Abstract
Zearalenone (ZEN) is a non-steroidal estrogenic mycotoxin produced by several species of Fusarium that are found in cereals and agricultural products. ZEN has been implicated in mycotoxicosis in farm animals and in humans. The toxic effects of ZEN are well known, but the ability of an alkaline Comet assay to assess ZEN-induced oxidative DNA damage in Chang liver cells has not been established. The first aim of this study was to evaluate the Comet assay for the determination of cytotoxicity and extent of DNA damage induced by ZEN toxin, and the second aim was to investigate the ability of N-acetylcysteine amide (NACA) to protect cells from ZEN-induced toxicity. In the Comet assay, DNA damage was assessed by quantifying the tail extent moment (TEM; arbitrary unit) and tail length (TL; arbitrary unit), which are used as indicators of DNA strand breaks in SCGE. The cytotoxic effects of ZEN in Chang liver cells were mediated by inhibition of cell proliferation and induction of oxidative DNA damage. Increasing the concentration of ZEN increased the extent of DNA damage. The extent of DNA migration, and percentage of cells with tails were significantly increased in a concentration-dependent manner following treatment with ZEN toxin (p < 0.05). Treatment with a low concentration of ZEN toxin (25 μM) induced a relatively low level of DNA damage, compared to treatment of cells with a high concentration of ZEN toxin (250 μM). Oxidative DNA damage appeared to be a key determinant of ZEN-induced toxicity in Chang liver cells. Significant reductions in cytolethality and oxidative DNA damage were observed when cells were pretreated with NACA prior to exposure to any concentration of ZEN. Our data suggest that ZEN induces DNA damage in Chang liver cells, and that the antioxidant activity of NACA may contribute to the reduction of ZEN-induced DNA damage and cytotoxicity via elimination of oxidative stress.
Keywords: Zearalenone, An alkaline single cell gel electrophoresis (SCGE) Comet assay, DNA damaging, Cytotoxicity, N-Acetylcysteine amide (NACA), Chang liver cell
INTRODUCTION
Zearalenone (ZEN) is a fusarotoxin produced mainly by Furarium graninearum and Fusarium culmorum, which is frequently found in maize and small grains such as barley, wheat, sorghum, millet and rice, as well as in soybeans (1). ZEN is one of the most widely distributed fusarial mycotoxins which are encountered at a high incidence in many important crops intended for human and animal consumptions (2,3). While much attention has been given to the study of aflatoxins and ochratoxins produced by Aspergillus and Penicillium, but much less concern has been paid to fusarial toxins. It is known that ZEN is of relatively low toxicity (4). ZEN is mainly metabolized in the liver (5), which seems to be a main target. Thus, ZEN was found to be hepatotoxic and to induce liver lesions (6-8). Recently, several studies have been conducted and have shown that ZEN is cytotoxic; it inhibits cell proliferation and macromolecules synthesis in different cell lines (9,10) and exhibits a geneotoxic potential in vitro and in vivo through induction of micronuclei, chromosome aberrations, DNA fragmentation, cell cycle arrest, etc. (9,11-16).
A number of techniques for detecting DNA damage have been used to identify substance with genotoxic activity. Until recently, the most frequently used methods involve either the detection of DAN repair synthesis (so-called unscheduled DAN synthesis or UDS) in individual cells, or the detection of DNA SSB and ALS in pooled cell populations using the alkaline elution assay. The UDS technique is based on the replication of DNA during the excision repair of certain type of DNA lesions, as demonstrated by the incorporation of titrated thymidine into the DNA repair sites. While providing information at the level of the individual cell, the technique is technically cumbersome, requires the use of radioactivity, and is limited in sensitivity. The alkaline elution assay ignores the critical importance of intercellular differences in DNA damage and requires relatively large numbers of cells. A more useful approach for assessing DNA damage is the single-cell gel (SCG) or Comet assay. The terms “SCG” or Comet” are used interchangeable; the term “Comet” is used to identify the individual cell DNA migration patterns produced by this assay (17). It was first developed by Östling & Johansson in 1984 and later modified by Singh et al. in 1988. It has since increased in popularity as a standard technique for evaluating DNA damage/repair, biomonitoring and genotoxicity testing. It involves the encapsulation of cells in a low-melting- point agarose suspension, lysis of the cells in neutral or alkaline (pH > 13) conditions, and electrophoresis of the suspended cell lysates. In this technique, cells embedded in agarose were placed on a microscope-slide, the cells were lysed by detergents and high salt, and the liberated DNA was electrophoresed under neutral conditions. Cells with increased frequently of DNA double-strand breaks (DSB) displayed increased migration of DNA toward the anode. The DNA migration was quantified by staining with DAPI or ethidium bromide and by measuring the intensity of fluorescence at two fixed positions within the migration pattern using a microscope photometer. The neutral conditions greatly limited the general utility of the assay. Subsequently, Singh et al. (18) introduced a microgel technique involving electrophoresis under alkaline (pH > 13) conditions for detecting DNA damage in single cells. At this pH, increased DNA migration is associated with incomplete excision repair sites, and ALS. The alkaline Comet assay is also widely accepted xeno-biotics-testing method for novel pharmaceuticals or other chemicals, and is a simple and sensitive procedure for detecting DNA strand breaks that requires a small numbers of cells and a short time to complete the study (19-21). Compared with other toxicity assays, the advantage of this technique include: (1) its demonstrated sensitivity for detecting low levels of DNA damage; (2) the requirement for small numbers of cells per sample; (3) flexibility; (4) low costs; (5) ease of application; (6) the ability to conduct studies using relatively small amounts of a test substance; and (7) the relatively short time period needed to complete an experiment. Both methods to assess DNA damage induced in vitro or in vivo were readily adapted to virtually any cell population feasible of being obtained as a single cell suspension, including lymphocytes isolated either from the spleen or peripheral blood (19, 22-24). These test methods also have been used in screening for cancer-inducing compounds in the environment (24).
