Abstract
The process of drug discovery and development requires substantial resources and time. The drug industry has tried to reduce costs by conducting appropriate animal studies together with molecular biological and genetic analyses. Basic science research has been limited to in vitro studies of cellular processes and ex vivo tissue examination using suitable animal models of disease. However, in the past two decades new technologies have been developed that permit the imaging of live animals using radiotracer emission, Xrays, magnetic resonance signals, fluorescence, and bioluminescence. The main objective of this review is to provide an overview of small animal molecular imaging, with a focus on nuclear imaging (single photon emission computed tomography and positron emission tomography). These technologies permit visualization of toxicodynamics as well as toxicity to specific organs by directly monitoring drug accumulation and assessing physiological and/or molecular alterations. Nuclear imaging technology has great potential for improving the efficiency of the drug development process.
Keywords: Nuclear imaging, microSPECT, microPET, Animal experimental model, Molecular imaging, Drug development
INTRODUCTION
The drug discovery and development is a relatively long process that requires on average 12 years and 800 million US dollars (1,2).
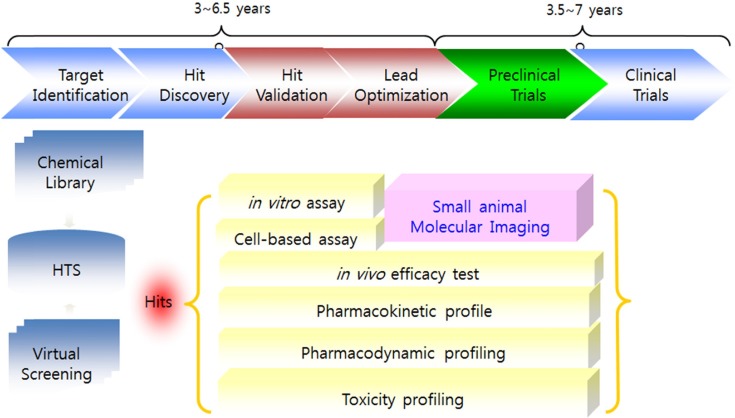
Rational predefined steps have been streamlined for drug development in recent years. In general, target identification, target validation, hit &lead identification, lead optimization, preclinical and clinical trial steps are included before FDA approval (Fig. 1).
Fig. 1. Rational steps for drug discovery and development.
During the past two decades, molecular imaging, which can give non-invasive, quantitative, and repetitive analytic information on molecules and biological processes in living organisms, has emerged as a new technology for both research and clinical drug development (3,4).
Many imaging techniques have been routinely used in the drug discovery process to monitor the drug in blood, normal organs, tissues, and tumor and to evaluate the effects of the drug in the context of tumor (5,6).
Molecular imaging, defined as the visual representation, characterization, and quantification of biological processes at the cellular and subcellular levels within intact living organisms, can be obtained by imaging techniques such as optical imaging, nuclear imaging, magnetic resonance imaging (MRI), ultrasound imaging and computed tomography (CT) (7,8) (Table 1).
Table 1.
Small animal imaging techniques for drug discovery and development
| Technique | Resolution | Depth | Sensitivity (moles of label detected) | Agent | Target |
|---|---|---|---|---|---|
| MRI | 10~100 μm | No limit | 10−9~10−6 | Gadolinium, Dysprosium, iron oxide particles | A1, P2, M3 |
| CT | 50 μm | No limit | 10−6~10−3 | Lodine | A, P |
| SPECT | 1~2 mm | No limit | 10−14~10−10 | 99mTc, 111In | P, M |
| PET | 1~2 mm | No limit | 10−15~10−12 | 18F, 11C, 15O | P, M |
| Optical | 1 mm | < 10 cm | 10−12~10−11 | Fluorescence, NIR | P, M |
| Ultrasound | 50 μm | Millimeters | 10−8~10−6 | Microbubble | A, P |
1: anatomical change, 2: physiological change, 3: molecular level change
Molecular imaging techniques can improve the efficiency of drug screening, assess pharmacokinetics, and evaluate drug effects to markedly reduce the costs and time required for the new drug development (5,9-11).
