicas.
*: Significantly different from control at p<0.05.
+: Significantly different from Vac at p<0.05.
Fig. 5. Changes in cytokine content in mesenteric lymph nodes by administration of zearalenone to female Wistar rats. Rats in ZEA low, medium and high groups were administered with 1mg, 5mg and 30 mg ZEA/kg bw by gavage once per day for 35 days. Vac groups were administered 2.0ml of inactivated porcine parvovirus vaccine subcutaneously on day 8 (Vac, ZEA Low+ Vac, ZEA Medium + Vac and ZEA High + Vac groups). Values are mean ± S.D. of 5 replicas. *: Significantly different from control at p< 0.05. +: Significantly different from Vac at p< 0.05. Ig: cytokines involved in primarily immunoglobulin actions; BC: cytokines involved in primarily blood cell actions; APP: cytokines involved in primarily acute phase protein actions.
Table 2.
Summary of cytokines showing changes by the administration of ZEA in female Wistar rats injected with inactivated parvovirus vaccine
| Parameters | Serum | Thymus | Spleen | Mesenteric lymph node | ||||
|---|---|---|---|---|---|---|---|---|
| ZEA | ZEA with Vac | ZEA | ZEA with Vac | ZEA | ZEA with Vac | ZEA | ZEA with Vac | |
| IL-1α | NT | NT | - | - | ⬇ | ⇩ | - | ⇩ |
| IL-1β | - | - | ⬇ | ⇩ | NT | NT | ||
| IL-2 | - | - | ⬇ | ⇩ | - | ⇩ | - | - |
| IL-4 | - | - | NT | NT | NT | NT | - | - |
| IL-6 | - | - | ⬇ | ⇩* | - | - | - | - |
| IL-10 | - | - | ⬇ | - | ⬇ | ⇩ | - | - |
| IFN-γ | ⬇ | ⇩* | ⬇ | - | ⬇ | ⇩ | - | - |
| GM-CSF | NT | NT | - | ⇩ | - | - | - | - |
| TNF-α | NT | NT | - | ⇩ | NT | NT | - | - |
Solid arrows represent significant decrease between ZEA treated rats and control, and blank arrows between ZEA Vac and control groups.
-, no change.
NT, not tested.
* Decreased, but not significantly.
DISCUSSION
ZEA is a non-steroidal estrogenic mycotoxin biosynthesized by multiple species of Fusarium fungi. ZEA causes reproductive disorders. There could be concern that it causes immune suppression in livestock. ZEA can decrease resistance to L. monocytogenes without affecting splenic plaque-forming or delayed hypersensitivity responses in the B6C3F1 mice (Pestka, Tai et al., 1987). A single dose of ZEN (40 mg/kg bw) reduced the immunoglobulin profile (IgA and IgG) and T cells subtypes (CD3+, CD4+, CD8+ and CD56+) in the Balb/c mouse (Abbes et al., 2006). Mice treated with ZEA (40 mg/kg) for 2 weeks showed a decrease in the lymphocyte count, the total white blood cell count, immunoglobulins (IgG and IgM), B cells, T cell subtypes (CD3+, CD4+ and CD8+) and natural killer cells, and pro-inflammatory cytokines (Ben Salah-Abbes et al., 2008). ZEA and ZEA derivatives altered several parameters of the humoral and cellular immune responses in vitro (Marin et al., 2011). ZEA decreased the TNF-α and IL-8 synthesis in the supernatant from stimulated porcine peripheral blood mononuclear cells (Marin et al., 2011). In our study, a medium dose of ZEA was associated with decreased antibody titers to porcine parvovirus in female Wistar rats but there was not a predictable dose-response effect. The results present herein suggest that ZEA does not consistently affect antibody production in Wistar rats. These finding is in line with other report that ZEA did not affect splenic plaqueforming responses in B6C3F1 mice (Pestka et al., 1987). But there are other reports that ZEA may affect T cell subsets and immunoglobulin production in mice. It is well documented that species difference exists in immune response (Heyes et al., 1997; Innes, 1997; Pestka and Smolinski, 2005; Chapes and Ganta, 2008). Based on these differences between the rat and mouse models, we could not exclude the possibility that the immune system of rats is less sensitive to ZEA compared to mice.