The thiol group plays an important role in biological system. Thiol oxidation can result in protein structure alteration leading to protein dysfunction. The thiol group appearing in a variety of proteins or non-proteins, e.g. glutathione (GSH), undergoes reversible thiol-disulfide interactions to mediate the oxidant-induce stress (25). The use of biothiols, such as GSH, N-acetyl-cysteine (NAC), homocysteine, cysteine (CYS), and γ-glutamyl cysteine, to mitigate acute oxidative stress induced by anticancer drugs has long been proposed, though their efficacies have not been fully evaluated. NAC did not provide significant antioxidant effects, presumably due to its low lipid solubility that limits its bioavailability (26). The carboxyl group in NAC is negatively charged at physiological pH, limiting its ability to cross cell membranes. Recently, N-acetylcysteine amide (NACA), a structural analogue of NAC, was synthesized and evaluated in a certain in vitro and in vivo models. Replacing the carboxyl group with an amide increase lipophilicity, allowing it to cross cell membranes. Two studies have shown that NACA could cross the blood-brain barrier, chelate Cu2+ (which catalyzes free radical formation), scavenge free-radicals, protect red blood cells from oxidative stress, and prevent ROSinduced activation of c-Jun N-terminal protein kinase (JNK), mitogen-activated protein kinase MAPK (p38), and matrix metalloproteinases (27-29). The ability of NACA to protect Chang liver cells from ZEN-induced toxicity (DNA damage) has not been investigated. To our knowledge, there have been no comprehensive studies of the preventive effects of NACA on DNA damage by ZEN-induced cytotoxicity in Chang liver cells. Therefore, we hypothesized that NACA would protect Chang liver cells by reducing cytotoxicity and DNA damage by ZEN toxicity. Accordingly, we determined the ability of NACA to mitigate the cytotoxicity of ZEN in Chang liver cells and correlated these effects with the attenuation of ZEN-induced DNA damage. The present study focuses on the preventive effects of NACA on induction of DNA damage and cytotoxicity by ZEN-induced toxicity.
MATERIALS AND METHODS
Chemicals and regents. ZEN was obtained from Sigma Chemical Co. (St Louis, MO, USA). RPMI-1640 medium, fetal calf serum (FCS), phosphate buffer saline (PBS), trypsine- EDTA, penicillin and streptomycin mixture, 3-4,5-dimethylthiazol- 2-yl, 2,5-diphenyl-tetrazolium bromide (MTT), were obtained from Sigma-Aldrich, USA. All chemicals used here were of analytical grade.
Cell culture and treatment. The Chang liver cell line (CCL 13) was obtained from the American Type Culture Collection (ATCC). The cells were cultured at 37℃ in a 5% CO2-95% air humidified atmosphere. The culture medium was Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma) supplemented with 10% fetal calf serum, penicillin (100 IU/ml), streptomycin (100 mg/L) and nystatin (8.2 mg/L). For testing, the cells were seeded on 96-well microtiter plates, 5000 or 10,000 cells in 100 μl per well (MTT-test).
Determination of cell viability. Cytotoxicity of ZEN was determined using the colorimetric method described by Carmichael et al. (30). This method assesses the ability of viable cells to form 3-4, 5-dimethylthiazol-2-yl, 2,5-diphenyl- tetrazolium bromide (MTT) formazan by the mitochondrial enzyme succinate dehydrogenase.
Cells were seeded on 96 well culture plates (Poly-labo, USA) at 105 cells/well and treated with increasing concentrations of ZEN, 1~200 μM for 24 at 37oC. At the end of the reaction time, the culture medium was replaced with 200 μl medium containing 0.5 mg/ml MTT and the plates were incubated for 3 h at 37℃. The medium was then removed and replaced with 100 μl of DMSO to dissolve the converted purple dye in culture plates. The absorbance was measured on a spectrophotometer microplater reader (Dynatech 4000) at a wavelength of 560 nm. Cell viability was expressed as the relative formazan formation in treated samples as compared to control cells [(A560 treated cells/ A560 control cells) 100%]. IC50 values defined as the concentration including 50% loss of cell viability, was estimated from the figure.
Single cell gel electrophoresis (SCGE, the Comet assay). DNA breaks were detected using in adaptation of the method of Singh et al. (18). Briefly, Chang liver cells (2 × 105 cells/well) were cultured in 6-well multi-dishes (Polylabo, USA) and were treated with vehicle alone or various concentrations of ZEN ranging from 25 to 200 μM for 24 hr. These concentrations were chosen from the range of non-cytotoxic concentrations as assessed by the MTT assay. A positive control was included where Chang live cells were treated with 20 μM H2O2 for 5 min on ice. The adherent cells were then trypsinized, centrifuged, counted and mixed with 1% (w/v) low-melting-point agarose in PBS, pH 7.4 at 37℃ and immediately pipetted onto a frosted glass microscope slide pre-coated with a layer of 1% (w/v) normal melting point agarose similarly prepared in PBS, The agarose was allowed to set at 4℃ for 5~10 min and the slide immersed in lysis solution (2.5 M NaCl, 100 mM Na2- EDTA, 10 mM Tris, NaOH to pH 10.0 and 1% (v/v) Triton X-100) at 4℃ for 1 hr to remove cellular proteins and membranes. They were then washed three times with enzyme buffer (0.1 M KCl, 0.5 mM Na-EDTA, 40 mM Hepes-KOH, 0.2 mg/ml BSA, pH 8.0) and incubated for 45 min glycosylase (FPG), or with buffer alone. Slides were then placed in horizontal electrophoresis tank containing 0.3 M NaOH and 1 mM Na2-EDTA for 40 min before electrophoresis at 25 V for 30 min at an ambient temperature of 4℃. The slides were then washed three times for 5 min each with 0.4 M Tris-HCl, pH 7.5 before staining with 20 μl ethidium bromide (20 μg/ml). The experiment was performed three times separately.
Quantification of the Comet assay. The stained slides were viewed under fluorescent microscope (Olympus Optical, Tokyo, Japan) at × 250 magnification with a green excitation filter. The images were transferred through a CCD video camera to a computer and analyzed by software, Kinetic imaging Komet Assay 5.5 image analysis system (Kinetic Imaging, Livepool, UK) to evaluate the degree of DNA migration. The tail length and the tail moment were used as the measures of the extent of DNA damage. Two hundred cells were randomly selected and measured (two slides made for one sample, 100 randomly-selected cells per slide) from one sample. Statistical calculations were performed by using the Excel 5.0 program (Microsoft, Redmond, USA), that produced the values of the mean tail length for sample and the values of percentage cells in five ranges of tail length, i.e., as undamaged cells without tail, cells with tiny tail, cells with a dim tail, cells with a clear tail, and only tail. The ranges of tail length and moment were divided arbitrarily in this study.
Evaluation of DNA.
