Abstract
Betaine supplementation has been shown to alleviate altered glucose and lipid metabolism in mice fed a high-fat diet or a high-sucrose diet. We investigated the beneficial effects of betaine in diabetic db/db mice. Alleviation of endoplasmic reticulum (ER) and oxidative stress was also examined in the livers and brains of db/db mice fed a betaine-supplemented diet. Male C57BL/KsJ-db/db mice were fed with or without 1% betaine for 5 wk (referred to as the db/db-betaine group and the db/db group, respectively). Lean non-diabetic db/db+ mice were used as the control group. Betaine supplementation significantly alleviated hyperinsulinemia in db/db mice. Betaine reduced hepatic expression of peroxisome proliferator-activated receptor gamma coactivator 1 alpha, a major transcription factor involved in gluconeogenesis. Lower serum triglyceride concentrations were also observed in the db/db-betaine group compared to the db/db group. Betaine supplementation induced hepatic peroxisome proliferator-activated receptor alpha and carnitine palmitoyltransferase 1a mRNA levels, and reduced acetyl-CoA carboxylase activity. Mice fed a betaine-supplemented diet had increased total glutathione concentrations and catalase activity, and reduced lipid peroxidation levels in the liver. Furthermore, betaine also reduced ER stress in liver and brain. c-Jun N-terminal kinase activity and tau hyperphosphorylation levels were lower in db/db mice fed a betaine-supplemented diet, compared to db/db mice. Our findings suggest that betaine improves hyperlipidemia and tau hyperphosphorylation in db/db mice with insulin resistance by alleviating ER and oxidative stress.
Keywords: Betaine, db/db mice, ER stress, Insulin resistance, Oxidative stress, Tau phosphorylation
INTRODUCTION
Diabetes mellitus is one of the most important and prevalent chronic diseases. Oxidative stress, reflected in increased levels of proinflammatory cytokines, the dysregulation of mitochondrial function, and the overproduction of reactive oxygen species, has also been implicated in insulin resistance (1). Endoplasmic reticulum (ER) stress is a key factor linking obesity and type 2 diabetes. Previous reports have shown that the administration of molecular or active chemical chaperones to obese and diabetic mice normalizes insulin signaling in the liver, skeletal muscle, and white adipose tissues (2,3). The markers of ER and oxidative stress are increased in db/db mice, a genetic model of type 2 diabetes, compared with those in lean mice (4,5).
It has been shown that obesity, diabetes mellitus, and non-alcoholic steatohepatitis can be associated with pathological changes in the brain (6,7). Insulin receptors are present in the hippocampus and the medial temporal cortex in rat, suggesting a role for insulin in cognitive function (8). A previous study indicated that mice with insulin deficiency induced by streptozotocin showed rapid tau hyperphosphorylation, similar to that seen in early Alzheimer’s disease (AD) (9). Whereas accumulating evidence continues to support the hypothesis that insulin dysregulation is a significant risk factor in the development of neurodegenerative diseases, the precise mechanisms by which insulin resistance affects AD remain unknown.
Betaine (N,N,N-trimethylglycine) has been shown to protect the liver by attenuating steatosis, oxidative stress, and fibrosis. It has three active methyl groups and is an alternative methyl donor for betaine-homocysteine methyltrans-ferase-mediated remethylation, which converts homocysteine to methionine (10). ER stress, fatty acid accumulation, and apoptosis were alleviated in the livers of animal models and in cultured hepatocytes supplemented with betaine (11,12). Betaine also ameliorates alcoholic and nonalcoholic pathological injuries by reducing peroxidation in the liver (13-15). Beneficial effects of betaine on fasting glucose and insulin levels, and hepatic insulin resistance have been reported in mice fed a high-fat diet or high-sucrose diet (16,17). Therefore, in the present study, we investigated the beneficial effects and the underlying mechanism(s) of betaine in the livers and brains of db/db mice.