Nuclear imaging, such as single photon emission tomography (SPECT), positron emission tomography (PET), provides the 3D distribution of radiopharmaceuticals and has excellent sensitivity and high resolution with excellent tissue penetration depth (8,12-14).
This review will discuss the roles of nuclear molecular imaging for drug discovery and development, particularly for drug screening, pharmacokinetic and preclinical drug evaluation.
MOLECULAR IMAGING
The past two decades have seen an immense growth of knowledge in the field of molecular biology, leading to a better understanding of pathologic processes at the molecular level. New genetic tests have been among the first translations of these discoveries into diagnostics. These tests can be used for prevention screening, provide prognostic information and predict disease in progeny. However, in vitro diagnostics in general does not provide anatomical information on the actual localization of the event. Imaging procedures will be necessary to visualize abnormalities identified by in vitro diagnostics. Obtaining a non-invasive diagnosis of preclinical or even pre-malignant disease states is highly desirable and could be achieved with molecular image (MI), which is capable of detection and visualization of molecular disease markers in vivo via specific probes (15).
While conventional imaging yields anatomical maps or a rendering of physiologic functions, molecular imaging provides additional information on the distribution and (in some cases) amount or activity of specific molecular markers in vivo and will thus expand the emphasis of radiological imaging beyond the anatomical and functional level to a molecular one. Since changes on the molecular level always precede anatomical restructuring which can be detected in conventional imaging methods, diagnosis at an earlier stage in the course of a disease is needed so that an earlier treatment starts. Obtaining a non-invasive diagnosis of preclinical or even reversible states of serious diseases is highly desirable and may be achieved with molecular imaging.
There are hundreds of potential molecular imaging targets and a variety of probes currently under development. Among the existing imaging modalities PET, SPECT, and also MRI are particularly suited for molecular imaging. A new technology is in vivo near infrared imaging (NIR), so far in preclinical use. Beside hardware adaptations, there is a need for dedicated post-processing tools for quantification, image fusion and parameter mapping for MI.
SMALL ANIMAL MOLECULAR IMAGING MODALITIES
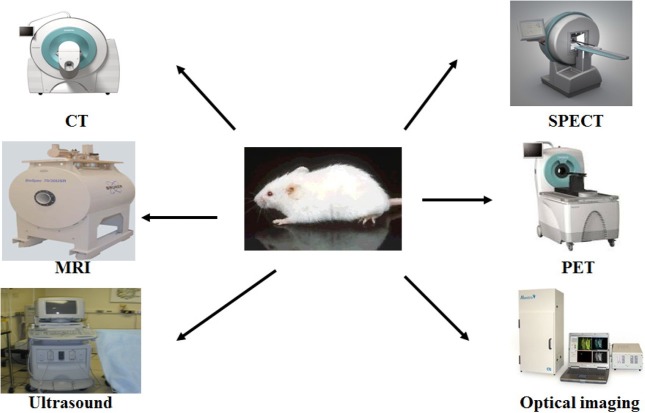
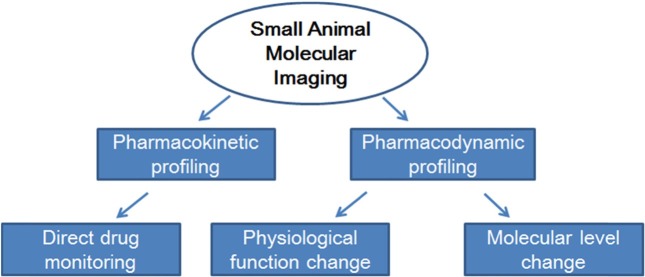
Academia and pharmaceutical companies are increasingly expressing aninterest in small animal molecular imaging (SAMI), because small animal models constitute established research tools for molecular imaging and the biological validation of new therapies. The small animal dedicated imaging modalities PET, SPECT, CT, MRI, optical imaging and also ultrasound are alreadyused for in vivo molecular imaging (Fig. 2). Fig. 3 represents that the SAMI is a promising technique that provides pharmacokinetic and pharmacodynamics profiles with direct in vivo drug monitoring and detecting the functional and/or molecular level change. SAMI allows for repetitive studies on the same animal and therefore enables the evaluation of disease progression or treatment response over time. It furthermore aids in target identification and early prioritization of candidate lead compound. Imaging instrumentation used for SAMI includes those modalities already used in clinical radiology. Dedicated scanners for SAMI are today available for all different modalities. MicroPET became commercially available around 1997, following by microSPECT in 2001. All these dedicated systems are specifically designed for the needs of SAMI. Most of the systems today are tomographic.
