Abstract
Inflammation is the immune system’s response to infection and injury-related disorders, and is related to pro-inflammatory factors (NO, PGE2, cytokines, etc.) produced by inflammatory cells. Atopic dermatitis (AD) is a representative inflammatory skin disease that is characterized by increasing serum levels of inflammatory chemokines, including macrophage-derived chemokine (MDC). Carpinus tschonoskii is a member of the genus Carpinus. We investigated the anti-inflammatory activity of C. tschonoskii by studying the effects of various solvent fractions prepared from its leaves on inflammatory mediators in HaCaT and RAW264.7 cells. We found that the chloroform fraction of C. tschonoskii inhibited MDC at both the protein and mRNA levels in HaCaT cells, acting via the inhibition of STAT1 in the IFN-γ signaling pathway. In addition, the chloroform fraction significantly suppressed the expression of inflammatory factors induced by lipopolysaccharide stimulation, except COX-2 and TNF-α. These results suggest that the chloroform fraction of C. tschonoskii leaves may include a component with potential anti-inflammatory activity.
Keywords: Carpinus tschonoskii, Inflammation mediators, HaCaT keratinocytes, RAW264.7 macrophages
INTRODUCTION
Inflammation is an essential defense mechanism against various insults to our body. However, an overreaction of inflammatory response leads to inflammatory disease such as rheumatoid arthritis, systemic lupus erythematous, Crohn’s disease, atopic dermatitis (AD). Various mediators, such as nitric oxidase, prostaglandins, inflammatory cytokines, and others induced by macrophages have important roles in inflammatory diseases (Coleman, 2001; Duffield, 2003; Maruotti et al., 2007).
AD is a chronic inflammatory skin disease with allergic and genetic etiology. It is characterized by the infiltration of inflammatory cells, like Th2-type cells, eosinophils, mast cells, and macrophages, into lesioned skin. Migration of inflammatory cells is regulated by chemokines. Chemokines are a group of small cytokines produced by various cell types (Kaplan, 2001; Pease and Williams, 2006). Especially, macrophage-derived chemokine (MDC/CCL22) and thymus- and activation-regulated chemokine (TARC/CCL17) are produced by keratinocytes, dendritic cells, endothelial cells, bronchial epithelial cells and play important roles in the recruitment of Th2-type cells expressed CC chemokine receptor 4 (CCR4) on the surface. It has been reported that the serum level of these Th2-type chemokines is associated with atopic diseases like AD (Kakinuma et al., 2002; Leung et al., 2003).
Carpinus tschonoskii belongs to the genus Carpinus and family Betulaceae and is distributed through southern regions, including Jeju, of Korea, China, and Japan. It is reported that the leaf extract of C. tschonoskii has cytoprotective effect on H2O2 induced oxidative stress and inhibitory effect on CpG-induced pro-inflammatory cytokines (Zhang et al., 2007; Koo et al., 2012). There is also a report that tannins from galls of C. tschonoskii inhibited the degranulation of mast cells (Yamada et al., 2012).
We investigated the effects and action mechanisms of the solvent extracts from C. tschonoskii leaves on the inflammatory mediators (MDC, NO, COX-2, TNF-α, IL-6, and IL-1β) in HaCaT keratinocytes and RAW264.7 macrophages.
MATERIALS AND METHODS
Reagents. Recombinant human interferon-γ (hIFN-γ) was obtained from GIBCO (Grand Island, NY). Lipopolysaccharide (LPS, E. coli 0111:B4) was purchased from Sigma Chemical Co. (St. Louis, MO). Fetal bovine serum (FBS) and Dulbecco’s modified Eagle’s medium (DMEM) were obtained from GIBCO. Human MDC/CCL22 primers were obtained from Bioneer (Daejeon, Korea). Human β-actin primers were purchased from Bionex (Seoul, Korea). Human MDC enzyme-linked immunosorbent assay (ELISA) kits were obtained from R&D Systems (St. Louis, MO). Anti-phospho-STAT1 antibody was purchased from Cell Signaling Technology (Beverly, MA); rabbit or mouse origin anti-STAT1 antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) and Becton Dickinson (San Diego, CA), respectively; β-actin was obtained from Sigma (St. Louis, MO); and DyLight488 conjugated Donkey anti-Rabbit antibody was purchased from BioLegend Inc. (San Diego, CA). All other reagents were reagent grade.
