Abstract
Cyanogenic glycosides are HCN-producing phytotoxins; HCN is a powerful and a rapidly acting poison. It is not difficult to find plants containing these compounds in the food supply and/or in medicinal herb collections. The objective of this study was to investigate the distribution of total cyanide in nine genera (Dolichos, Ginkgo, Hordeum, Linum, Phaseolus, Prunus, Phyllostachys, Phytolacca, and Portulaca) of edible plants and the effect of the processing on cyanide concentration. Total cyanide content was measured by ion chromatography following acid hydrolysis and distillation. Kernels of Prunus genus are used medicinally, but they possess the highest level of total cyanide of up to 2259.81 CN−/g dry weight. Trace amounts of cyanogenic compounds were detected in foodstuffs such as mungbeans and bamboo shoots. Currently, except for the WHO guideline for cassava, there is no global standard for the allowed amount of cyanogenic compounds in foodstuffs. However, our data emphasize the need for the guidelines if plants containing cyanogenic glycosidesare to be developed as dietary supplements.
Keywords: Cyanogenic compounds, Herbal medicines, Ion chromatography
INTRODUCTION
There are various forms of cyanogenic compounds that release hydrogen cyanide upon breakdown. The cyanogenic compound is present mainly as glycoside in more than 2650 plant species. Apricot kernel, peach kernel, cassava, almond, bamboo shoot, sorghum, Japanese apricot, flaxseed among others have been consumed by human worldwide either as food or as herbal medicine (1,2). About ten cyanogenic glycosides including amygdalin, prunasin, dhurrin, linamarin, and taxiphyllin have been reported in edible plants (3). Hydrogen cyanide derived from cyanogenic glycoside can cause health concerns including cell death by blocking cytochrome oxidase and the arrest of the ATP production. Several symptoms have been related to the consumption of cyanogen containing foodstuff including vomiting, nausea, dizziness, weakness, konzo, and occasional death. Chronic intake has been linked to goiter especially in iodine deficiency cases (4,5).
Various methods have been used for the quantitative analysis of cyanogenic compounds of edible plants. The colorimetric method via König reaction after acid hydrolysis, picrate method, and the chromatographic method are the most common ones (6-13). The analysis using the colorimetric method involves three steps: (1) extraction of cyanogenic compounds from the plants material, (2) acid hydrolysis of cyanogenic glycoside, and (3) the color development and detection of cyanide (14). The König reaction used in the color development step detects not only cyanide but also thiocyanate, other plant secondary metabolites, making the method less specific to cyanide. Also during the acid hydrolysis, HCN gas may dissipate, resulting in an underestimation. The colorimetric method may employ enzymatic hydrolysis either intrinsic or extrinsic, however, tannin from the plant tissue may inhibit the enzyme giving rise to another source of underestimation (14,15). A gas chromatographic measurement of hydrogen cyanide released by the hydrolysis has been tried but this method could not prevent the loss of the HCN gas (12). Liquid chromatography has been used to quantify cyanogenic glycosides. But it can only be used for the known cynogenic glycoside with standard materials and it is difficult to estimate the total cyanogenic compounds in plant. Moreover tannin, flavonoid and chlorophyll in plant tissue have been shown to interfere with the liquid chromatographic determination of the cyanogenic glycosides (11,13). The hydrolysis step seems to be inevitable to measure total cyanogen content. In this study, ion chromatogryphy following acid hydrolysis was employed to prevent thiocyanate interference of the colorimetric methods.
There has been no concern for the cyan toxicity from Korean staple. However, since there have been attempts to develop dietary supplements with some of the plants containing cyanogenic glycoside, it was imperative to determine cyanogens in plants consumed in Korea.