MATERIALS AND METHODS
Animals. Male C57BL/KsJ-db/db mice and their lean heterozygote non-diabetic control (db/+) mice were purchased from Japan SLC, Inc. (Japan) on five weeks of age. During acclimation period, mice were fed only standard chow diet. After two to three week acclimation period, blood glucose level was measured. Confirmed high glucose db/db mice (> 300 mg/dL) were randomly divided into two groups and fed either control AIN-93G diet or betaine supplemented diet (1 g betaine/100 g diet). db/+ mice were fed an AIN-93G diet. Diets and water were provided ad libitum. All mice were maintained in a temperature (22 ± 3℃) and humidity (50 ± 10%)-controlled room with a 12 h lightdark cycle. The experimental procedures used in the present study were approved by Seoul National University Institutional Animal Care and Use Committee. At the end of experiment, mice were sacrificed after an overnight fast. Blood samples were rapidly obtained from puncture of the right atrium and serum was obtained after centrifugation. Tissues were removed, snap-frozen immediately in liquid nitrogen, and stored at −80℃ until analysis.
Serum analysis. After blood collection, blood was centrifuged at 3000 rpm for 20 min and stored at −80℃ until the blood biochemistry was analyzed. Serum glucose and triglyceride levels were determined using commercial kits (Asan Pharmacy, Korea). Serum insulin levels were measured using an insulin ELISA kit (Millipore, USA). The insulin resistance index was estimated by homeostasis model assessment with the following formula: serum glucose × serum insulin/22.5, with serum glucose in mmol/ml and serum insulin in μU/ml.
Hepatic lipid analysis. Total lipids were extracted from 25 mg of tissue according to the method used by Folch et al. (18). Briefly, total lipids from tissues were extracted and homogenized in methanol-chloroform (1 : 2, v/v). The extraction solvent was concentrated by nitrogen gas, the lipid pellets were resuspended in isopropanol, and triglyceride and cholesterol contents were determined by an enzymatic colorimetric method using the commercial kit (Asan Pharmacy, Korea).
Antioxidant enzyme activity and total glutathione assays. Tissues were homogenized in 10 volumes (w : v) of homogenizing buffer containing 154 mM KCl, 50 mM Tris-HCl, and 1 mM EDTA (pH 7.4). Tissue homogenate was centrifuged at 600 ×g for 10 min at 4℃ and the supernatant was used for the following analyses. The activity of catalase was measured by the method of Abei (19). Total glutathione levels were measured by the method of Griffith (20). Protein contents were determined using a commercial kit from Bio-Rad (USA).
Lipid peroxidation analysis. The level of lipid peroxides in the liver and kidney homogenate was analyzed according to the method of Ohkawa et al. (21). Briefly, homogenates (10%, w : v) were mixed with sodium dodecyl sulfate, acetate buffer (pH 3.5), and thiobarbituric acid. After boiling at 95℃ for 1 h, the mixture was immediately placed on the ice to stop the reaction. The red pigment was extracted with n-butanol : pyridine mixture (15 : 1, v:v) after vigorous vortexing. Finally, the absorbance of butanol layer was measured at 532 nm using 1,1,3,3-tetraethoxypropane as a standard. The lipid peroxide level was expressed as malondialdehyde equivalents per gram tissue.
Total RNA extraction and semiquantitative RT-PCR. Total RNA of liver tissue was isolated using RNAiso reagent (Takara, Japan) and amount of RNA was measured using Quanti-iT™ RNA Assay Kit (Invitrogen, USA). cDNA was synthesized using 2 mg of total RNA with the Superscript®II Reverse Transcriptase (Invitrogen). For amplification of cDNA, primers for X-box-protein-1 (XBP-1; forward primer 5'-AAACAGAGTAGCAGCTCAGACTGC-3', reverse primer 5'-TCCTTCTGGGTAGACCTCTGGGAG-3') and peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α: forward primer 5'-AGCCGTGACCACTGACAACGAG- 3', reverse primer 5'-GCTGCATGGTTCTGAGTGCTAAG-3') were used. Expression of β-actin was examined as an internal control (forward primer 5'-TGACCCAGATCATGTTTGAGACC- 3', reverse primer 5'-CCATACCCAAGAAGGAAGGC- 3'). Amplified products of XBP- 1 were further digested by PstI to check whether a PstI restriction site was lost after inositol-requiring enzyme 1- mediated splicing of mRNA. The amplified products were separated on agarose gel and visualized with ethidium bromide staining under UV illumination. The bands were quantified with Quantity One software (Bio-Rad, USA).