IgM is the first antibody produced in response to an infection or exogenous protein, followed by IgG. In the present study, the concentration of IgM decreased significantly in the ZEA treatment groups at day 22 day of treatment, but remained decreased only in the ZEA medium (5 mg/kg) group at day 36. The IgG concentration was decreased in the ZEA only and ZEA with Vac groups, and significantly decreased in the ZEA with Vac group compared the Vac only groups. The results that ZEA can inhibit antibody production in response to vaccination in rats suggest that ZEA alone can affect the production of immunoglobulins.
IgA antibodies are found on mucosal surfaces of the body such the nose, airways, digestive tract, ears, eyes, and vagina, where they are important in mucosal immune system. Some mycotoxin, like deoxynivalenol, increases IgA concentrations in serum in laboratory animals (Pestka et al., 2004; Pinton et al., 2008). The increase in specific and total IgA concentrations was associated with increased susceptibility to viral infections in mice (Li et al., 2005). In the present study, the serum IgA concentration was not changed in ZEA with Vac groups and the Vac only group of rats, suggesting that ZEA may not affect mucosal immunity. IgE antibodies are found in the lungs, skin, and mucous membranes and are involved in parasitic and allergic reactions. The serum concentration of IgE normally present in mice favors immune sensitization of mast cells in an antigenindependent manner (Bryce et al., 2005). According to the present study, IgE production was decreased at treatment day 22 in all ZEA groups, but IgE production was not affected in ZEA with Vac and Vac only groups.
Cytokines are important in host defense, inflammatory response, and immune-mediated disease. Through this study, we determined the cytokine concentrations in lymphoid organs and serum in ZEN and ZEN with Vac groups in female Wistar rats. Acute phase protein (APP) is a group of proteins secreted by hepatocytes which are different biochemically and functionally unrelated. The APP concentration in the serum changes at the onset of a systemic inflammatory reaction. The interleukins IL-1, IL-6 and IL- 8, and TNF-α are cytokines which stimulate changes in production of acute phase protein (Ganapathi et al., 1988; Dusetti et al., 1995).
TNF-α and GM-CSF are primary involved in white blood cell action. TNF-α is produced primarily by macrophages involved in systemic inflammation and a member of a group of cytokines that stimulate the acute phase reaction. The primary role of TNF-α is in the regulation of immune cells by activating macrophages, endothelial cells, neutrophils and T lymphocytes to produce cytokines and by inducing apoptotic cell death (Elenkov and Chrousos, 2002; O’Shea et al., 2002). GM-CSF can mobilize hematopoietic stem cells circulating in the peripheral blood (Ahmed et al., 1992) and function as a white blood cell growth factor. GM-CSF stimulates stem cells to produce granulocytes and monocytes.
IL-2, IL-4, IL-6, IL-10 and IFN-γ are involved in immunoglobulin production. IL-2 binds to T cell receptors (TCR) and stimulates the growth, differentiation and survival of antigen-selected cytotoxic T cells (O’Shea et al., 2002), IL- 4 promotes growth and antibody production in B cells (Bende et al., 1992). IL-6 is also involved in immunoglobulin synthesis and secretion from B lymphocytes (Shiao et al., 1996), and Th2-type anti-inflammatory cytokines, such as IL-4, IL-5, IL-6, and IL-10, induce humoral immunity and play a role in inducing IgG1 antibody production (Finkelman et al., 1990). These cytokines facilitate production of immunoglobulins made by B cells and induce differentiation and proliferation of natural killer cells (Waldmann, 2006). Zearalenone stimulates the production of IL-2 and IL-5 in vitro (Marin et al., 1996). In the present study, the amount of IL-1 in thymus and spleen, INF-γ in serum, IL-2 and IL-6, and IL-10 in thymus, and IL-10, IFN-γ in spleen were decreased by ZEA treatment while the amounts of IL- 1α in spleen and mesenteric lymph node, IL-1β in thymus, IL-2 in thymus and spleen, IL-6 in thymus, IL-10 and IFN-γ in spleen, and GM-CSF and TNF-α in thymus were decreased by vaccination to rats exposed to ZEA compared to those of group treated vaccine alone. Based on this study, we propose that cytokine expression are more affected by ZEA in the thymus and spleen than in the lymph nodes, and IL-1, IL-2, IL-10, and INF-γ are the most sensitive cytokines to ZEA treatment.