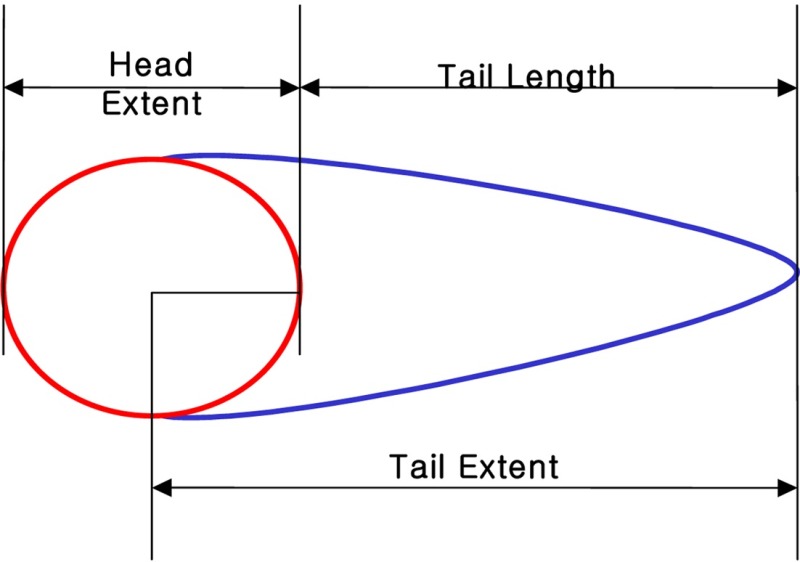
TEM : TL X TD/100
TL: Tail extent (Tail from center ) + Head extent /2
TD : (Head optical intensity/Head optical intensity + Tail optical intensity) × 100
Thiobarbituric acid reactive substance (TBARS) and glutathione (GSH) content analysis. The TBARS and GSH levels were determined according to the corresponding assay kit protocol, (BioVision-Milpitas, CA, USA).
Statistical analysis. Each experiment was done three times separately. Values are presented as means ± S.D. Statistical differences between control and treated groups for the expression of Hsp70 or 90 were determined by χ2-test. Difference were considered significant at p < 0.05.
RESULTS
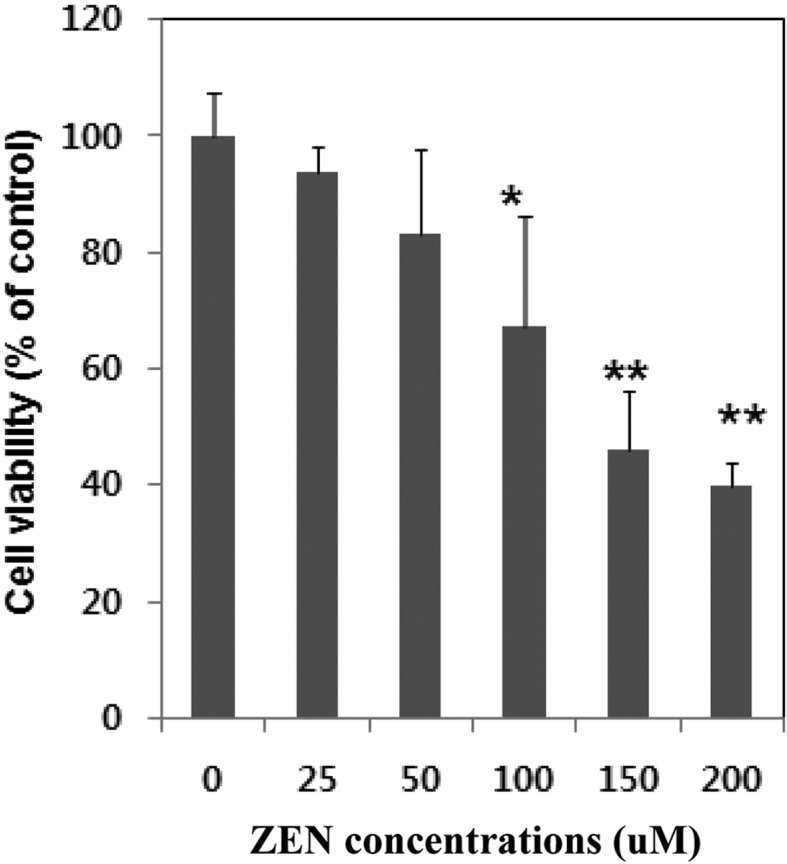
Inhibition of cell proliferation of ZEN on Chang liver cells. The cytotoxic effect of ZEN on Chang liver cells after 24 hr incubation as measured by MTT assay is shown in Fig. 1. ZEN caused a marked decrease of cell viability in a dose-dependent manner. After 24 hr exposure to concentrations ranging from 0 μM to 200 μM, the number of viable cells falls to 54% and to 60.3% at 150 μM and 200 μM, respectively. The inhibition of cell proliferation was most pronounced at 200 μM concentration suggesting the dose dependency of the inhibition of cell proliferation. The IC50 values were approximately 150 μM after 24 hr of treatment.
Fig. 1. Cytotoxic effects of ZEN on Chang liver cells. Cells were treated with ZEN at the indicated concentrations for 24 h. Cell viability was determined using MTT assay and expressed as percentages of control, which was exposed to vehicle only. Control value was taken as 100%. Data are expressed as mean ± S.D of three independent experiment (n = 3). *Significantly different at p < 0.05, ** p<0.01.