Real-time quantitative RT-PCR. All reactions were performed using a StepOne™ Real Time PCR System (Applied Biosystems, USA). To determine gene expression using SYBR® premix Ex Taq™, the amplification reactions were performed according to manufacturer’s protocol. For amplification of cDNA, primers for peroxisome proliferator- activated receptor alpha (PPARα; forward primer 5'- CCTCAGGGTACCACTACGGAGT-3', reverse primer 5'- GCCGAATAGTTCGCCGAA-3'), carnitine palmitoyltransferase 1a (CPT1a: forward primer 5'-ACGGAGTCCTGCAACTTTGT- 3', reverse primer 5'-GTACAGGTGCTGGTGCTTTTC- 3'), stearoyl-CoA desaturase 1 (SCD1: forward primer 5'-ATCTCCAGTTCTTACACGACCACC-3', reverse primer 5'-CGTCTTCACCTTCTCTCGTTCATT-3'), and heme oxygenase 1 (HO-1: forward primer 5'-CCTCACTGGCAGGAAATCATC- 3', reverse primer 5'-CCTCGTGGAGACGCTTTACATA- 3') were used. Expression of ribosomal protein L19 (RPL19) was examined as an internal control (forward primer 5'-TCAGGCTACAGAAGAGGCTTGC- 3', reverse primer 5'-ATCAGCCCATCCTTGATCAGC- 3'). Quantification of relative changes in expression was determined following the manufacturer’s suggested protocol for a ΔΔCt assay.
Tissue extract preparation and immunoblotting analysis. Liver tissues were homogenized in ice-cold protein lysis buffer. After centrifugation for 30 min at 10,000 ×g at 4℃, the protein content of the supernatant was determined with Bio-Rad protein Assay Reagent. Equal amounts of protein were loaded into the lanes of a SDS-PAGE gel, separated, and blotted onto a PVDF membrane. After the membranes had been blocked with either 5% nonfat milk or bovine serum albumin, they were probed with a specific antibody, as follows: anti-eukaryotic translation initiation factor 2 alpha (eIF2α; Cell Signaling, USA), anti-p-eIF2α (Cell Signaling), anti-dephosphorylated tau (clone tau-1; Chemicon, USA), anti-phosphorylated acetyl-CoA carboxylase (ACC; Millipore, USA), anti-KDEL (Stressgen, USA), anti-c-Jun Nterminal kinase (JNK; Cell Signaling), anti-p-JNK3 (Millipore), or anti-beta-actin (Sigma). The membranes were then incubated with a horseradish peroxidase-conjugated secondary antibody for chemiluminescent detection. The band intensities were quantified using Quantity One software.
Statistical analysis. The data were analyzed using SPSS software (version 16.0). For all experiments, one-way ANOVA followed by Duncan’s multiple range test was employed to determine the statistical significances among groups. Data were expressed as means ± SEM and differences were considered statistically significant at p < 0.05.
RESULTS
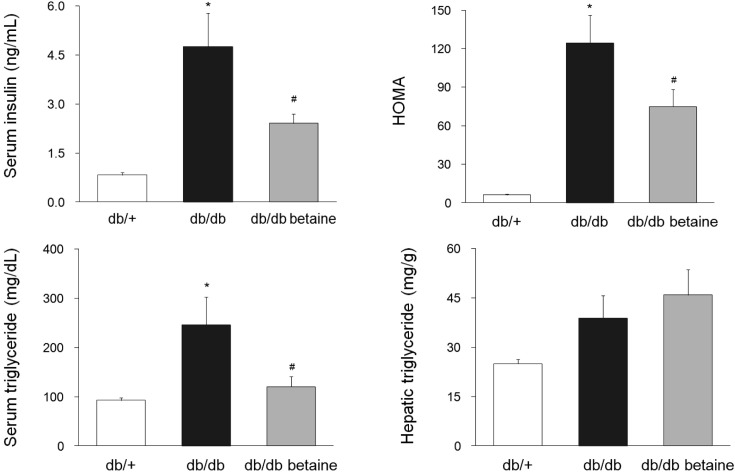
Effect of betaine on serum and hepatic biochemical parameters. The body weight changes and final relative perirenal fat weights did not differ among the groups (data not shown). However, both the absolute and relative liver weights were significantly increased in the db/db mice, and betaine supplementation did not cause a change in the liver weight (data not shown). Serum insulin levels and homeostatic model assessment of insulin resistance (HOMA-IR) of db/db mice were significantly reduced in the betaine-supplemented group (Fig. 1). Serum triglyceride levels of the db/db group were significantly higher than those of the db/db betaine group. However, supplementation with betaine did not significantly block an increase of hepatic triglyceride accumulation.