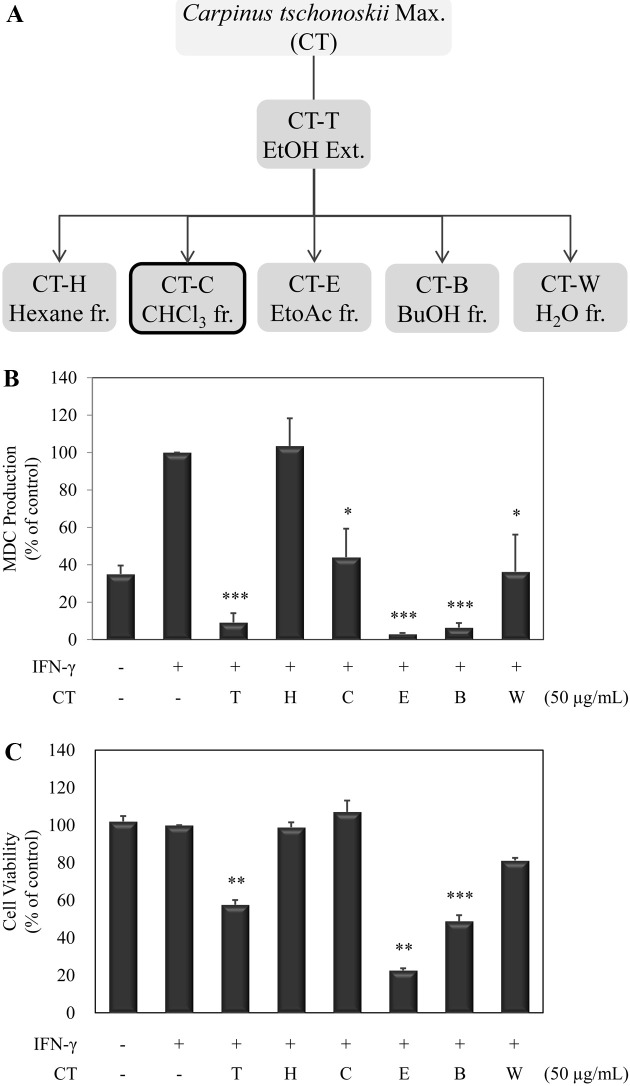
Preparation of solvent fractions. Each fraction isolated from Carpinus tschonoskii was provided from COSMAX Inc. The leaves of C. tschonoskii were harvested and identified at Halla Arboretum in Jeju. The samples were washed, dried and ground to powder (150 g). The powder was extracted with 80% ethanol (5 L) by sonication for 4 hr and filtered through filter paper (Advantech No. 4, Toyo Roshi Kaisha, Ltd.). The filtrate was evaporated to dryness in a Rotavapor (CT-T, 19.3 g). The ethanol extract (12 g) was further extracted using hexane (CT-H; 1.29 g), chloroform (CT-C; 0.82 g), ethyl acetate (CT-E; 1.98 g), n-butanol (CT-B; 2.53 g), and water (CT-W; 1.74 g). The systematic preparation scheme is shown in Fig. 1A.
Fig. 1. Effects of various fractions from Carpinus tschonoskii on the protein production of MDC in IFN-γ-stimulated HaCaT human keratinocytes. (A) The Solvent fractionation of leaves of Carpinus tschonoskii. (B) Protein production of MDC was determined from the culture supernatant of cells stimulated with IFN-γ (10 ng/ml) in the presence of various fractions (T, EtOH; H, Hexane; C, chloroform; E, EtoAc; B, BuOH; W, H2O) of C. tschonoskii (50 μg/ml) for 24 hr. MDC production was determined by ELISA and was done in triplicate. (C) Cell viability was determined from the cells stimulated with IFN-γ (10 ng/ml) in the presence of various fractions of C. tschonoskii (50 μg/ml). Cell viability was analyzed by MTT assay. Error bars indicate ± S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.001, significant when compared with IFN-γ positive control.
Cell culture. The immortalized human keratinocyte cell line, HaCaT, was cultured in DMEM supplemented with 10% FBS and penicillin-streptomycin (100 U/ml) at 37℃ in a 5% CO2 atmosphere. The mouse macrophage cell line, RAW264.7, was cultured in DMEM supplemented with 10% heat-inactivated FBS, penicillin-streptomycin (100 U/ ml) at 37℃ in a 5% CO2 atmosphere.
Cell viability assay. Cell viability was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assays. HaCaT or RAW264.7 cells were stimulated with IFN-γ or LPS in the absence or presence of C. tschonoskii fractions. After incubating for 24 hr, cells were treated with 20 μl MTT for 4 hr. The formazan precipitate was dissolved in 200 μl of dimethyl sulphoxide (DMSO) for 30 min, and the absorbance of the contents of each well was measured at 540 nm using ELISA reader.
Lactate dehydrogenase (LDH) assay. The levels of LDH in the extracellular space were determined using a Cytotox 96® Non-Radioactive Cytotoxicity Assay kit (Promega, Madison, WI). A cell supernatant of 50 μl was mixed with an equal volume of the substrate solution [0.054 M L-(+)-lactic acid, 1.3 mM β-NAD+, 0.66 mM INT and 0.28 mM phenazine methosulfate dissolved in 0.2 M Tris (pH 8.2)]. After 30 min shaking at room temperature, the enzymatic reaction was stopped by adding 50 μl of 1 M acetic acid. The absorbances of the samples were measured at 490 nm to detect the presence of reduced INT, and 9% Triton X-100 was used to determine the maximal LDH content of the cells by allowing complete lysis.
NO assay. Nitrite, which is the end-point of NO generation by activated macrophage, was measured by a colorimetric assay. 100 μl of Griess reagent (1% sulfanilamide and 0.1% N-[1-naphthyl]-ethylenediamine dihydrochloride in 5% phosphoric acid) was added to 100 μl cell supernatant. After incubation for 10 min in RT, the absorbance was determined at 540 nm using an ELISA reader. The concentration of
![]()
was calculated by comparison with a standard curve prepared using NaNO2.