MATERIALS AND METHODS
Food materials and Herbal medicines reported to contain cyanogenic compounds were purchased from the various markets in Seoul. Edible plants from 14 genera, Dolichos, Ginkgo, Hordeum, Linum, Malus, Manihot, Phaseolus, Phyllostachys, Phytolacca, Portulaca, Prunus, Sorghum, Vicia, and Vitis, have been included in the study (4,7,11,16). The origins of the plants were Korea (both South and North) and China. Cyanide standard for the ion chromatography (1000 ppm) was obtained from Fluka Co, USA. Phosphoric acid, barbituric acid, isonicotinic acid and chloramine-T were obtained from Sigma-Aldrich Chemical Co, USA. Micro Dist, from Lachat Co (USA) and Dionex (USA) ion chromatograph, DX-2500, were used for the cyanide distillation and quantification, respectively.
Detection of total cyanide in edible plants. Acid hydrolysis of Brandbury (8) and distillation used by Hong et al. (6) has been combined and used for the pre-chromatographic step. Each sample (about 1 g) was homogenized with 30 ml of 0.1 M phosphoric acid and the solution was centrifuged for 20 min at 8000 rpm. 3 ml of the supernatant was transferred to the tight capped vial and the equal amount of 4 M sulfuric acid was added. Hydrolysis was started by heating to 100℃ and continued for 50 mins. The reaction mixture was allowed to be cooled in ice. The hydrolysis mixture was transferred to the Micro Dist tube (Lachat, USA) with 0.75 ml of 0.79 M MgCl2 and heated for 45 min. After cooling at ambient temperature, cyanide collected in sodium hydroxide solution was analyzed by ion chromatography. The chromatographic condition was summarized in Table 1. Calibration curve was constructed with ion chromatographic cyanide standard. The working standards were diluted with 0.2 M sodium hydroxide solution.
Table 1.
Ion Chromatographic Condition to analyze CN– anion
| Item | Conditions |
|---|---|
|
| |
| IC | Dionex DX-2500 system |
| Column | Guard: IonPac AG7 (40 mm × 50 mm) |
| Analytical: IonPac AS7 (40 mm × 250 mm) | |
| Detector | ED40, DC Amperometry |
| Flow rate | 1.0 ml/min (isocratic), 200 μl injection |
| Electrode cell | Silver working electrode |
| 0.00 V vs Ag/AgCl reference | |
| Mobile phase | 0.5 M Sodium Acetate/0.1 M Sodium Hydroxide/ |
| 0.5% (v/v) | |
| Ethylenediamine | |
RESULTS AND DISCUSSION
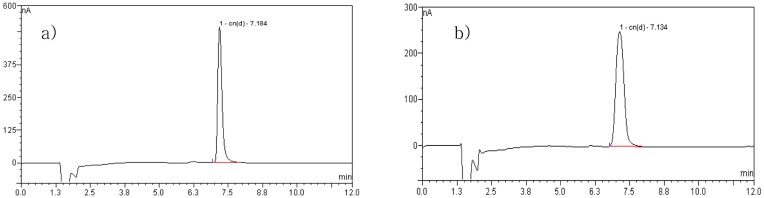
Cyanide analysis using ion chromatography. The ion chromatograms of cyanide standard (a) and cyanide in apricot kernel (b) are given in Fig. 1. Unlike colorimetric method, thiocyanate ion did not interfere with the detection (data not shown). Limit of detection, determined as signal to noise ratio(S/N) of 3 was 0.005 mg CN−/L.
Fig. 1. Ion chromatogram of (a) cyanide standard and (b) cyanide from apricot kernel.
Total cyanide in herbal medicines. Herbal medicines used in Korea, which belong to the genera known to con-tain cyanogenic glycosides, were purchased from the professional market in Seoul. All of the samples were originated from either Korea (South and North) or China. The results are shown in Table 2. Each analysis was duplicated and a mid-point value was reported for each sample. Coefficient of variance was lower than 10% for each of the samples. Since the cyanogen contents varied greatly from sample to sample, ranges of the values as well as the mean are reported. In cases where both domestic and imported samples were analyzed, there was no difference in cyanogens contents by origin.
Table 2.