Fig. 1. Effect of betaine supplementation on selected serum and hepatic parameters in db/db mice. Each bar represents the mean ± SEM. *p < 0.05 compared with db/+ mice. #p < 0.05 compared with db/db mice.
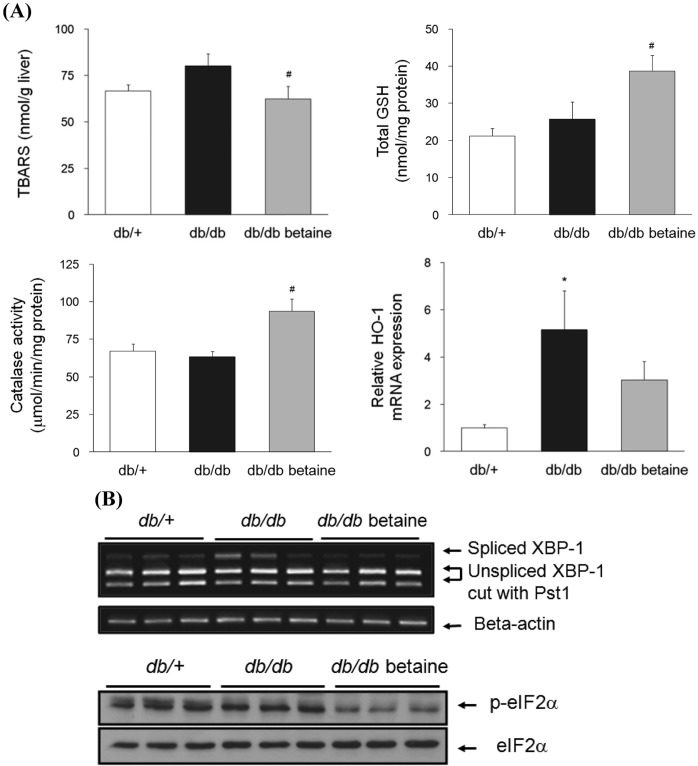
Effect of betaine on oxidative stress and ER stress in the livers of db/db mice. A significantly decreased hepatic thiobarbituric acid reactive substance (TBARS) levels was observed in the db/db group by betaine supplementation (Fig. 2A). Hepatic total glutathione levels were also significantly increased in the betaine-supplemented group. Consistently, catalase activity was significantly increased by betaine supplementation. A significant induction of HO- 1 expression was observed in db/db mice. Betaine tended to reduce mRNA levels of HO-1, but the differences did not reach statistical significance.
Fig. 2. (A) Effect of betaine supplementation on oxidative stress markers in the livers of db/db mice (n = 8-10). Relative mRNA levels of HO-1/RPL-19 were determined by quantitative RT-PCR (n = 3). Each bar represents the mean ± SEM. *p < 0.05 compared with db/+ mice. #p < 0.05 compared with db/db mice. (B) Effect of betaine supplementation on ER stress markers in the livers of db/db mice. Top: Relative mRNA levels of spliced XBP-1 were determined by semiquantitative RT-PCR. Equal RNA loading was confirmed with beta-actin. Bottom: The protein levels of p-eIF2α and total eIF2α were determined by immunoblotting analysis.
To determine the effects of betaine on ER stress, we examined the signaling in the ER stress pathway. As shown in Fig. 2B, the ER stress-induced splicing of XBP-1 mRNA was markedly increased in db/db mice. Betaine alleviated ER stress in the livers of db/db mice. We also determined the phosphorylation of eIF2α, which is typically associated with the stress responses and causes a reduction in protein synthesis (22). Betaine supplementation significantly reduced the levels of p-eIF2α in the livers of db/db mice. Total eIF2α levels were also assessed and indicated equal loading.