Extraction of total RNA and Reverse transcription polymerase chain reaction (RT-PCR). Total RNA from IFN-γ-treated HaCaT or LPS-treated RAW264.7 cells was isolated using the easy-BlueTM Total RNA Extraction kit (iNtRON Biotechnology, Seongnam, Korea) according to the manufacturer’s instructions. Total RNA was stored at −70℃ until use. Reverse transcription was performed using a First-Standard cDNA Synthesis kit (Promega). Total RNA (1 μg) was incubated with oligo(dT)18 primer at 70℃ for 5 min and cooled on ice for 5 min. After addition of the RT premix, reactions were incubated at 42℃ for 60 min. The reactions were terminated at 70℃ for 15 min. The PCR reaction was conducted using i-TaqTM DNA polymerase (iNtRON Biotechnology) with the appropriate sense and antisense primers for MDC, iNOS, COX-2, TNF-α, IL-1β, IL-6, and β-actin (for human and mouse). PCR was performed using a C1000 instrument (Bio-Rad, Hercules, CA) for 32 cycles (30 cycles for β-actin). Each cycle included denaturing at 94℃ for 30 sec, annealing at 55~60℃ for 30 sec, and extending at 72℃ for 2 min. A final extension at 72℃ for 10 min was performed at the end of the cycles. The reaction products were visualized by electrophoresis on a 1.2% agarose gel (Promega) and UV light illumination after staining with ethidium bromide. The relative intensity was analyzed using Quantity One software version 4.2.1.
Enzyme-linked immunosorbent assay (ELISA). Protein production of MDC in the supernatant was measured with an ELISA kit according to the manufacturer’s instructions. Cell culture supernatants were added to each well and incubated for 2 hr at room temperature (RT). After washing, anti-MDC antibody conjugated to biotin was added to each well. The plates were incubated for 2 hr at room temperature. After washing, Streptavidin conjugated to horseradish-peroxidase was added and incubated for 20 min. After washing, a substrate solution (including tetramethylbenzidine) was added and incubated for 20 min. The reaction was stopped by 2 N sulfuric acid and the optical density of each well was determined using an ELISA reader.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and western blot analysis. After incubation, cells were washed twice with ice-cold PBS and were lysed in a protein lysis buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 2 mM EDTA, 1 mM EGTA, 1 mM NaVO3, 10 mM NaF, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 25 μg/ml aprotinin, 25 μg/ml leupeptin]. The whole-cell lysates were then isolated by centrifugation. Protein concentration was measured using the Bradford method. Aliquots of the lysates (20~ 30 μg of protein) were separated on a 10% SDS-polyacrylamide gel and transferred onto a polyvinylidene fluoride (PVDF) membrane (Bio-Rad) with a glycine transfer buffer [192 mM glycine, 25 mM Tris-HCl (pH 8.8), 20% MeOH (v/v)]. After blocking the nonspecific site with 5% non-fat skim milk solution, the membrane was then incubated with rabbit anti-human phospho-STAT1 (1 : 1000, Cell Signaling), mouse anti-STAT1 (1:1000, BD) or mouse anti-human β-actin (1 : 1000, Sigma) specific primary antibodies at 4℃ for overnight. The membrane was further incubated for 60 min with a peroxidase-conjugated anti-rabbit (1 : 5000, Cell Signaling) or anti-mouse (1 : 5000, Santa Cruz Biotechnology) secondary antibody at room temperature. Immunoactive proteins were detected using the WEST-ZOL (plus) Western Blot Detection System (iNtRON Biotechnology).
Confocal microscopy analysis. The cells were seeded onto coverslips at 6-well plate and were fixed with freshly prepared 3.5% paraformaldehyde for 30 min and premeabilized with 0.1% Triton X-100 for 10 min. After 1 hr incubation with 3% BSA/0.1% triton X-100/PBS, the primary antibody against STAT1 (1 : 200) was applied overnight at 4℃. The cells was washed and then incubated with DyLight488 conjugated donkey anti-rabbit secondary antibody (1 : 300) for 30 min at room temperature. After wash steps, coverslips were mounted in VECTASHIELD Mounting Media with DAPI (Vector Labs, Burlingame, CA). The results were visualized using FV500 confocal microscopy (Olympus, Tokyo, Japan).
Statistical analysis. Quantity One version 4.2.1 and Image-Pro plus version 4.5 were used to transform images into numerical values. Student’s t-test and two-way analysis of variance were used to determine the statistical significance of differences between values for the experimental and control groups.
RESULTS
Effects of solvent fractions from C. tschonoskii on the inflammatory chemokine in HaCaT keratinocyte. We investigated effects of solvent fractions from C. tschonoskii on the inflammatory chemokines in IFN-γ-stimulated HaCaT cells. MDC production is markedly increased by IFN-γ stimulation in HaCaT cells and decreased when treated with solvent fractions from C. tschonoskii, except hexane fraction (Fig. 1B). Especially, EtOH, EtoAc, and BuOH fractions strongly inhibited the MDC production. We next examined whether these inhibitory effects of solvent fractions of C. tschonoskii are related with cell cytotoxicity. HaCaT cells were treated with solvent fractions (50 μg/ml) for 24 hr and cell viability was affirmed by MTT assay. As shown in result, the fractions had an inhibitory activity on MDC production, with cell cytotoxicity activity at 50 μg/ml on the HaCaT cells, excluding chloroform fraction (Fig. 1C). Thereafter, we used only the chloroform fraction in further study.