Fig. 2. Small animal molecular imaging modalities.
Fig. 3. Scheme representing the role of small animal molecular imaging techniques for drug development.
NUCLEAR IMAGING
Nuclear techniques include planar scintigraphy, SPECT and PET. All of these systems have a lower resolution (1 to 2 mm) than MRI and CT, but are highly sensitive and inherently quantitative. Picomolar concentration of isotopes can be detected with no depth limit in vivo. Because of low resolution, nuclear imaging provides only limited anatomical information. The output is merely a map of activity in the animal, the exact anatomical location of which can only be derived from the shape of the accumulated activity. This makes combinations with other modalities such as CT or MRI that deliver very valuable anatomical information. PET and SPECT are currently the most advanced methods to image molecular events in patients.
Nuclear imaging has always been at the forefront of molecular imaging. Many of the clinical probes have been already designed to detect molecular mechanisms, e.g. radiolabeled annexin to image apoptosis (16-20). For PET, positron-emitting isotopes are needed (15O, 13N, 18F, 94mTc, 11C). Most of these isotopes are produced in a cyclotron and have short half lives (e.g. 18F has a half life of 110 min), thus cannot be transported over long distance and require rapid chemical synthesis of the radio-labeled probe and ideally a cyclotron on side. On the other hand, the short halflife allows for repetitive experiments within shorter time cycles. Small molecules are widely used as probes, which are recognized by enzymes, receptors or other targets (21).
IMAGING PROBES FOR MOLECULAR IMAGING
In order to visualize specific cellular and molecular events non-invasively in living subjects, appropriate imaging probes need to be designed (8,22). In general, nonspecific probes are the most commonly used imaging probes and do not interact with a specific molecular target. They accentuate differences between tissues in permeability or perfusion. These probes are helpful to characterize physiological processes. For characterization of early changes in disease development, imaging visualization of specific biological processes at the cellular or molecular level is required. Thus, molecular imaging probes need to interact specifically with targets on these levels, leading to the development of targeted probes. In principle, targeted probes are used to localize biomolecules and to reveal their distribution. Such probes are detectable regardless of their interaction with the target. Therefore, background noise can target and remaining unbound probe is eliminated.
IDENTIFICATION OF SUITABLE TARGETS FOR MOLECULAR IMAGING
To find novel targets for drug development, the pharmaceutical industry undertakes screening tests in the magnitude of millions of samples. Using high throughput screening, thousands of samples can be evaluated simultaneously, quantitatively and rapidly against approximately 500 different molecular targets, of which 45% are receptors, 30% are enzymes and 25% are other molecules. Recently, screening technologies are also being extended to identify targets for molecular imaging (23).
Potential molecular targets are primarily nucleic acid sequences (DNA and mRNA) or proteins, but can also include any other specific cell structure such as carbohydrates (e.g. integrins, epitopes) or lipids. Several features of these biomolecules need to be considered, including the localization of the target molecules (on the cell surface or within the target cells) and the amount of copies of the same molecule in a single cell (quantity) (22).
LABELING A LIGAND THAT BINDS TO A MOLECULAR TARGET
Once a suitable target has been identified, a molecule (ligand) that specifically binds to this target has to be confirmed. Ligands can be small molecules, such as receptor ligands and substrates for enzymes, or larger ones such as recombinant proteins or monoclonal antibodies. Depending on the imaging modality, a detectable signal molecule (label) has to be bound to the ligand. For nuclear imaging, a variety of appropriate radioisotopes are available and already in clinical use, simplifying potential clinical applications. Numerous labeling techniques assist in probe design whereby radioisotopes are either chelated or covalently coupled to the ligand (24).