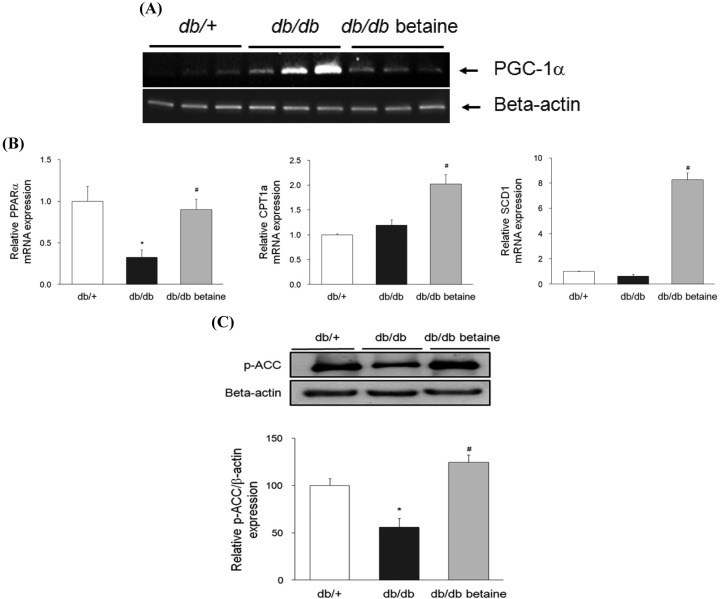
Effect of betaine on glucose and lipid metabolism in the livers of db/db mice. PGC-1α has been identified as an important mediator of glucose regulation and its expression in the liver is dramatically increased in diabetes or during fasting (23). Betaine alleviated PGC-1α mRNA expression, which was markedly increased in db/db mice (Fig. 3A). The expression of PPARα pathway genes was also significantly increased in db/db mice fed a betaine-supplemented diet (Fig. 3B). PPARα promotes the hydrolysis of circulating triglyceride, increases the partitioning of fatty acids for β-oxidation, and reduces the secretion of very-low-density lipoprotein (VLDL) in the liver (24). Betaine supplementation significantly increased expression of CPT1a, the key regulatory enzyme for fatty acid oxidation. Betaine also increased ACC phosphorylation, confirming that betaine increased hepatic fatty acid oxidation in db/db mice (Fig. 3C). Interestingly, we also observed that db/db mice fed a betaine-supplemented diet exhibited significantly increased mRNA levels of SCD1, which is responsible for the conversion of saturated fatty acids into monounsaturated fatty acids (Fig. 3B).
Fig. 3. Effect of betaine supplementation on glucose and lipid metabolism in the livers of db/db mice. (A) Relative mRNA levels of PGC-1α were determined by semiquantitative RT-PCR. Equal RNA loading was confirmed with beta-actin. (B) Relative mRNA levels of PPARα, CPT1a, and SCD1 were determined by real-time quantitative RT-PCR (n = 3-4). (C) The protein levels of p-ACC and beta-actin were determined by immunoblotting analysis (n = 3). Each bar represents the mean ± SEM. *p < 0.05 compared with db/+ mice. #p < 0.05 compared with db/db mice.