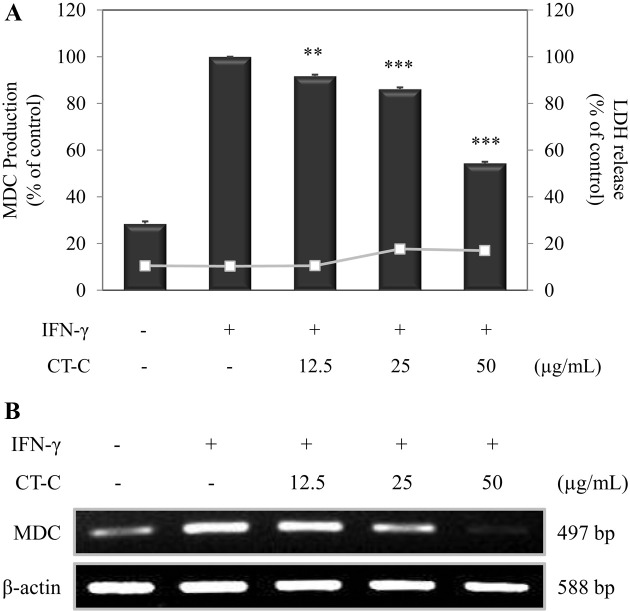
Effect of chloroform fraction from C. tschonoskii on the inflammatory chemokine in HaCaT keratinocyte. We next investigated the effects of chloroform fraction from C. tschonoskii on the inflammatory chemokines in IFN-γ- stimulated HaCaT cells. To confirm the cytotoxicity of chloroform fraction on the HaCaT cells, we performed a LDH assay. Chloroform fraction did not affect the cytotoxicity (Fig. 2A).
Fig. 2. Effect of chloroform fraction from Carpinus tschonoskii on the mRNA and protein level of MDC in IFN-γ-stimulated HaCaT human keratinocytes. (A) Cells (3.0 × 105 cells/ml) were pre-incubated for 18 hr, and the protein production of MDC was determined from the culture supernatant of cells stimulated by IFN-γ (10 ng/ml) in the presence of chloroform fraction (12.5, 25, 50 μg/ ml) of C. tschonoskii. MDC production was determined by ELISA and was done in triplicate. Error bars indicate ± S.D. **, p < 0.01; ***, p < 0.001, significant when compared with IFN-γ positive control. (B) mRNA expression of MDC was determined from the cells stimulated with IFN-γ (10 ng/mL) in the presence of chloroform fraction (12.5, 25, 50 μg/ml) of C. tschonoskii for 18 hr.
Therefore, we examined the effect of chloroform fraction on the protein production and the mRNA expression of MDC. HaCaT cells were treated with chloroform fraction and stimulated with IFN-γ for 24 hr. Increased MDC production by IFN-γ stimulation was reduced by treatment of chloroform fraction (Fig. 2A). We next analyzed the effect of chloroform fraction on IFN-γ-induced mRNA expression of MDC after the treatment of chloroform fraction for 18 hr by RT-PCR. Consistent with the ELISA result, chloroform fraction from C. tschonoskii significantly suppressed IFN-γ-induced mRNA expression of MDC with increasing dose (Fig. 2B). These results suggest that chloroform fraction from C. tschonoskii can modulate expression of Th2 inflammatory chemokine, MDC, in IFN-γ-stimulated HaCaT keratinocytes.
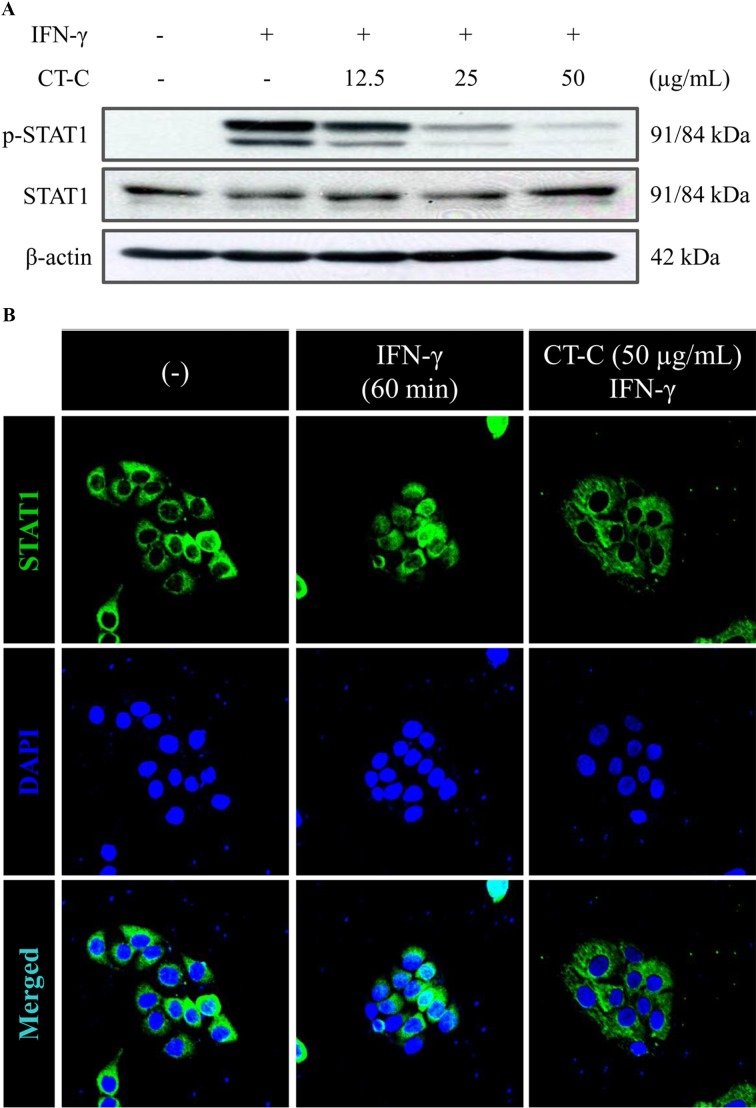
Effect of chloroform fraction from C. tschonoskii on IFN-γ-activated STAT1 in HaCaT keratinocyte. We further studied the effect of chloroform fraction on the signaling pathway that leads to expression of MDC in IFN-γ- stimulated HaCaT cells. Previous studies have reported that Jak/STAT, NF-κB, and p38 MAPK pathways contribute to production of MDC in IFN-γ and/or TNF-α-stimulated HaCaT cells. Also, Jak/STAT1 signaling pathway is a major signaling pathway of IFN-γ signaling (Ju et al., 2009; Jeong et al., 2010; Hongqin et al., 2011). Therefore, we investigated the effect of chloroform fraction on IFN-γ-induced STAT1 activation. chloroform fraction from C. tschonoskii pre-treated for 2 hr, and then HaCaT cells were stimulated with IFN-γ for 30 min. Chloroform fraction dramatically suppressed the IFN-γ-induced phosphorylation of STAT1 (Fig. 3A). Also, we confirmed the effect of chloroform fraction on nuclear translocation of STAT1 by immunofluorescence analysis. STAT1 protein at cytosol was trans-located to nuclear after IFN-γ stimulation and was inhibited by chloroform fraction treatment (Fig. 3B). These results indicate that chloroform fraction from C. tschonoskii inhibits IFN-γ-induced MDC production through inhibition of STAT1 activation in HaCaT keratinocytes.