Out of the cytokines involved in immunoglobulin actions, INF-γ was affected in the serum, spleen and thymus in ZEA treated rats, IL-2 was decreased in all lymphoid organs, and IL-10 was decreased in the thymus and spleen. In terms of cytokines associated with APP production, IL-1 was affected in the thymus, spleen and lymph nodes, IL-6 was decreased only in the thymus, and the concentrations of GM-CSF and TNF-α, which are also involved in leukocyte actions were decreased only in ZEA with Vac groups compared to the Vac only group. Based on the present study, we propose that ZEA exposure in rats inhibits the production of cytokines involved in immune response initiated by vaccination by decreasing IL-1 and IL-2 in thymus, spleen and mesenteric lymph node, and IL-10 and IFN-γ in spleen, and GM-CSF and TNF-α in spleen. Since IFN-γ is the only cytokine that undergoes a dose-dependent decrease, it could imply that Th1 response is specially decreased following exposure to ZEA.
To the best of our knowledge, a few studies on the effects of ZEA on cytokine production in lymphoid organs have been reported. ZEA could also affect resistance to Listeria monocytogenes infection and delayed hypersensitivity in mice (Pestka et al., 1987). ZEA and its derivatives may have divergent effects on important parameters of innate immunity in swine, such as cell proliferation and IL-8 synthesis (Marin et al., 2010). In the present study, rats were injected with inactivated porcine parvovirus vaccine, which does not proliferate in body, thus limiting the immune response expected. So, further studies on the effects of ZEA on secretion of cytokines involved in the lymphoid immune response to active viral and bacterial challenge are strongly recommended.
In conclusion, even though ZEA did not significantly inhibit the production of antibodies to porcine parvovirus in vaccinated rats, it could cause immune suppression by modulating the immunoglobulins in serum and cytokines in lymphoid organs. However, further study is needed to elucidate the mechanism of immune modulation and cytological effects of ZEA in lymphoid organs.
Acknowledgments
This project was supported by research funds from National Veterinary Research and Quarantine Service, Republic of Korea. The authors also express deep thanks to Dr. Bischoff at Cornell University for her kind review on this paper.
References
- 1.Abbès S., Ouanes Z., Salah-Abbès J., Houas Z., Oueslati R., Bacha H., Othman O. The protective effect of hydrated sodium calcium aluminosilicate against haematological, biochemical and pathological changes induced by Zearalenone in mice. Toxicon. (2006);47:567–574. doi: 10.1016/j.toxicon.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Abbès S., Salah-Abbès J.B., Ouanes Z., Houas Z., Othman O., Bacha H., Abdel-Wahhab M.A., Oueslati R. Preventive role of phyllosilicate clay on the Immunological and Biochemical toxicity of zearalenone in Balb/c mice. Int. Immunopharmacol. (2006);6:1251–1258. doi: 10.1016/j.intimp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed T., Wuest D., Ciavarella D. Peripheral blood stem cell mobilization by cytokines. J. Clin. Apher. (1992);7:129–131. doi: 10.1002/jca.2920070306. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson H.A., Miller K. Inhibitory effect of deoxynivalenol, 3-acetyldeoxynivalenol and zearalenone on induction of rat and human lymphocyte proliferation. Toxicol. Lett. (1984);23:215–221. doi: 10.1016/0378-4274(84)90129-2. [DOI] [PubMed] [Google Scholar]
- 5.Ben Salah-Abbès J., Abbès S., Houas Z., Abdel-Wahhab M.A., Oueslati R. Zearalenone induces immunotoxicity in mice: possible protective effects of radish extract (Raphanus sativus). J. Pharm. Pharmacol. (2008);60:761–770. doi: 10.1211/jpp.60.6.0012. [DOI] [PubMed] [Google Scholar]