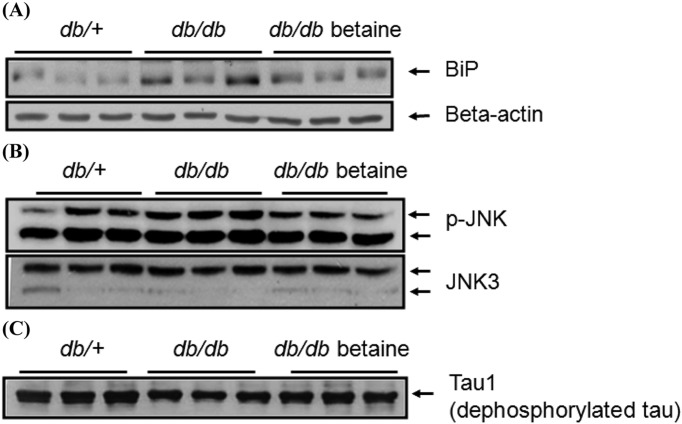
Effect of betaine on tau hyperphosphorylation in the brains of db/db mice. ER stress is involved in neuronal toxicity, as well as in insulin resistance in the liver and brain. We observed an alleviation of ER stress by betaine in the cortex and hippocampus based on the reduced expression of immunoglobin heavy chain-binding protein (BiP) (Fig. 4A). JNK is activated in response to ER stress in the brain. The activity of JNK, a serine/threonine kinase involved in tau hyperphosphorylation, was also measured by immunoblotting using a specific antibody directed against p-JNK. Consistent with the ER stress indicator BiP, we observed the activation of JNK in the cortices and hippocampi of db/db mice and the reduction of JNK activity by betaine (Fig. 4B). We also determined the phosphorylation status of tau with immunoblotting. Protein levels of dephosphorylated tau were significantly increased in the db/db betaine group compared to those in the db/db group, suggesting that betaine alleviated tau hyperphosphorylation in the cortices and hippocampi (Fig. 4C).
Fig. 4. (A) Effect of betaine supplementation on ER stress marker in the brains of db/db mice. Protein levels of BiP and beta-actin were determined by immunoblotting analysis. (B) Effect of betaine supplementation on JNK activation in the brains of db/db mice. The protein levels of p-JNK and total JNK3 were determined by immunoblotting analysis. (C) Effect of betaine supplementation on tau hyperphosphorylation in the brains of db/db mice. The protein levels of dephosphorylated tau were determined by immunoblotting analysis.
DISCUSSION
In this study, the administration of betaine averted ER and oxidative stress responses and attenuated the pathological features of the diabetic livers and brains in db/db mice. We observed a significant increase in fatty acid oxidation in betaine group, as determined by PPARα and CPT1a mRNA levels, and phosphorylated ACC protein level, indicating a possible mechanism of betaine action in the alleviation of hyperlipidemia. This observation is consistent with previous reports that have shown that activation of AMP-activated protein kinase, which phosphorylates and inhibits ACC, can alleviate hepatic steatosis in animal models (16,17). Impaired mitochondrial β-oxidation of fatty acids, but the induction the tricarboxylic acid cycle and pyruvate carboxylase have been shown to contribute to elevated gluconeogenesis in diabetic ZDF rats (25). Furthermore, betaine supplementation dramatically increased SCD1 mRNA levels, suggesting that increased accumulation of monounsaturated fatty acids may responsible for alleviation of insulin resistance in db/db mice fed a betaine-supplemented diet. Previous studies have reported that fatty acid composition, rather than fatty acid oversupply, is related to insulin sensitivity (26,27). In this study, we observed significantly reduced serum insulin levels and HOMA-IR after betaine supplementation. Betaine supplementation also reduced the expression of PGC-1α mRNA, which correlates well with hepatic gluconeogenesis (28). A recent study reported that betaine reduced fasting glucose and insulin and reversed hepatic insulin resistance in mice with nonalcoholic fatty livers (16).
Betaine reduced ER and oxidative stress in the livers of db/db mice. Consistently, betaine supplementation has a protective effect against oxidative stress and steatosis in the livers of rats fed a high-fat diet (14), suggesting a role for betaine in increased glutathione synthesis by supplying its substrates, cysteine and glycine. As an osmolyte, betaine increases the water retention of cells and acts like a chaperone to stabilize protein structures against environmental stress (29,30). We also observed that ER stress in the cortices and hippocampi of db/db mice was alleviated by betaine supplementation. db/db mice have been shown to be more sensitive to seizure-induced hippocampal damage than wildtype, suggesting a role for leptin receptors in cell survival signaling (31). Furthermore, betaine supplementation alleviated JNK activation and tau hyperphosphorylation in db/db mice. Previous studies reported that increased tau hyperphosphorylation in the brains of db/db mice (32) and streptozotocin- mediated diabetic mice (33).
In conclusion, betaine supplementation improved serum insulin and triglyceride levels of db/db mice. We also observed the alleviation of tau hyperphosphorylation in the brains of db/db mice fed a betaine-supplemented diet. The ER and oxidative stress evident in db/db mice were alleviated by betaine, which may have contributed to the beneficial effects of betaine. Therefore, betaine may have potential application in the treatment of type 2 diabetes.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (#2009-0069120).