Fig. 3. Effect of chloroform fraction from Carpinus tschonoskii on the STAT1 signal in IFN-γ-stimulated HaCaT human keratinocytes. (A) Cells were pretreated with the indicated concentration of chloroform fraction of C. tschonoskii for 2 hr, and then the phosphorylation of STAT1 was determined in cells stimulated by IFN-γ (10 ng/ml) for 30 min. The levels of STAT1 and β-actin were identified using Western blotting analysis. (B) Cells were pretreated with the chloroform fraction (50 μg/ml) of C. tschonoskii for 2 hr, and then the translocation of STAT1 protein was determined in cells stimulated by IFN-γ (10 ng/ml) for 60 min. Immunofluorescence stain of STAT1 was stained with DyLight488-conjugated 2nd antibody and the fluorescence was identified using confocal microscopy (FV500, OLYMPUS) and the images were acquired at constant conditions (PMT, gain, offset, magnification (20 × objective with zoom factor of 2.5) and resolution, etc.). These data are representative of three independent experiments.
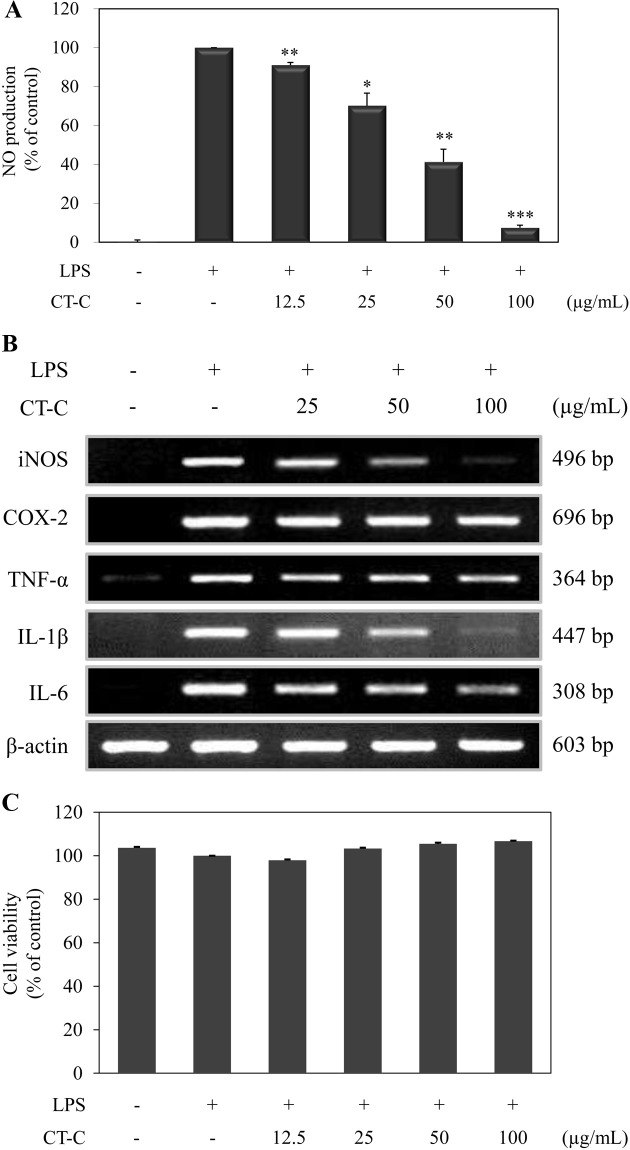
Effect of chloroform fraction from C. tschonoskii on the inflammatory mediators in RAW264.7 macrophage. Next, we investigated whether chloroform fraction from C. tschonoskii has an anti-inflammatory effect in RAW264.7 macrophage. We first measured the effect of chloroform fraction on LPS-induced NO production in RAW264.7 cells by Griess method in culture medium. Stimulation of cells with LPS for 24 hr increased NO production in the medium and chloroform fraction dose-dependently inhibited NO production by LPS in RAW264.7 cells (Fig. 4A). To examine whether this suppression of NO production was due to reduced iNOS expression, we performed RT-PCR analysis of LPS-stimulated cells. Consistent with the NO assay result, the chloroform fraction suppressed the mRNA expression of iNOS in a dose-dependent manner (Fig. 4B).