DELIVERY OF PROBE
Imaging probes must have the ability to reach the intended target at sufficient concentration and time to be detectable in living subjects. After administration, imaging probes are subject to pharmacokinetic precepts: absorption, distribution, metabolism, and elimination. The “fate” of imaging probes in vivo depends on properties of the probes such as molecular size, water solubility or binding characteristics. The main process by which the body eliminates “foreign” substances is excretion. Hydrophilic materials are eliminated by kidneys, whereas lipophilic compounds are usually metabolized by liver, the principal organ for metabolism. Lipophilic compounds are also redistributed into fatty tissue.
In order to leave the vascular system, the vascular wall needs to be traversed. However, extravasation can be challenging, especially in the brain when the blood-brain-barrier is intact (25). In tumors or inflammatory processes, the vasculature is “leaky” and therefore easier to traverse (26). Once the probe crosses over into the interstitial space of the target tissue, it needs to reach the target cells.
APPLICATION OF NUCLEAR IMAGING FOR DRUG DISCOVERY AND DEVELOPMENT
18F-fluorodeoxyglucose (FDG) is the most widely used tracer molecule for PET imaging in clinical studies to detect metabolically active tumors. This glucose analogue is transported into the cell by a specific transporter molecule, overexpressed in many cancers, which leads to an accumulation of the tracer within the tumor cells. FDG might serve as a surrogate marker to assess treatment response after chemotherapy. PET has been further applied to study multi-drug resistance, apoptosis (27) and gene expression in animal studies using reporter genes (28). For example, to study gene expression with PET, herpes simplex virus thymidine kinase (HSV-TK) is utilized as a marker gene. If HSV-TK is expressed, nucleoside analogs are phosphorylated and become trapped within the cell due to the associated changes in charge. The accumulation of activity is therefore an indicator of HSV-TK presence and consequently successful transfection. Those marker genes can also be utilized for tracking of transfected cells in vivo (29).
SPECT utilizes gamma-emitting radioisotopes (99mTc, 111In, 123I, 131I, etc.), which are produced in generators without needing a cyclotron. These isotopes are generally cheaper and have a longer half-life than the positron emitting isotopes used for PET imaging (e.g. 111In has a half life of 2.8 days). This longer half life makes SPECT easier to work with and facilitates imaging of probes with slow kinetics such as antibody fragments. Isotopes suitable for SPECT imaging on the other hand emit gamma rays of different energies, which allows for the simultaneous detection of several isotope, e.g. simultaneous imaging of different molecular targets. SPECT has been used to track cells or molecules such as radiolabelled annexin-V to image apoptosis (16) or tumor specific peptides (30,31) in vivo. It has also been deployed to image oncogene expression. Preliminary reports show that SPECT imaging might play a role as surrogate marker after chemotherapy as well (32).
Radioisotope labeling is the method of choice as modification can be isotopical, producing the same drug molecule with the same pharmacological effects and providing accurate pharmacokinetic information. In addition, nuclear imaging is highly sensitive and requires doses that are orders of magnitude lower than the pharmacological dose to obtain an image (3).
However, radiolabeling of a drug without changing pharmacokinetic characteristics is not always possible, and the radiochemistry is often complex. In addition, radiolabeled metabolites can produce same imaging signals as the original radiolabeled drug, and some isotopically labeled drugs have short windows for imaging acquisition (33).
Gene or protein targets related to disease development and therapeutic response can be exploited through molecular imaging and dynamics of the ligands in vivo and allows screening for agonistic and antagonistic ligand candidates for the receptor (34). Protein-protein interaction can be in vivo imaged with PET technology using thymidine kinase, therefore it can be applied to human subjects (35).