- 6.Bende R.J., Jochems G.J., Frame T.H., Klein M.R., van Eijk R.V., van Lier R.A., Zeijlemaker W.P. Effects of IL-4, IL-5, and IL-6 on growth and immunoglobulin production of Epstein-Barr virus-infected human B cells. Cell. Immunol. (1992);143:310–323. doi: 10.1016/0008-8749(92)90028-N. [DOI] [PubMed] [Google Scholar]
- 7.Bennett G.A., Nelsen T.C., Miller B.M. Enzymelinked immunosorbent assay for detection of zearalenone in corn, wheat, and pig feed: collaborative study. J. AOAC. Int. (1994);77:1500–1509. [PubMed] [Google Scholar]
- 8.Bodine A.B., Alberty C.F., Buck C.S., Richardson M.E., Wright R.E. Possible “immuno-protection” of the bovine parvovirus in the uterus: Preliminary communication. Theriogenology. (1981);16:201–206. doi: 10.1016/0093-691X(81)90102-3. [DOI] [PubMed] [Google Scholar]
- 9.Bondy G.S., Pestka J.J. Immunomodulation by fungal toxins. J. Toxicol. Environ. Health B. (2000);3:109–143. doi: 10.1080/109374000281113. [DOI] [PubMed] [Google Scholar]
- 10.Brown K.E., Young N.S. Parvovirus B19 infection and hematopoiesis. Blood Rev. (1995);9:176–182. doi: 10.1016/0268-960X(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 11.Bryce P.J., Miller M.L., Miyajima I., Tsai M., Galli S.J., Oettgen H.C. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE on mast cells. Novartis Found. Symp. (2005);271:15–24. doi: 10.1002/9780470033449.ch3. [DOI] [PubMed] [Google Scholar]
- 12.Chapes S.K., Ganta R.R. Defining the immune response to Ehrlichia species using murine models. Vet Parasitol. (2008);158:344–359. doi: 10.1016/j.vetpar.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chisaka H., Morita E., Yaegashi N., Sugamura K. Parvovirus B19 and the pathogenesis of anaemia. Rev. Med. Virol. (2003);13:347–359. doi: 10.1002/rmv.395. [DOI] [PubMed] [Google Scholar]
- 14.Dusetti N.J., Ortiz E.M., Mallo G.V., Dagorn J.C., Iovanna J.L. Pancreatitis-associated protein I (PAP I), an acute phase protein induced by cytokines. Identification of two functional interleukin-6 response elements in the rat PAP I promoter region. J. Biol. Chem. (1995);270:22417–22421. doi: 10.1074/jbc.270.38.22417. [DOI] [PubMed] [Google Scholar]
- 15.Elenkov I.J., Chrousos G.P. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann. N. Y. Acad. Sci. (2002);966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 16.Finkelman F.D., Holmes J., Katona I.M., Urban J.F. Jr., Beckmann M.P., Park L.S., Schooley K.A., Coffman R.L., Mosmann T.R., Paul W.E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu. Rev. Immunol. (1990);8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 17.Forsell J.H., Pestka J.J. Relation of 8-ketotrichothecene and zearalenone analog structure to inhibition of mitogen- induced human lymphocyte blastogenesis. Appl. Environ. Microbiol. (1985);50:1304–1307. doi: 10.1128/aem.50.5.1304-1307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganapathi M.K., Schultz D., Mackiewicz A., Samols D., Hu S.I., Brabenec A., Macintyre S.S., Kushner I. Heterogeneous nature of the acute phase response. Differential regulation of human serum amyloid A, C-reactive protein, and other acute phase proteins by cytokines in Hep 3B cells. J. Immunol. (1988);141:564–569. [PubMed] [Google Scholar]
- 19.Heyes M.P., Saito K., Chen C.Y., Proescholdt M.G., Nowak T.S. Jr., Li J., Beagles K.E., Proescholdt M.A., Zito M.A., Kawai K., Markey S.P. Species heterogeneity between gerbils and rats: quinolinate production by microglia and astrocytes and accumulations in response to ischemic brain injury and systemic immune activation. J. Neurochem. (1997);69:1519–1529. doi: 10.1046/j.1471-4159.1997.69041519.x. [DOI] [PubMed] [Google Scholar]
- 20.Innes E.A. Toxoplasmosis: comparative species susceptibility and host immune response. Comp. Immunol. Microbiol. Infect. Dis. (1997);20:131–138. doi: 10.1016/S0147-9571(96)00038-0. [DOI] [PubMed] [Google Scholar]