Fig. 4. Effect of chloroform fraction of Carpinus tschonoskii on the inflammatory mediators in the LPS-stimulated RAW264.7 murine macrophage. (A) Cells were treated with LPS (1 μg/ml) only or LPS plus different concentrations (12.5, 25, 50, 100 μg/ ml) of chloroform fraction for 24 hr. At the end of incubation, NO assay was determined from the supernatant. NO production was determined by Griess method. The measurement of NO was done in triplicate. Error bars indicate ± S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.001, significant when compared with LPS positive control. (B) mRNA expression of inflammatory mediators was determined from the cells stimulated by LPS (1 μg/ml) in the presence of chloroform fraction (25, 50, 100 μg/ml) for 24 hr. (C) Cell viability was determined from the cells stimulated with LPS (1 μg/ml) in the presence of chloroform fraction of C. tschonoskii (12.5, 25, 50, 100 μg/ml) for 24 hr. Cell viability was analyzed by MTT assay.
Furthermore, we investigated the effect of chloroform fraction from C. tschonoskii on the other pro-inflammatory factors (COX-2, TNF-α, IL-1β, and IL-6). The mRNA expression of pro-inflammatory factors was evaluated in RAW264.7 cells incubated with LPS in the presence of various concentration of chloroform fraction. The stimulation of RAW264.7 cells with LPS led to the increased expression of the proinflammatory factors. Chloroform fraction from C. tschonoskii dramatically decreased the mRNA expression of IL-1β and weakly inhibited the mRNA expression of TNF-α and IL-6. However, COX-2 mRNA expression by LPS stimulation was not suppressed by the treatment of chloroform fraction from C. tschonoskii (Fig. 4B).
To determine the effect on cell viability by chloroform fraction in RAW264.7 cell, we performed MTT assay. As a result, chloroform fraction did not affect on the cell viability (Fig. 4C). These results indicated that chloroform fraction from C. tschonoskii shows the anti-inflammatory activity through the inhibition on pro-inflammatory factors in RAW264.7 macrophages.
DISCUSSION
In this study, we found that chloroform fraction from C. tschonoskii has an inhibitory activity on various inflammatory mediators.
Chemokines are secreted basic proteins of 8~10 kDa and are critically important in allergic inflammation via leukocyte trafficking and leukocyte activation. Among them, I- 309 (CCL1), monocyte chemotactic protein-1 (MCP-1/ CCL2), regulated and normal T cell expressed and secreted (RANTES/CCL5), eotaxin(CCL11), MCP-4 (CCL13), thymus and activation regulated chemokine (TARC/CCL17), macrophage inflammatory protein-3α (MIP-3α/CCL20), macrophage-derived chemokine (MDC/CCL22), and cutaneous T-cell-attracting chemokine (CTACK/CCL27) are originated from keratinocytes and may be an effective target for treatment of inflammatory skin diseases (Kaplan, 2001; Pease and Williams, 2006; Luster, 2001). Particularly, an MDC level is closely related with AD and is one of the characteristic features of AD (Kakinuma et al., 2002; Leung et al., 2003; Mantovani et al., 2000). Therefore, we firstly tested the effect of solvent extracts of C. tschonoskii on MDC expression in HaCaT keratinocytes and we found that chloroform fraction from C. tschonoskii inhibits the protein production and mRNA expression of MDC in IFN- γ-stimulated HaCaT keratinocytes (Fig. 1C and 2A and 2B).
IFN-γ is well-known to trigger antiviral and adaptive immune responses through a Jak-STAT signaling pathway. IFN-γ binds to IFNGR1 and R2 on cell surface and then activates Jak/STAT, ERK, p38 MAPK, and NF-κB pathways (van Boxel-Dezaire and Stark, 2007; Gough et al., 2008). Also, these activated pathways can modulate the expression of numerous inflammatory mediators, including MDC (Jeong et al., 2010; Hongqin et al., 2011; Kang et al., 2011). Accordingly, to confirm the action mechanism of chloroform fraction from C. tschonoskii, we next explored the effect of chloroform fraction from C. tschonoskii on the STAT1 activated by IFN-γ stimulation in HaCaT cells. As expected, chloroform fraction from C. tschonoskii potently inhibited the phosphorylation and translocation of STAT1 (Fig. 3A and 3B). These results indicate that chloroform fraction of C. tschonoskii can affect the STAT1 signaling cascade.
Numerous studies demonstrated that TNF-α, IL-6, NO, and COX-2 play key roles in inflammation in vitro and in vivo. TNF-α and IL-6 are the cytokines that act as coordinate the inflammatory responses. High NO production by macrophages can damage host cells and tissues and COX-2 appears to be the dominant source of prostaglandin formation in inflammation. (MacMicking et al., 1995, Lee et al., 2009, Damte et al., 2011, Ricciotti and FitzGerald, 2011, Li et al., 2012; Marcuzzi et al., 2012). We lastly explored the activity of chloroform fraction from C. tschonoskii on inflammatory mediators in LPS-stimulated RAW264.7 macrophages. Although there is diverseness among inhibitory activities on inflammatory mediators induced by LPS stimulation in RAW264.7 cells, chloroform fraction of C. tschonoskii inhibited inflammatory mediators induced by LPS stimulation (Fig. 4A and 4B). Our results indicate that chloroform fraction from C. tschonoskii has anti-inflammatory activity in LPS-stimulated RAW264.7 cells.