Deficiencies in bioavailability and pharmacokinetic properties cause drug development failures, and early warning systems for new drug candidates are essential for drug development (3). To generate accurate and reproducible pharmacokinetic studies, an exact input function of the labeled drug is critical for kinetic modeling.
APPLICATION OF NUCLEAR IMAGING FOR TOXICITY STUDY
Toxicology is heavily influenced by advances in many scientific displines. Currently increasing array of molecular methods provides deeper insights into toxicity mechanism. Many in vivo imaging techniques are used for visualizing toxicodynamics. Nuclear imaging has outstanding sensitivity as levels of picomole to femtomole for detection in vivo are possible without any depth limitation. The excellent sensitivity gives advantage to toxicologist to use tiny amount of radioactive compound for toxicokinetic study instead of overdose against detection limit. Visualization property can contribute to easy and reliable detection of the organ specific accumulation and toxicity. In addition, these techniques can contribute to the toxicodynamic profiling through early and reliable detection of the changes of physiological function and/or molecular level. For example, even in clinics, 18F-FDG imaging is the gold standard to detect the metastatic tumor for cancer patient. For this purpose, many FDA approved radiopharmaceuticals to be used for assessment of physiological functional changes and furthermore many nuclear molecular imaging probes are already in the pipeline.
CONCLUSION
Although nuclear molecular imaging technologies have to overcome challenges related to resolution, specificity, and accuracy to improve the process of drug discovery and development process, close collaborations from molecular biologist, chemist, physicist, and imaging scientist provide more potential imaging target and process. Efficient collaboration of all groups can promote target discovery and validation, drug screening, and preclinical trial. Molecular imaging is being integrated into the pharmaceutical industry infrastructure and will reduce the costs and time required for new drug development. Additionally, the potential of molecular imaging can contribute the prediction of the organ specific toxicity since they can reveal the distribution of compound in vivo easily and provide the evidence based approach for organ toxicity screening.
Acknowledgments
This work was supported by the Korea Atomic Energy Research Institute and grant no. 2012M2B2B1055245 from the Nuclear R&D Program of Ministry of Education, Science and Technology, South Korea
References
- 1.DiMasi J.A., Hansen R.W., Grabowski H.G. The price of innovation: new estimates of drug development costs. J. Health Econ. (2003);22:151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 2.Ahn B.C. Applications of molecular imaging in drug discovery and development process. Curr. Opin. Biotechnol. (2011);12:459–468. doi: 10.2174/138920111795163904. [DOI] [PubMed] [Google Scholar]
- 3.Rudin M. Noninvasive structural, fuctional, and molecular imaging in drug development. Curr. Opin. Chem. Biol. (2009);13:360–371. doi: 10.1016/j.cbpa.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Czernin J., Weber W.A., Herschman H.R. Molecular imaging in development of cancer therapeutics. Annu. Rev. Med. (2006);57:99–118. doi: 10.1146/annurev.med.57.080904.190431. [DOI] [PubMed] [Google Scholar]
- 5.Rudin M., Weissleder R. Molecular imaging in drug discovery and development. Nat. Rev. Drug Discov. (2003);2:123–131. doi: 10.1038/nrd1007. [DOI] [PubMed] [Google Scholar]