- 21.Kuiper-Goodman T., Scott P.M., Watanabe H. Risk assessment of the mycotoxin zearalenone. Regul. Toxicol. Pharmacol. (1987);7:253–306. doi: 10.1016/0273-2300(87)90037-7. [DOI] [PubMed] [Google Scholar]
- 22.Li M., Cuff C.F., Pestka J. Modulation of murine host response to enteric reovirus infection by the trichothecene deoxynivalenol. Toxicol. Sci. (2005);87:134–145. doi: 10.1093/toxsci/kfi225. [DOI] [PubMed] [Google Scholar]
- 23.Marin D.E., Taranu I., Burlacu R., Manda G., Motiu M., Neagoe I., Dragomir C., Stancu M., Calin L. Effects of zearalenone and its derivatives on porcine immune response. Toxicol. In Vitro. (2011);25:1981–1988. doi: 10.1016/j.tiv.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Marin D.E., Taranu I., Burlacu R., Tudor D.S. Effects of zearalenone and its derivatives on the innate immune response of swine. Toxicon. (2010);56:956–963. doi: 10.1016/j.toxicon.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Marin M.L., Murtha J., Dong W., Pestka J.J. Effects of mycotoxins on cytokine production and proliferation in EL-4 thymoma cells. J. Toxicol. Environ. Health. (1996);48:379–396. doi: 10.1080/009841096161267. [DOI] [PubMed] [Google Scholar]
- 26.O’Shea J.J., Ma A., Lipsky P. Cytokines and autoimmunity. Nat. Rev. Immunol. (2002);2:37–45. doi: 10.1038/nri702. [DOI] [PubMed] [Google Scholar]
- 27.Pestka J.J., Smolinski A.T. Deoxynivalenol: toxicology and potential effects on humans. J. Toxicol. Environ. Health B. (2005);8:39–69. doi: 10.1080/10937400590889458. [DOI] [PubMed] [Google Scholar]
- 28.Pestka J.J., Tai J.H., Witt M.F., Dixon D.E., Forsell J.H. Suppression of immune response in the B6C3F1 mouse after dietary exposure to the Fusarium mycotoxins deoxynivalenol (vomitoxin) and zearalenone. Food Chem. Toxicol. (1987);25:297–304. doi: 10.1016/0278-6915(87)90126-8. [DOI] [PubMed] [Google Scholar]
- 29.Pestka J.J., Zhou H.R., Moon Y., Chung Y.J. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: unraveling a paradox. Toxicol. Lett. (2004);153:61–73. doi: 10.1016/j.toxlet.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 30.Pinton P., Accensi F., Beauchamp E., Cossalter A.M., Callu P., Grosjean F., Oswald I.P. Ingestion of deoxynivalenol (DON) contaminated feed alters the pig vaccinal immune responses. Toxicol. Lett. (2008);177:215–222. doi: 10.1016/j.toxlet.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Prelusky D.B., Scott P.M., Trenholm H.L., Lawrence G.A. Minimal transmission of zearalenone to milk of dairy cows. J. Environ. Sci. Health B. (1990);25:87–103. doi: 10.1080/03601239009372678. [DOI] [PubMed] [Google Scholar]
- 32.Shiao R.T., McLeskey S.B., Khera S.Y., Wolfson A., Freter C.E. Mechanisms of inhibition of IL-6-mediated immunoglobulin secretion by dexamethasone and suramin in human lymphoid and myeloma cell lines. Leuk. Lymphoma. (1996);21:293–303. doi: 10.3109/10428199209067610. [DOI] [PubMed] [Google Scholar]
- 33.Swamy H.V., Smith T.K., MacDonald E.J. Effects of feeding blends of grains naturally contaminated with Fusarium mycotoxins on brain regional neurochemistry of starter pigs and broiler chickens. J. Anim. Sci. (2004);82:2131–2139. doi: 10.2527/2004.8272131x. [DOI] [PubMed] [Google Scholar]
- 34.Urraca J.L., Benito- Peña E., Pèrez-Conde C., Moreno-Bondi M.C., Pestka J.J. Analysis of zearalenone in cereal and Swine feed samples using an automated flow-through immunosensor. J. Agric. Food Chem. (2005);53:3338–3344. doi: 10.1021/jf048092p. [DOI] [PubMed] [Google Scholar]
- 35.Waldmann T.A. The biology of interleukin-2 and interleukin- 15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. (2006);6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 36.Zinedine A., Soriano J.M., Moltó J.C., Mañes J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: an oestrogenic mycotoxin. Food Chem. Toxicol. (2007);45:1–18. doi: 10.1016/j.fct.2006.07.030. [DOI] [PubMed] [Google Scholar]