There is a report that MeOH extract of C. tschonoskii inhibits CpG-induced inflammatory cytokines (IL-6 and TNF-α) through blocking NF-κB signaling in bone marrow- derived macrophages (BMDMs) (Koo et al., 2012). Viral DNA is rich in unmethylated CpG motifs, which stimulates TLR9 that is expressed intracellularly within the endosomal compartments and functions to al-ert the immune system of viral and bacterial infections. CpG-recognized TLR9 pathway principally activates an expression of proinflammatory cytokines via the activation of MyD88-dependent pathway. Nevertheless, lipopolysaccharide (LPS) is an important structural component of the outer membrane of Gram-negative bacteria and is recognized by TLR4. This is largely mediated via two downstream pathways: MyD88- dependent and -independent pathway (Pålsson-McDermott and O’Neill, 2004; Takeshita et al., 2004; Michelsen et al., 2004; Ulevitch et al., 2004). Moreover, LPS stimulation can activate a Jak/STAT pathway and regulates the transcription of target genes encoding proinflammatory cytokines, chemokines, and inducible enzymes such as iNOS and COX-2 (Stempelj et al., 2007; Hu et al., 2007; Hammer et al., 2010). Although further study is needed on the action mechanism of chloroform fraction from C. tschonoskii in RAW264.7 macrophage, taken together with the results in HaCaT cells, the inhibitory effect of chloroform fraction on LPS-induced inflammatory mediators may be the effect through an inhibition of NF-κB and STAT1.
Previous study showed that genus Carpinus contained various flavonols (myricetin 3-O-glycoside, quercetin 3-O-glycoside, kaempferol 3-O-glycoside, etc), flavanone (naringenin), and isoflavone (genistein). Especially, C. tschonoskii exclusively involved another flavonol, myricetin (Jeon and Chang, 2000). Therefore, study is needed that confirms the activity component in chloroform fraction from C. tschonoskii.
In summary, chloroform fraction of C. tschonoskii suppresses the inflammatory chemokine, MDC, expression through the regulation of STAT1 signal in HaCaT keratinocytes and the inflammatory mediators in RAW264.7 macrophages. The biological effects of the chloroform fraction from C. tschonoskii confirmed in this study indicate that an extract or its components might be useful for the treatment of inflammatory disorders.
References
- 1.Coleman J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. (2001);1:1397–1406. doi: 10.1016/S1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 2.Damte D., Reza M.A., Lee S.J., Jo W.S., Park S.C. Anti-inflammatory activity of dichloromethane extract of auricularia auricula-judae in RAW264.7 cells. Toxicol. Res. (2011);27:11–14. doi: 10.5487/TR.2011.27.1.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffield J.S. The inflammatory macrophage: a story of Jekyll and Hyde. Clin. Sci. (Lond) (2003);104:27–38. doi: 10.1042/CS20020240. [DOI] [PubMed] [Google Scholar]
- 4.Gough D.J., Levy D.E., Johnstone R.W., Clarke C.J. IFNgamma signaling-does it mean JAK-STAT? Cytokine Growth Factor Rev. (2008);19:383–394. doi: 10.1016/j.cytogfr.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Hammer K.D., Yum M.Y., Dixon P.M., Birt D.F. Identification of JAK-STAT pathways as important for the antiinflammatory activity of a Hypericum perforatum fraction and bioactive constituents in RAW 264.7 mouse macrophages. Phytochemistry. (2010);71:716–725. doi: 10.1016/j.phytochem.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hongqin T., Xinyu L., Heng G., Lanfang X., Yongfang W., Shasha S. Triptolide inhibits IFN-gamma signaling via the Jak/STAT pathway in HaCaT keratinocytes. Phytother. Res. (2011);25:1678–1685. doi: 10.1002/ptr.3471. [DOI] [PubMed] [Google Scholar]
- 7.Hu X., Chen J., Wang L., Ivashkiv L.B. Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. J. Leukocyte Biol. (2007);82:237–243. doi: 10.1189/jlb.1206763. [DOI] [PubMed] [Google Scholar]
- 8.Jeon J.I., Chang C.S. Foliar flavonoids of genus carpinus in eastern Asia. Kor. J. Plant Tax. (2000);30:139–153. [Google Scholar]
- 9.Jeong S.I., Choi B.M., Jang S.I. Sulforaphane suppresses TARC/CCL17 and MDC/CCL22 expression through heme oxygenase-1 and NF-kappaB in human keratinocytes. Arch. Pharm. Res. (2010);33:1867–1876. doi: 10.1007/s12272-010-1120-6. [DOI] [PubMed] [Google Scholar]
- 10.Ju S.M., Song H.Y., Lee S.J., Seo W.Y., Sin D.H., Goh A.R., Kang Y.H., Kang I.J., Won M.H., Yi J.S., Kwon D.J., Bae Y.S., Choi S.Y., Park J. Suppression of thymusand activation-regulated chemokine (TARC/CCL17) production by 1,2,3,4,6-penta-O-galloyl-beta-D-glucose via blockade of NF-kappaB and STAT1 activation in the HaCaT cells. Biochem. Biophys. Res. (2009);387:115–120. doi: 10.1016/j.bbrc.2009.06.137. [DOI] [PubMed] [Google Scholar]
- 11.Kakinuma T., Nakamura K., Wakugawa M., Mitsui H., Tada Y., Saeki H., Torii H., Komine M., Asahina A., Tamaki K. Serum macrophage-derived chemokine (MDC) levels are closely related with the disease activity of atopic dermatitis. Clin. Exp. Immunol. (2002);127:270–273. doi: 10.1046/j.1365-2249.2002.01727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang G.J., Han S.C., Yi E.J., Kang H.K., Yoo E.S. The inhibitory effect of premature Citrus unshiu extract on atopic dermatitis in vitro and in vivo. Toxicol. Res. (2011);27:173–180. doi: 10.5487/TR.2011.27.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan A.P. Chemokines, chemokine receptors and allergy. Int. Arch. Allergy Immunol. (2001);124:423–431. doi: 10.1159/000053777. [DOI] [PubMed] [Google Scholar]