References
- 1.Kumashiro N., Tamura Y., Uchida T., Ogihara T., Fujitani Y., Hirose T., Mochizuki H., Kawamori R., Watada H. Impact of oxidative stress and peroxisome proliferatoractivated receptor gamma coactivator-1alpha in hepatic insulin resistance. Diabetes. (2008);57:2083–2091. doi: 10.2337/db08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozcan U., Cao Q., Yilmaz E., Lee A.H., Iwakoshi N.N., Ozdelen E., Tuncman G., Görgün C., Glimcher L.H., Hotamisligil G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Sci. (2004);306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 3.Nakatani Y., Kaneto H., Kawamori D., Yoshiuchi K., Hatazaki M., Matsuoka T.A., Ozawa K., Ogawa S., Hori M., Yamasaki Y., Matsuhisa M. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J. Biol. Chem. (2005);280:847–851. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- 4.Han M.S., Chung K.W., Cheon H.G., Rhee S.D., Yoon C.H., Lee M.K., Kim K.W., Lee M.S. Imatinib mesylate reduces endoplasmic reticulum stress and induces remission of diabetes in db/db mice. Diabetes. (2009);58:329–336. doi: 10.2337/db08-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han K.L., Choi J.S., Lee J.Y., Song J., Joe M.K., Jung M.H., Hwang J.K. Therapeutic potential of peroxisome proliferators--activated receptor-alpha/gamma dual agonist with alleviation of endoplasmic reticulum stress for the treatment of diabetes. Diabetes. (2008);57:737–745. doi: 10.2337/db07-0972. [DOI] [PubMed] [Google Scholar]
- 6.Profenno L.A., Porsteinsson A.P., Faraone S.V. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol. Psychiatry. (2010);67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Beydoun M.A., Beydoun H.A., Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes. Rev. (2008);9:204–218. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao W.Q., Chen H., Quon M.J., Alkon D.L. Insulin and the insulin receptor in experimental models of learning and memory. Eur. J. Pharmacol. (2004);490:71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 9.Planel E., Tatebayashi Y., Miyasaka T., Liu L., Wang L., Herman M., Yu W.H., Luchsinger J.A., Wadzinski B., Duff K.E., Takashima A. Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms. J. Neurosci. (2007);27:13635–13648. doi: 10.1523/JNEUROSCI.3949-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueland P.M. Choline and betaine in health and disease. J. Inherited Metab. Dis. (2011);34:3–15. doi: 10.1007/s10545-010-9088-4. [DOI] [PubMed] [Google Scholar]
- 11.Ji C., Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. (2003);124:1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 12.Ji C., Shinohara M., Kuhlenkamp J., Chan C., Kaplowitz N. Mechanisms of protection by the betainehomocysteine methyltransferase/betaine system in HepG2 cells and primary mouse hepatocytes. Hepatology. (2007);46:1586–1596. doi: 10.1002/hep.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erman F., Balkan J., Cevikba U., Koçak-Toker N., Uysal M. Betaine or taurine administration prevents fibrosis and lipid peroxidation induced by rat liver by ethanol plus carbon tetrachloride intoxication. Amino Acids. (2004);27:199–205. doi: 10.1007/s00726-004-0105-5. [DOI] [PubMed] [Google Scholar]
- 14.Kwon do Y., Jung Y.S., Kim S.J., Park H.K., Park J.H., Kim Y.C. Impaired sulfur-amino acid metabolism and oxidative stress in nonalcoholic fatty liver are alleviated by betaine supplementation in rats. J. Nutr. (2009);139:63–68. doi: 10.3945/jn.108.094771. [DOI] [PubMed] [Google Scholar]
- 15.Lakshman R., Cederbaum A.I., Hoek J.B., Konishi M., Koop D., Donohu T.M. Use of CYP2E1-transfected human liver cell lines in elucidating the actions of ethanol. Alcohol. Clin. Exp. Res. (2005);29:1726–1734. doi: 10.1097/01.alc.0000179379.03078.8f. [DOI] [PubMed] [Google Scholar]