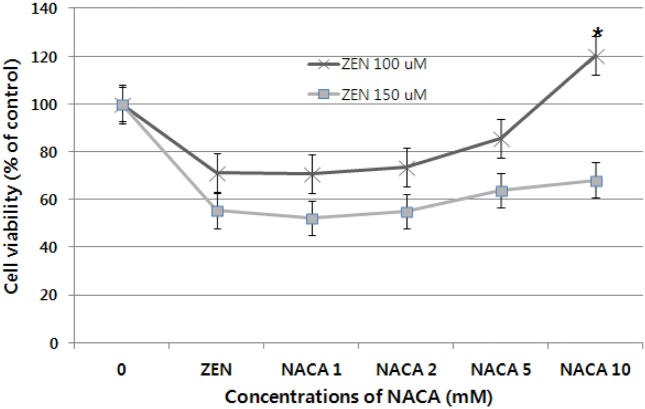
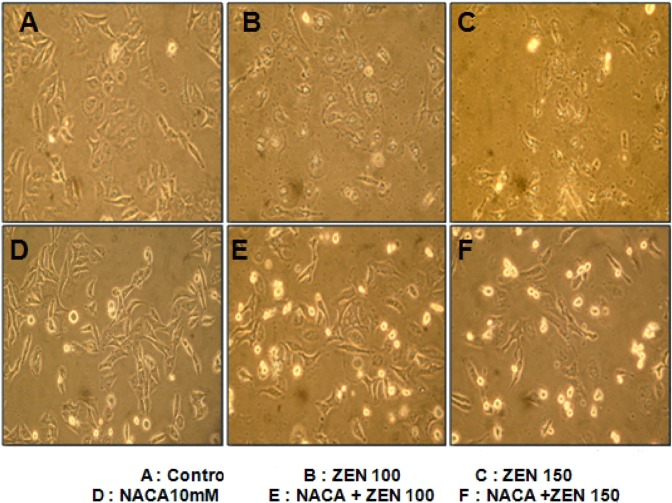
Cytoprotection by NACA. The cytoprotective effect of NACA was determined after exposure of cells pre-treated 2 hr by the NACA to 100 and 150 μM ZEN. As shown in Fig. 2, 3, and 4, NACA efficiently increases cell viability in a dose-dependent manner. But no significant increase in cell viability was observed under concentrations of 5 mM NACA in the presence of 100 and 150 μM ZEN. 10 mM of NACA had maximal protective effect (43.3%) on cytotoxicity compared to 150 μM concentration of ZEN (p < 0.01, n = 4) and over a period of 24 hr exposure. Cell viability at all concentration showed significant (p < 0.01, n = 4) decrease about 32~48% from 1~10 mM of NACA compare to control group, respectively, presence of 150 μM ZEN. From above result obtained, 10 mM is optimal concentration for cytoprotective effect of NACA against ZEN 100 and 150 μM cytolethality on Chang liver cell. To check cells viability, cells pretreated with 10 mM NACA for 2 hr prior ZEN treatment at the designated concentrations for 24 hr at 37℃. The effect of NACA on cell viability and morphology were determined by the MTT assay and convert microscopy, respectively. Significant increase of cell viability (about 200%) was observed in 10 mM NACA alone treatment. Increase of ZEN concentrations from 25 to 100 μM for 24 hr, cell viability was increased, but even at the high dose of 150 μM, decrease of cell viability was observed over 24 hr of incubation (Fig. 3). In morphological examination, treatment groups of NACA 10 mM in ZEN 100 and 150 μM (E and F) showed proliferation of cell viability and clearly exhibited marked cell membranes compared with ZEN alone treat groups (B and C). The NACA alone treat group (D) showed high cell viability and clear cell membrane than control (A) and ZEN alone treat groups (B, C) (Fig. 4).
Fig. 2. Cytoprotective effect of NACA against ZEN 100 and 150 μM cytolethality on Chang liver cell. Cell viability was determined using the MTT assay in cells that were pre-treated for 2 hr by NACA or not before ZEN treatment. Data are expressed as the mean ± S.D. *Significantly different at p< 0.01.
Fig. 3. Cytoprotective effect of NACA against ZEN at 25, 50, 100 and 150 μM on Chang liver cells. Cells were pre-treated for 2 hr by NACA (10mM). Values are mean ± SD from six independent experiments. Significance indicated by * p<0.05, ** p < 0.01. treat vs control group.
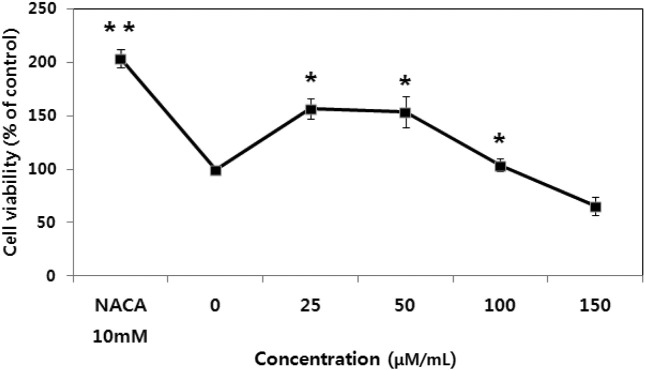
Fig. 4. Microscopic study of cellular morphology of Chang liver cell viability. The cells were treated in the absence (A) or presence of ZEN 100 μM (B), ZEN 150 μM (C), NACA alone (10mM) (D), ZEN 100 μM plus NACA 10 mM (E), ZEN 150 μM plus NACA 10mM (F) and cultured for 48 hr.
DNA damage assay and its preventive effect of NACA against ZEN-induced oxidative stress. DNA damage was analyzed by the Comet assay, a sensitivity method for detecting DNA strand breaks in individual cells and a versatile tool that is highly efficacious in human bio-monitoring of nature or environmental compounds (18). The high sensitivity of the Comet assay, and the provided ability to measure DNA damage in individual cells, has destined it to become a tool for rapidly predicting xenobiotics toxicity of compounds of interest. For the evaluation of preventive effect of NACA, migration of Head DNA, Tail DNA, tail moment, and tail length levels as marked of DNA damage in the ZEN treated cell samples were compared with control and NACA treat groups by counting 100 Comet images (cells) per sample. The ranges of tail length, tail moment, head DNA, and tail DNA was divided arbitrarily in this study.
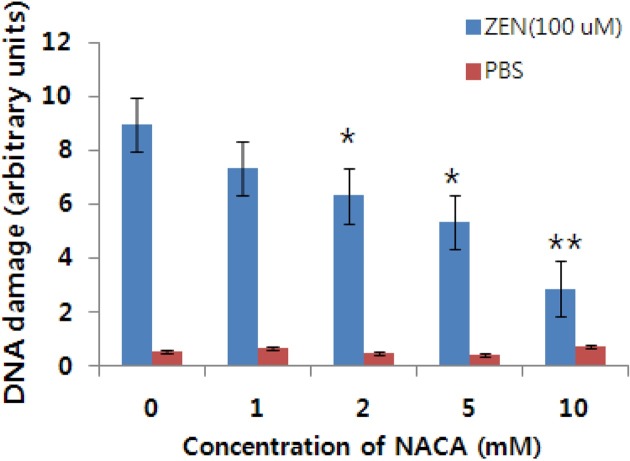
The ZEN treated groups produced concentration-dependent increases in DNA damage in Chang liver cells. DNA damage caused by ZEN increased markedly compared to control and NACA addition groups, as evident from an increase in the tail length and moment. A similar pattern was observed for head DNA and tail DNA (arbitrary units Fig. 4, Table 1 and Table 2). However, no significant DNA damage was observed in the Chang liver cell treated with the lowest dose of ZEN, i.e., 25 μM which was evident from the tail length and moment values (arbitrary units Table 1 and Table 2). The trends of DNA damage in five concentrations were different from each other and were decreased to nearly normal levels by adding NACA (Fig. 4 and Fig. 5, Table 1 and Table 2). Comet tail length, tail moment, head DNA and tail DNA mean the DNA damage levels for each DNA (cell). The mean tail length of the Comet reflected approximately the increasing patterns of DNA damage. A significantly (p < 0.01) increase in tail length was produced in the concentration range of 50~200 μM ZEN. Tail length and moment in ZEN at 150~200 μM exhibited statistically significance (p < 0.01) (Table 1 and Table 2).