- 6.Ding H., Wu F. Image guide biodistribution and pharmacokinetic studies of theranostics. Theranostics. (2012);2:1040–1053. doi: 10.7150/thno.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mankoff D.A. A definition of molecular imaging. J. Nucl. Med. (2007);48:18N, 12N. [PubMed] [Google Scholar]
- 8.Massoud T.F., Gambhir S.S. Molecular imaging in living subjects: seeing fundermental biological processes in a new light. Genes Dev. (2003);17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 9.Vanderheyden J.L. The use of imaging in preclinical drug development. Q. J. Nucl. Med. Mol. Imaging. (2009);53:374–381. [PubMed] [Google Scholar]
- 10.Massoud T.F., Gambhir S.S. Integrating noninvasive molecular imaging into molecular medicine: an evolging paradigm. Trends Mol. Med. (2007);13:183–191. doi: 10.1016/j.molmed.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Cai W., Rao J., Gambhir S.S., Chen X. How molecular imaging is speeding up antiagiogenic drug development. Mol.Cancer Ther. (2006);5:2624–2633. doi: 10.1158/1535-7163.MCT-06-0395. [DOI] [PubMed] [Google Scholar]
- 12.Lecchi M., Ottobrini L., Martelli C., Del Sole A., Lucignani G. Instrumentation and probes for molecular and cellular imaging. Q. J. Nucl. Med. Mol. Imaging. (2007);51:111–126. [PubMed] [Google Scholar]
- 13.Pichler B.J., Wehrl H.F., Judenhofer M.S. Latest advances in molecular imaging instrumentation. J. Nucl. Med. (2008);49:5S–23S. doi: 10.2967/jnumed.108.045880. [DOI] [PubMed] [Google Scholar]
- 14.Koba W., Jelicks L.A., Fine E.J. MicroPET/SPECT/CT imaging of small animal models of disease. Am. J. Pathol. (2013);182:319–324. doi: 10.1016/j.ajpath.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Koba W., Kim K., Lipton M.L., Jelicks L., Das B., Herbst L., Fine E. Imaging devices for use in small animals. Semin. Nucl. Med. (2011);41:151–165. doi: 10.1053/j.semnuclmed.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 16.van de Wiele C., Lahorte C., Vermeersch H., Loose D., Mervillie K., Steinmetz N.D., Vanderheyden J.L., Cuvelier C.A., Slegers G., Dierck R.A. Quantitative tumor apoptosis imaging using technetium-99m-HYNIC annexin V single photon emission computed tomography. J. Clin. Oncol. (2003);21:3483–3487. doi: 10.1200/JCO.2003.12.096. [DOI] [PubMed] [Google Scholar]
- 17.Riemann B., Schafers K.P., Schober O., Schafers M. Small animal PET in preclinical studies: oppertunities and chanllenges. Q. J. Nucl. Med. Mol. Imaging. (2008);52:215–221. [PubMed] [Google Scholar]
- 18.Franc B.L., Acton P.D., Mari C., Hasegawa B.H. Small animal SPECT and SPECT/CT: important tools for preclinical investigation. J. Nucl. Med. (2008);49:1651–1663. doi: 10.2967/jnumed.108.055442. [DOI] [PubMed] [Google Scholar]
- 19.Toppomg G.J., Dinelle K., Kornelsen R., McCormick S., Holden J.E., Sossi V. Positron emission tomography kinetic modeling algorithms for small animal dopaminergic system imaging. Synapses. (2010);64:200–208. doi: 10.1002/syn.20716. [DOI] [PubMed] [Google Scholar]
- 20.Stacy M.R., Maxfield M.W., Sinusas A.J. Targeted molecular imaging of angiogenesis in PET and SPECT: a review. Yale J. Biol. Med. (2012);85:75–86. [PMC free article] [PubMed] [Google Scholar]
- 21.Haubner R., Wester H.J., Weber W.A., Mang C., Ziegler S.I., Goodman S.L., Senekowitsch-Schmidtke R., Kessler H., Schwaiger M. Noninvasive imaging of alpha(v)beta3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res. (2001);61:1781–1785. [PubMed] [Google Scholar]
- 22.Nolting D.D., Nickels M.L., Guo N., Pham W. Molecular imaging probe development: a chemistry perspective. Am. J. Nucl. Med. Mol. Imaging. (2012);2:273–306. [PMC free article] [PubMed] [Google Scholar]