- 14.Koo J.E., Hong H.J., Mathema V.B., Kang H.K., Hyun J.W., Kim G.Y., Kim Y.R., Maeng Y.H., Hyun C.L., Chang W.Y., Koh Y.S. Inhibitory effects of Carpinus tschonoskii leaves extract on CpG-stimulated pro-inflammatory cytokine production in murine bone marrow-derived macrophages and dendritic cells. In Vitro Cell. Dev. Biol. Anim. (2012);48:197–202. doi: 10.1007/s11626-012-9495-y. [DOI] [PubMed] [Google Scholar]
- 15.Lee H.J., Dang H.T., Kang G.J., Yang E.J., Park S.S., Yoon W.J., Jung J.H., Kang H.K., Yoo E.S. Two enone fatty acids isolated from Gracilaria verrucosa suppress the production of inflammatory mediators by down-regulating NFkappaB and STAT1 activity in lipopolysaccharide-stimulated RAW 264.7 cells. Arch. Pharm. Res. (2009);32:453–462. doi: 10.1007/s12272-009-1320-0. [DOI] [PubMed] [Google Scholar]
- 16.Leung T.F., Ma K.C., Hon K.L., Lam C.W., Wan H., Li C.Y., Chan I.H. Serum concentration of macrophagederived chemokine may be a useful inflammatory marker for assessing severity of atopic dermatitis in infants and young children. Pediatr. Allergy Immunol. (2003);14:296–301. doi: 10.1034/j.1399-3038.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 17.Li C.C., Hsiang C.Y., Lo H.Y., Pai F.T., Wu S.L., Ho T.Y. Genipin inhibits lipopolysaccharide-induced acute systemic inflammation in mice as evidenced by nuclear factor-kappaB bioluminescent imaging-guided transcriptomic analysis. Food Chem. Toxicol. (2012);50:2978–2986. doi: 10.1016/j.fct.2012.05.054. [DOI] [PubMed] [Google Scholar]
- 18.Luster A.D. Antichemokine immunotherapy for allergic diseases. Curr. Opin. Allergy Clin. Immunol. (2001);1:561–567. doi: 10.1097/00130832-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 19.MacMicking J.D., Nathan C., Hom G., Chartrain N., Fletcher D.S., Trumbauer M., Stevens K., Xie Q.W., Sokol K., Hutchinson N. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. (1995);81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 20.Mantovani A., Gray P.A., Van Damme J., Sozzani S. Macrophage-derived chemokine (MDC). J. Leukocyte Biol. (2000);68:400–404. [PubMed] [Google Scholar]
- 21.Marcuzzi A., Secchiero P., Crovella S., Zauli G. TRAIL administration down-modulated the acute systemic inflammatory response induced in a mouse model by muramyldipeptide or lipopolysaccharide. Cytokine. (2012);60:43–46. doi: 10.1016/j.cyto.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Maruotti N., Cantatore F.P., Crivellato E., Vacca A., Ribatti D. Macrophages in rheumatoid arthritis. Histol. Histopathol. (2007);22:581–586. doi: 10.14670/HH-22.581. [DOI] [PubMed] [Google Scholar]
- 23.Michelsen K.S., Doherty T.M., Shah P.K., Arditi M. TLR signaling: an emerging bridge from innate immunity to atherogenesis. J. Immunol. (2004);173:5901–5907. doi: 10.4049/jimmunol.173.10.5901. [DOI] [PubMed] [Google Scholar]
- 24.Pålsson-McDermott E.M., O’Neill L.A. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunol. (2004);113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pease J.E., Williams T.J. Chemokines and their receptors in allergic disease. J. Allergy Clin. Immunol. (2006);118:305–318. doi: 10.1016/j.jaci.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Ricciotti E., FitzGerald G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. (2011);31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stempelj M., Kedinger M., Augenlicht L., Klampfer L. Essential role of the JAK/STAT1 signaling pathway in the expression of inducible nitric-oxide synthase in intestinal epithelial cells and its regulation by butyrate. J. Biol. Chem. (2007);282:9797–9804. doi: 10.1074/jbc.M609426200. [DOI] [PubMed] [Google Scholar]
- 28.Takeshita F., Gursel I., Ishii K.J., Suzuki K., Gursel M., Klinman D.M. Signal transduction pathways mediated by the interaction of CpG DNA with Toll-like receptor 9. Semin. Immunol. (2004);16:17–22. doi: 10.1016/j.smim.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Ulevitch R.J., Mathison J.C., da Silva Correia J. Innate immune responses during infection. Vaccine. (2004);22(Suppl 1):S25–S30. doi: 10.1016/j.vaccine.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 30.van Boxel-Dezaire A.H., Stark G.R. Cell type-specific signaling in response to interferon-gamma. Curr. Top. Microbiol. Immunol. (2007);316:119–154. doi: 10.1007/978-3-540-71329-6_7. [DOI] [PubMed] [Google Scholar]
- 31.Yamada P., Ono T., Shigemori H., Han J., Isoda H. Inhibitory effect of tannins from galls of Carpinus tschonoskii on the degranulation of RBL-2H3 Cells. Cytotechnology. (2012);64:349–356. doi: 10.1007/s10616-012-9457-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang R., Kang K.A., Piao M.J., Park J.W., Shin T., Yoo B.S., Yang Y.T., Hyun J.W. Cytoprotective activity of Carpinus tschonoskii against H2O2 induced oxidative stress. Nat. Prod. Sci. (2007);13:118–122. [Google Scholar]