Table 1.
ZEN-induced DNA damage and the preventive effect of NACA in Chang liver cells (Means ± SD)
| Compounds | Scored cells | Tail moment (TM) | Tail length (TL) | |
|---|---|---|---|---|
| Control | 100 | 0.32 ± 0.14a | 6.52 ± 2.48a | |
| H2O2 (20 μM/ml) | 100 | 18.24 ± 3.21d | 48.17 ± 5.23d | |
| ZEN | 25 μM | 100 | 1.87 ± 0.74a | 7.51 ± 3.47a |
| 50 μM | 100 | 7.43 ± 0.35b | 12.72 ± 3.61b | |
| 100 μM | 100 | 14.51 ± 2.15c | 27.11 ± 4.14c | |
| 150 μM | 100 | 17.36 ± 3.64d | 41.54 ± 4.28d | |
| 200 μM | 100 | 19.01 ± 4.21d | 50.25 ± 5.41d | |
| ZEN (25 μM) + NACA (10 mM) | 100 | 1.57 ± 0.11a | 6.72 ± 1.24a | |
| ZEN (50 μM) + NACA (10 mM) | 100 | 4.62 ± 0.25b | 8.21 ± 2.65b | |
| ZEN (100 μM) + NACA (10 mM) | 100 | 7.52 ± 2.41b | 13.82 ± 3.71c | |
| ZEN (150 μM) + NACA (10 mM) | 100 | 8.38 ± 3.78b | 17.24 ± 3.61c | |
| ZEN (200 μM) + NACA (10 mM) | 100 | 10.03 ± 3.06c | 26.34 ± 4.01d | |
abcd Values with different superscript within the same column are significantly different (p<0.01).
Table 2.
Effects of ZEN and prevention of NACA on the percentage of head DNA and tail DNA in Chang liver cells
| Compounds | Scored cells | Head DNA (%) | Tail DNA (%) | |
|---|---|---|---|---|
| Control | 100 | 87.76 ± 3.26a | 12.24 ± 1.15a | |
| H2O2 (20 μM/ml) | 100 | 34.85 ± 2.43d | 65.15 ± 4.32d | |
| ZEN | 25 μM | 100 | 80.57 ± 4.28a | 19.43 ± 2.21a |
| 50 μM | 100 | 61.64 ± 4.26b | 38.36 ± 3.47b | |
| 100 μM | 100 | 49.76 ± 2.36c | 50.24 ± 3.46c | |
| 150 μM | 100 | 37.57 ± 3.25d | 62.43 ± 3.14d | |
| 200 μM | 100 | 30.66 ± 2.58d | 69.34 ± 3.27d | |
| ZEN (25 μM) + NACA (10 mM) | 100 | 83.79 ± 4.02a | 16.21 ± 1.83a | |
| ZEN (50 μM) + NACA (10 mM) | 100 | 70.59 ± 3.51b | 29.41 ± 2.1b | |
| ZEN (100 μM) + NACA (10 mM) | 100 | 59.76 ± 4.24b | 40.24 ± 2.45b | |
| ZEN (150 μM) + NACA (10 mM) | 100 | 49.69 ± 3.23c | 50.31 ± 3.27cd | |
| ZEN (200 μM) + NACA (10 mM) | 100 | 41.79 ± 2.86d | 58.21 ± 4.28d | |
abcd Values with different superscript within the same column are significantly different (p<0.01).
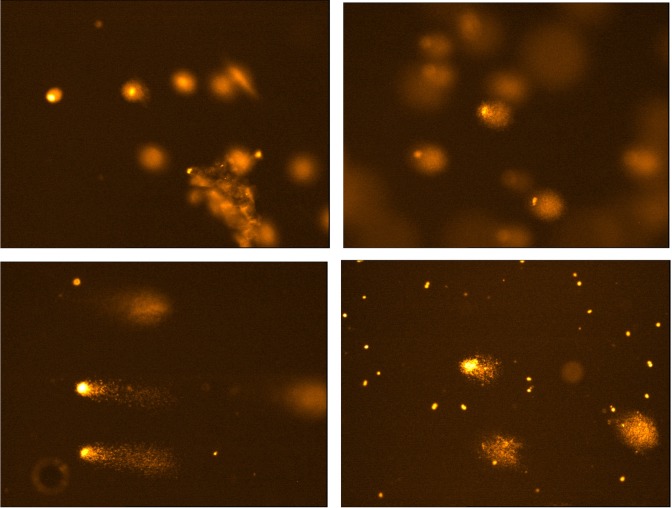
Fig. 5. Representative images of ZEN toxin induced oxidative DNA damage in Chang liver cells. Typical Comet are shown in group non-treated (upper left) and positive control (upper right) and treated with 150 μM ZEN (bottom left) and treated with ZEN 150 μM plus NACA 10mM (bottom right).
Analysis of TBARS and GSH levels. The level of TBARS is a marker of lipid peroxidation, and GSH is the most relevant antioxidant agent in the cell. The TBARS levels were significantly increased by ZEN treatment when compared to the control, but this effect was decreased with 10 mM NACA treatment. ZEN treatment at concentrations ranging from 25 to 200 μM significantly decreased GSH levels when compared to the control. Treatment with 200 μM ZEN decreased GSH levels to approximately 71.1% of the control level (Table 3).
Table 3.
The effects of ZEN treatment alone or in combination with NACA on TBARS and GSH levels in Chang liver cells
| Treatment | TBARS (nM/mg protein) | GSH (μM) | |
|---|---|---|---|
| Control | 1.25 ± 0.12a | 41.19 ± 1.14b | |
| ZEN | 25 μM | 3.15 ± 0.46b | 36.54 ± 0.19a |
| 50 μM | 4.08 ± 0.45b | 21.48 ± 2.24c | |
| 100 μM | 5.21±0.52c | 15.37 ± 0.36c | |
| 150 μM | 5.62±0.62c | 13.64 ± 0.28c | |
| 200 μM | 7.37 ± 0.24d | 11.89 ± 1.75cd | |
| NACA 10mM | 1.37 ± 0.29a | 45.24 ± 1.34a | |
| ZEN + NACA | 25 μM+ 10mM | 2.03 ± 0.34b | 40.16 ± 1.36b |
| 50 μM+ 10mM | 2.18 ± 0.16b | 36.49 ± 0.37b | |
| 100 μM+ 10mM | 2.09 ± 0.62b | 38.32 ± 0.21c | |
| 150 μM+ 10mM | 4.26 ± 0.36c | 32.58 ± 1.32d | |
| 200 μM+ 10mM | 5.18 ± 0.47d | 30.75 ± 1.54d | |
abcd Mean ± SD with different superscripts in the same row are significantly different (p < 0.01).