- 23.Lindsay M.A. Target discovery. Nat. Rev. Drug Discovery. (2003);2:831–838. doi: 10.1038/nrd1202. [DOI] [PubMed] [Google Scholar]
- 24.Haberkorn U., Altmann A. Radionuclide imaging in the post-genomic era. J. Cell. Biochem. Suppl. (2002);39:1–10. doi: 10.1002/jcb.10386. [DOI] [PubMed] [Google Scholar]
- 25.Begley D.J., Brightman M.W. Structural and functional aspects of the blood-brain barrier. Prog. Drug Res. (2003);61:39–78. doi: 10.1007/978-3-0348-8049-7_2. [DOI] [PubMed] [Google Scholar]
- 26.Jain R.K. Transport of molecules, particles, and cells in solid tumors. Annu. Rev. Biomed. Eng. (1999);1:241–263. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- 27.Murakami Y., Takamatsu H., Taki J., Tatsumi M., Noda A., Ichise R., Tait J.F., Nishimura S. 18F-labelled annexin V: a PET tracer for apoptosis imaging. Eur. J. Nucl. Med. Mol. Imaging. (2004);31:469–474. doi: 10.1007/s00259-003-1378-8. [DOI] [PubMed] [Google Scholar]
- 28.Green L.A., Yap C.S., Nguyen K., Barrio J.R., Namavari M., Satyamurthy N., Phelps M.E., Sandgren E.P., Herschman H.R., Gambhir S.S. Indirect monitoring of endogenous gene expression by positron emission tomography (PET) imaging of reporter gene expression in transgenic mice. Mol. Imaging Biol. (2002);4:71–81. doi: 10.1016/s1095-0397(01)00071-1. [DOI] [PubMed] [Google Scholar]
- 29.Koehne G., Doubrovin M., Doubrovina E., Zanzonico P., Gallardo H.F., Ivanova A, Balatoni J., Teruya-Feldstein J., Heller G., May C., Ponomarev V., Ruan S., Finn R., Blasberg R.G., Bornmann W., Riviere I., Sadelain M., O’Reilly R.J., Larson S.M., Tjuvajev J.G. Serial in vivo imaging of the targeted migration of human HSV-TK-transduced antigen-specific lymphocytes. Nat. Biotechnol. (2003);21:405–413. doi: 10.1038/nbt805. [DOI] [PubMed] [Google Scholar]
- 30.Rogers B.E., Rosenfeld M.E., Khazaeli M.B., Mikheeva G., Stackhouse M.A., Liu T., Curiel D.T., Buchsbaum D.J. Localization of iodine-125-mIP-Des-Met14-bombesin (7-13)NH2 in ovarian carcinoma induced to express the gastrin releasing peptide receptor by adenoviral vector-mediated gene transfer. J. Nucl. Med. (1997);38:1221–1229. [PubMed] [Google Scholar]
- 31.Yazaki P.J., Shively L., Clark C., Cheung C.W., Le W., Szpikowska B., Shively J.E., Raubitschek A.A., Wu A.M. Mammalian expression and hollow fiber bioreactor production of recombinant anti-CEA diabody and minibody for clinical applications. J. Immunol. Methods. (2001);253:195–208. doi: 10.1016/s0022-1759(01)00388-x. [DOI] [PubMed] [Google Scholar]
- 32.Kao C.H., Hsieh J.F., Tsai S.C., Ho Y.J., Lee J.K. Quickly predicting chemotherapy response to paclitaxel- based therapy in non-small cell lung cancer by early technetium-99m methoxyisobutylisonitrile chest single-photon- emission computed tomography. Clin. Cancer Res. (2000);6:820–824. [PubMed] [Google Scholar]
- 33.Wong D.F., Tauscher J., Gründer G. The role of imaging in proof of concept for CNS drug discovery and development. Neuropsychopharmacology. (2009);34:187–203. doi: 10.1038/npp.2008.166. [DOI] [PubMed] [Google Scholar]
- 34.Paulmurugan R., Gambhir S.S. An intramolecular folding sensor for imaging estrogen receptor-ligand interactions. Proc. Natl. Acad. Sch. U.S.A. (2006);103:15883–15888. doi: 10.1073/pnas.0607385103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massoud T.F., Paulmurugan R., De A, Ray P., Gambhir S.S. Reportor gene imaging of protein-protein interactions in living subjects. Curr. Opin. Biotechnol. (2007);18:31–37. doi: 10.1016/j.copbio.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]