- 16.Kathirvel E., Morgan K., Nandgiri G., Sandoval B.C., Caudill M.A., Bottiglieri T., French S.W., Morgan T.R. Betaine improves nonalcoholic fatty liver and associated hepatic insulin resistance: a potential mechanism for hepatoprotection by betaine. Am. J. Physiol. Gastrointest. Liver Physiol. (2010);299:G1068–G1077. doi: 10.1152/ajpgi.00249.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song Z., Deaciuc I., Zhou Z., Song M., Chen T., Hill D., McClain C.J. Involvement of AMP-activated protein kinase in beneficial effects of betaine on high-sucrose diet-induced hepatic steatosis. Am. J. Physiol. Gastrointest. Liver Physiol. (2007);293:G894–G902. doi: 10.1152/ajpgi.00133.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. (1957);226:497–509. [PubMed] [Google Scholar]
- 19.Aebi H. Catalase in vitro. Methods Enzymol. (1984);105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 20.Griffith O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyri-dine. Anal. Biochem. (1980);106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 21.Ohkawa H., Ohish N., Yagi K. Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal. Biochem. (1979);95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 22.Zhang P., McGrath B., Li S., Frank A., Zambito F., Reinert J., Gannon M., Ma K., McNaughton K., Cavener D.R. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol. Cell. Biol. (2002);22:3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang H., Ward W.F. PGC-1alpha: a key regulator of energy metabolism. Adv. Physiol. Educ. (2006);30:145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- 24.Duval C., Müller M., Kersten S. PPARalpha and dyslipidemia. Biochim. Biophys. Acta. (2007);1771:961–971. doi: 10.1016/j.bbalip.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Satapati S., He T., Inagaki T., Potthoff M., Merritt M.E., Esser V., Mangelsdorf D.J., Kliewer S.A., Browning J.D., Burgess S.C. Partial resistance to peroxisome proliferator- activated receptor-α agonists in ZDF rats is associated with defective hepatic mitochondrial metabolism. Diabetes. (2008);57:2012–2021. doi: 10.2337/db08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benhamed F., Denechaud P.D., Lemoine M., Robichon C., Moldes M., Bertrand-Michel J., Ratziu V., Serfaty L., Housset C., Capeau J., Girard J., Guillou H., Postic C. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J. Clin. Invest. (2012);122:2176–2194. doi: 10.1172/JCI41636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J.Y., Cho H.K., Kwon Y.H. Palmitate induces insulin resistance without significant intracellular triglyceride accumulation in HepG2 cells. Metab. (2010);59:927–934. doi: 10.1016/j.metabol.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Yoon J.C., Puigserver P., Chen G., Donovan J., Wu Z., Rhee J., Adelmant G., Stafford J., Kahn C.R., Granner D.K., Newgard C.B., Spiegelman B.M. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nat. (2001);413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 29.Craig S.A. Betaine in human nutrition. Am. J. Clin. Nutr. (2004);80:539–549. doi: 10.1093/ajcn/80.3.539. [DOI] [PubMed] [Google Scholar]
- 30.Natalello A., Liu J., Ami D., Doglia S.M., de Marco A. The osmolyte betaine promotes protein misfolding and disruption of protein aggregates. Proteins. (2009);75:509–517. doi: 10.1002/prot.22266. [DOI] [PubMed] [Google Scholar]
- 31.Guo Z., Jiang H., Xu X., Duan W., Mattson M.P. Leptin-mediated cell survival signaling in hippocampal neurons mediated by JAK STAT3 and mitochondrial stabilization. J. Biol. Chem. (2008);283:1754–1763. doi: 10.1074/jbc.M703753200. [DOI] [PubMed] [Google Scholar]
- 32.Kim B., Backus C., Oh S., Hayes J.M., Feldman E.L. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinol. (2009);150:5294–5301. doi: 10.1210/en.2009-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clodfelder-Miller B.J., Zmijewska A.A., Johnson G.V., Jope R.S. Tau is hyperphosphorylated at multiple sites in mouse brain in vivo after streptozotocin-induced insulin deficiency. Diabetes. (2006);55:3320–3325. doi: 10.2337/db06-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]