Fig. 6. Inhibitory effect of NACA on DNA damage induced by ZEN in Chang liver cells. *; p< 0.05 vs control, **; p<0.01 vs control.
DISCUSSION
Zearalenone (ZEN) is a fusariotoxin occurring worldwide in cereals, animal food and forages (31) that adversely affects reproduction (32,33). Thus, ZEN was first known to be a potent disrupter of the reproductive system. It is also associated with several human diseases of unknown etiology (32). In humans, several observations have associated ZEN to precocious pubertal changes (34) and to endometrial adenocarcinoma and hyperplasia (35), hepatocarcinoma (6,8). The Chang liver cell line which derivate from normal human liver cell line was chosen as a model since it is reported to retain hepatic metabolis ability of a large number of toxins (36,37). The aim of this study was to demonstrate the ability of ZEN, adversely effects on Chang liver cell line and whether oxidative DNA damage could be involved in the toxicity of ZEN. In this content, cell viability after ZEN treatment of Chang liver cell was assessed by MTT assay and the oxidative DNA damage was determined with alkaline single cell gel electrophoresis (SCGE, Comet assay). Although the Comet assay is one of the standard methods for assessing DNA damage including singleand double-strand DNA breaks and alkali-labile sites (18), the introduction of lesion-specific endonuclease allows detection of oxidized bases and specific measure of oxidative DNA damage.
Our results clearly show that ZEN is cytotoxic on Chang liver cells by diminishing cell proliferation as provided by the MTT assay (IC50 150 μM). This cytolethality was dosedependent (Fig. 1). This finding agree with other studies (10), Vero cells (9,38-40), DOK cells (38), Caco-2 cells (38), bovine lymphocytes (41). This inhibition of cells viability was recovered by addition of NACA and increases cell viability in a dose-dependent manner. But no significant increase in cell viability was observed under concentrations of 5 mM NACA in the presence of 100 and 150 μM ZEN. 10 mM of NACA had maximal protective effect (43.3%) on cytotoxicity compared to 150 μM concentration of ZEN (p < 0.01, n = 4) in 100 μM and over a period of 24 hr exposure. Recently, many other papers reported the protective effect using various extract from natural products such as a cactus (Opuntia ficus-indica) cladodes (42,43), Raphanus sativus (15), adsorbents (13,14) and vitamin E (12,38,39) on ZEN-induce cytotoxicity. Because definitions of the contents of the extracts and the dosage used may vary from paper to paper, the results reported herein might not be directly comparable with those in other published papers. However, our results seem consistent with those of others (9,12-14,39). In agreement with these findings, we suggest that the observed effects may be due to the major antioxidant ingredient and adsorbent property of samples used in each experiment and antioxidant activity of NACA in this current study (Fig. 3). These facts were supported by our results of GSH and TBARS levels (Table 3) and Shi et al (29) study that NACA, although cell and induced cytotoxic agent used in their experiment are different, exhibited potential antioxidant activity decreasing oxidative stress and decrease cell death induced by doxorubicin in H9c2 cardiomyocytes. Our results clearly demonstrate the protective effect of NACA which efficiently protect Chang liver cell from DNA damage of ZEN. The protection afforded by NACA against ZEN-induced DNA damage is likely due to its ability to inhibit oxidative process induced by the mycotoxin ZEN. In fact, recently it has been demonstrated that ZEN induces oxidative damage both in vitro and in vivo including lipid peroxidation and oxidative DNA damage and a similar phenomenon of NACA activity protection by NACA was observed in a study on doxorubicin- induced oxidative DNA damage toxicity (29,35). Therefore, the oxidative DNA damage is suggested as a key determinant of ZEN induced toxicity (40,42). Protective effects of NACA against ZEN-induced DNA damage and cytotoxicity may be though associated to the presence of thiol group in NACA, which plays an important role in biological system mitigating the oxidative-induced DNA damage induced by anticancer drugs (25). We compared the prevention of ZEN toxicity (DNA damage) obtained by NACA to the prevention exerted by vitamin E (12,38) and by a variety of clay (HSCAS) (14) described as a compound able to adsorption and to sequester ZEN leading to the reduction of toxin bioavailability (44). As compared with other papers, NACA (43.3%) appears more efficient than them (vitamin: 36% and clay: 20.6%). The prevention potential of NACA is probably in relation, not only, with its high lipid solubility that could cross the blood-brain barrier, chelate Cu2+ (which catalyzes free radical formation), scavenge free-radicals and to neutralize toxic process induced by ZEN, but also with the inhibition of oxidative DNA damage effect. The observed effect of NACA on the cytoprotective effect and DNA damage protection of ZEN treated Chang liver cells is unlikely due to the activity of estrogen receptor, but rather through NACA activity as antioxidant component, Because ZEN binding affinity to cytosolic estrogen receptors is only about 5% of that of 17β-estradiol and the estrogenic potency is about 0.1% (45). Therefore, it is unlikely that ZEN toxicity (DNA damage) is due to its estrogenic activity solely suggesting other mechanism of ZEN toxicity may be involved such as antioxidant protective system from ZEN induce cytotoxicity.
In conclusion, our experiments using Chang liver cells exhibited that ZEN is cytotoxic by inhibiting cell proliferation and inducing oxidative DNA damage at low concentrations. ZEN dependant oxidative DNA damage preceded the loss of cell viability indicating that the oxidative DNA damage may contribute to ZEN induced cytotoxicity. These results were ameliorated by pre-incubation of NACA (10 mM) before the exposure to ZEN. Although, the mechanism of the protective effect of the NACA against ZENinduced cytotoxicity was not fully elucidated, we expect that the results will be useful information for the use of cytoprotective test from ZEN-induce toxicoty.
References
- 1.Wood G.E. Mycotoxins in foods and feeds in the United States. J. Anim. Sci. (1992);70:3941–3949. doi: 10.2527/1992.70123941x. [DOI] [PubMed] [Google Scholar]
- 2.Muller H.M., Reimann J., Schumacher U., Schwadorf K. Natural occurrence of Fusarium toxins in oats harvested during five years in an area of southwest Germany. Food Addit. Contam. (1998);15:801–806. doi: 10.1080/02652039809374713. [DOI] [PubMed] [Google Scholar]
- 3.Scudamore K.A., Patel S. Survey for aflatoxins, ochratoxin A, zearalenone and fumonisins in maize imported into the United Kingdom. Food Addit. Contam. (2000);17:407–416. doi: 10.1080/026520300404824. [DOI] [PubMed] [Google Scholar]
- 4.Felix D’Mello J.P., Duffus C.M., Duffus J.H. Toxic substances in crop plants. The Royal Society of Chemistry; Cambridge: (1991). pp. 226–257. [Google Scholar]
- 5.Kiessling K.H., Pettersson H. Metabolism of zearalenone in rat liver. Acta Pharmacol. Toxicol. (1978);43:285–290. doi: 10.1111/j.1600-0773.1978.tb02267.x. [DOI] [PubMed] [Google Scholar]
- 6.Marroufi K., Chekir L., Creppy E.E., Ellouz F., Bacha H. Zearalenone induces modifications of haematological and biochemical parameters in rats. Toxicon. (1996);34:535–540. doi: 10.1016/0041-0101(96)00008-6. [DOI] [PubMed] [Google Scholar]
- 7.Obremski K., Zielonka L., Zaluska G., Zwierzchowski W., Pirus K., Gajecki M. The influence low doses of zearalenone on liver enzyme activities in gilts.; In: proceeding of the X conference “Microscopy Fungi-plant pathogens and their metabolites”; (1999). p. 66. [Google Scholar]
- 8.Conková E., Laciaková A., Pástorová B., Seidel H., Kovác G. The effect of zearalenone on some enzymatic parameters in rabbits. Toxicol. Lett. (2001);121:145–149. doi: 10.1016/s0378-4274(01)00312-5. [DOI] [PubMed] [Google Scholar]
- 9.Abid-Essefi S., Quanes Z., Hassen W., Baudrimont I., Creppy E., Bacha H. Cytotoxicity, inhibition of DNA and protein syntheses and oxidative damage in culture cells exposed to zeararenolne. Toxicol. In Vitro. (2004);18:467–474. doi: 10.1016/j.tiv.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Hassen W., El Golli E., Baudrimont I., Mobio A.T., Ladjimi M.M., Creppy E.E., Bacha H. Cytotoxicity and Hsp70 induction in HepG2 cells in response to zearalenone and cytoprotection by sub-lethal heat shock. Toxicol. (2005);207:293–301. doi: 10.1016/j.tox.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Ouanes Z., Abid S., Ayed I., Anane R., Mobio T., Creppy E.E., Bacha H. Induction of micronuclei by zearalenone in Vero monkey kidney cells and in bone marrow cells of mice: protective effect of vitamin E. Mutat. Res. (2003);538:63–70. doi: 10.1016/s1383-5718(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 12.Ouanes Z., Ayed-Boussema I., Baati T., Creppy E.E., Bacha H. Zearalenone induces chromosome aberrations in bone marrow: preventive effect of 17β estradiol, progesterone and vitamin E. Mutat. Res. (2005);565:139–149. doi: 10.1016/j.mrgentox.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Abbès S., Quanes Z., Salah-Abbès J., Houau Z., Queslati R., Bacha H., Othman O. The protective effect of hydrated sodium calcium aluminosilicate against hematological, biochemical and pathological changes induced by zearalenone in mice. Toxicon. (2006a);47:567–574. doi: 10.1016/j.toxicon.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Abbès S., Salah-Abbès J.B., Quanes Z., Houau Z., Othman O., Bacha H., Addel-Wahhab M.A., Queslati R. Preventive role of phyllosilicate clay on the immunological and biochemical toxicity of zearenolone in Balb C/mice. Int. ImmunoPharmacol. (2006b);6:1251–1258. doi: 10.1016/j.intimp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Abbès S., Quanes Z., Salah-Abbes J.B., Addel-Wahhab M.A., Queslati R., Bacha H. Preventive role of aluminoslicate clay against induction of micronuclei and chromosome aberrations in bone marrow cells of Balb/c mice treated with zearalenone . Mutat. Res. (2007);631:85–92. doi: 10.1016/j.mrgentox.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Ayed-Boussema I., Ouanes Z., Bacha H., Abid A. Toxicities induced in cultured cell exposed to zearaleone: apoptosis or mutagenesis? J. Biochem. Mol. Toxicol. (2007);21:136–144. doi: 10.1002/jbt.20171. [DOI] [PubMed] [Google Scholar]
- 17.Tice R.R., Agurell E., Anderson D., Burlison B., Hartmann A., Kobayashi H., Miyamae Y., Rojas E., Ryu J.C., Sasaki Y.F. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. (2000);35:206–221. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. (1988);175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 19.Collins A., Dusinská M., Franklin M., Somorovaská M., Petrovská S., Duthi L., Fillion L., Panayiotidis M., Raslová K., Vaughan N. Comet assay in human biomonitoring studies: reliability, validation, and applications. Environ. Mol. Mutagen. (1997);30:139–146. doi: 10.1002/(sici)1098-2280(1997)30:2<139::aid-em6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Dixon D.R., Pruski A.M., Dixon L.R., Jha A.N. Marine invertebrate eco-genotoxicology: a methological overview. Mutagenesis. (2002);17:495–507. doi: 10.1093/mutage/17.6.495. [DOI] [PubMed] [Google Scholar]
- 21.Lee R.F., Steinert S. Use of the single cell gel electrophoresis/Comet assay for detecting DNA damage in aqatic (marine and freshwater) animals. Mutat. Res. (2003);544:43–64. doi: 10.1016/s1383-5742(03)00017-6. [DOI] [PubMed] [Google Scholar]
- 22.Gedik C.M., Ewen S.W., Collins A.R. Single cell gel electrophoresis applied to the analysis of UV-C damage and it repair in human cells. Int. J. Radiat. Biol. (1992);62:313–320. doi: 10.1080/09553009214552161. [DOI] [PubMed] [Google Scholar]
- 23.Jojte J., Zmyoelony M., Palus J., Dziubaltowska E., Rajkowska E. Protective effect of melatonin against in vitro iron ions and 7 mT 50 Hz magnetic field-induced DNA damage in rat lymphocytes. Mutat. Res. (2001);483:57–64. doi: 10.1016/s0027-5107(01)00230-5. [DOI] [PubMed] [Google Scholar]
- 24.Mohankumar M.N., Janani S., Prabhu B.K., Kumar P.R., Jeevanram R.K. DNA damage and intergrity of UV-induced DNA repair in lymphocytes of smokers analyzed by the comet assay. Mutat. Res. (2002);520:179–187. doi: 10.1016/s1383-5718(02)00201-2. [DOI] [PubMed] [Google Scholar]
- 25.Lupetti A., Paulusma-Annema A., Senesi S., Campa M., Van Dissel J.T., Nibbering P.H. Reactive oxygen species and internal thiols in the candidacidal activity of a Nterminal peptide of human lactoferrin. Antimicrob. Agents Chemother. (2002);46:1634–1639. doi: 10.1128/AAC.46.6.1634-1639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cotgreave I.A. N-acetylcysteine: Pharmacological considerations and experimental and clinical applications. Adv. Pharmacother. (1997);38:205–227. [PubMed] [Google Scholar]
- 27.Offen D., Gilgun-Sherki Y., Barhum Y., Benhar M., Grinberg L., Reich R., Mealmed E., Atlas D. A low molecular weight copper chelator crosses the blood-brain barrier and attenuates experimental autoimmune encephalomyelitis. J. Neurochem. (2004);89:1241–1251. doi: 10.1111/j.1471-4159.2004.02428.x. [DOI] [PubMed] [Google Scholar]
- 28.Grinberg L., Fibach E., Amer J., Atlas D. N-acetylcysteine amide, a novel cell-permeating thiol, restores cellular glutathione and protects human red blood cells from oxidative stress. Free Radical Biol. Med. (2005);38:136–145. doi: 10.1016/j.freeradbiomed.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Shi R., Huang C.C., Aronstam R.S., Ercal N., Martin A., Huang Y.W. N-acetylcysrein amide decrease oxidative stress but not cell death induced by doxorubicin in H9c2 cardiomyocytes. BMC Pharmacol. (2009);9:7. doi: 10.1186/1471-2210-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carmichael J., de Graff W.G., Gadza A.F., Minna J.D., Mitchell J.B. Evaluation of a tetrazolium-based semiautomatic colorimetric assay: assessment of chemosensitivity testing. Cancer Res. (1987);47:936–942. [PubMed] [Google Scholar]
- 31.Bottalico A. Fusarium diseases of cereal: Species complex and related mycotoxin profiles in Europe. J. Plant Pathol. (1998);80:85–103. [Google Scholar]
- 32.Placinta C.M., D’Mello J.P.F., Macdonald A.M.C. A review of world contamination of cerael grains and animal feed with Fusarium mycotoxins. Anim. Feed Sci. Technol. (1999);78:21–37. [Google Scholar]
- 33.Creppy E.E. Update of survey, reguration and toxic effects of mycotoxina in Europe. Toxicol. Lett. (2002);127:19–28. doi: 10.1016/s0378-4274(01)00479-9. [DOI] [PubMed] [Google Scholar]
- 34.Sáenz de Rodríguez C.A., Bougovanni A.M., Conde de Borrego L. An epidemic of precocious development in Puerto Rican children. J. Pediatr. (1985);107:393–396. doi: 10.1016/s0022-3476(85)80513-8. [DOI] [PubMed] [Google Scholar]
- 35.Tomaszewski J., Miturski R., Semezuk A., Koarski J., Jakowicki J. [Tissue zearalenone concentration in normal hyperplasic and neoplastic human endometrium]. Ginekol. Pol. (1998);69:363–366. [PubMed] [Google Scholar]
- 36.Chang R.S. Continous subcultivation of epithelial-like cells from normal human tissue. Proc. Soc. Exp. Biol. Med. (1954);87:440–443. doi: 10.3181/00379727-87-21406. [DOI] [PubMed] [Google Scholar]
- 37.Jukes T.H. Letter: Evolutionary changes in insulin. Nat. (1976);259:250. doi: 10.1038/259250a0. [DOI] [PubMed] [Google Scholar]
- 38.Abid-Essefi S., Baudrimont I., Hassen W., Quanes Z., Mobio T.A., Anane R., Creppy E.E., Bacha H. DNA fragmentation, apoptosis and cell cycle arrest induced by zearalenone in cultured DOK, Vero and Caco-2 cells: prevention by vitamin E. Toxicol. (2003);192:237–248. doi: 10.1016/s0300-483x(03)00329-9. [DOI] [PubMed] [Google Scholar]
- 39.El Golli E., Hassen E., Bouslimi W., Bouaziz A., Ladjimi M.M., Bacha H. Induction of Hsp 70 in Vero cells in response to mycotoxins cytoprotection by sub-lethal heat shock and by vitamin E. Toxicol. Lett. (2006);166:122–130. doi: 10.1016/j.toxlet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Hassen W., Ayed-Boussema I., Oscoz A.A., Lopez Ade C., Bacha H. The role of oxidative stress in zeralenone- meditated toxicity in Hep G2 cells: Oxidative DNA damage, gluthatione depletion and stress proteins induction. Toxicol. (2007);232:294–302. doi: 10.1016/j.tox.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Lioi M.B., Santoro A., Barbieri R., Salzano S., Ursini M.V. Ochratoxin A and zeralenone: a comparative study on genotoxic effects and cell death induced in bovine lymphocytes. Mutat. Res. (2004);557:19–27. doi: 10.1016/j.mrgentox.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 42.Zourgui L., Golli E.E., Bouaziz C., Bacha H., Hassen W. Cactus (opuntia ficus-indica) caladodes prevent oxidative damage induced by the mycotoxin zearalenone in Balb/C mice. Food Chem. Toxicol. (2008);46:1817–1824. doi: 10.1016/j.fct.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 43.Zourgui L., Ayed-Boussema I., Ayed Y., Bacha H., Hassen W. The antigenotoxic activities of Cactus (opuntia ficus-indica) caladodes against the mycotoxin zearalenone in Balb/C mice. : Preventive of micronuclei, chromosome aberrations and DNA fragmentation. Food Chem. Toxicol. (2009);47:662–667. doi: 10.1016/j.fct.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 44.Phillips T.D. Dietary clay in the chemoprevention of aflatoxin induced disease. Toxicol. Sci. (1999);52:118–126. doi: 10.1093/toxsci/52.suppl_1.118. [DOI] [PubMed] [Google Scholar]
- 45.Kuiper G.G., Lemmen J.G., Carlsson B., Corton J.C., Safe S.H., van der Saag P.T., van der Burg B., Gustafsson J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinol. (1998);139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]