Cyanogenic Compounds in Medicinal Plants Sold in Korea
| Scientific name | Sample name | N | Total Cyan Liberated by Acid Hydrolysis (μg CN–/g dry wt.)* | ||
|---|---|---|---|---|---|
|
| |||||
| Range | Mean ± SD† | ||||
|
| |||||
| Dolichos | D. lablab | Hyacinth bean | 3 | n.d. - trace | |
| Ginkgo | G. biloba | Ginko semen | 4 | trace - 1.00 | 0.25 ± 0.5 |
| Dried Ginko | 1 | trace | |||
| Hordeum | H. vulgare | Hordei germinates | 1 | 2.65 | |
| Linum | L. usitatissimum | Flaxseed | 4 | 261.9 - 345.4 | 299.82 ± 37.58 |
| Phyllostachys | P. nigra | Bamboo inner skin | 3 | n.d. - trace | trace |
| P. bambusoides | Bamboo leaf | 3 | trace | ||
| Phytolacca | P. esculenta | Phytolacca radix | 1 | 149.55 | |
| Portulaca | P. grandiflora | Rose moss | 1 | 14.81 | |
| P. oleraceae | Pulselane | 3 | 1013.39 - 1371.85 | 1152.25 ± 192.39 | |
| Prunus | P. armeniaca | Apricot kernel | 10 | 6.42 - 2259.81 | 1679.27 ± 697.41 |
| Apricot kernel, skinned | 13 | n.d. - 152.70 | 22.93 ± 41.98 | ||
| P. nakii | P. nakaii semem | 9 | 226.64 - 1305.34 | 872.38 ± 419.41 | |
| P. mume | Mume fruits | 4 | 22.63 - 88.27 | 45.87 ± 30.45 | |
| P. persica | Peach kernel | 14 | 442.72 - 1044.45 | 884.89 ± 159.42 | |
| P. tomentosa | Cherry, dried | 1 | trace | ||
* N: The number of samples; n.d.: Not Detected. trace: a peak can be seen but its area was less than limit of detection.
† Where there was a single sample, the value represent the mid-point of duplicates for the sample. In such cases C.V. was less than 10%.
The highest amount was found in apricot kernels (P. armeniaca), followed by P. domestica and P. nakii. Peach kernels (P. persica) also showed high amount of high amount of cyanogens. In case of apricot kernel, both peeled and intact dried kernels were available in the market. The peeled kernel showed average 22.9 μg CN−/g, while intact kernel showed average 1679 μg CN−/g . It seemed that most of cynogenic compounds were localized in the skin. In Korea, these medicinal plants can be purchased without prescription in the open market. Since apricot kernel is said to have cosmetic effect, the consumption of the apricot kernel as folk medicine both in topical and oral use is not rare among middle aged Korean women. Moreover, some of the plants listed in Table 2, though the cyanogens contents were low, are permitted as foods as well. Ever since the phytoestrogenic activities of the flaxseed lignin were reported, the consumption of flaxseed has been increasing.
The cyanogenic compounds were also detected in other herbal medicines. Prunus mume was dried form of a Japanese apricot, and showed 45.87 μg CN−/g dry weight. Cyanogenic compounds were detected in the kernels of all of the tested species of prunus.
The difference between cyanogens contents in the samples of the single species purchased in different locations ranged greatly, up to thousand fold, probably reflecting the variations of cultivation, harvest and/or storage.
Total cyanide analysis in dietary plants. Including Prunus genera, many other plants known to contain cyanogens are consumed as food as well as herbal medicines. Food originated from Hordeum, Marlus, Manihut, Phaseolus, Prunus, Sorghum, and Vitis are analyzed for the total cyanogens and shown in Table 3. While kernels from the Prunus genera, which are generally consumed as medicine, contained large amount of cyan, the edible parts including peel and flesh showed much smaller amount. However seeds from the fruits, apple, grape, peach, plum, and apricot, showed larger amount of cyanogens than flesh (data not shown), necessitating caution in food preparation. Cassava and the starch derived from it are not typical food items in Korea, but gaining popularity through the globalization of the food supply.
Table 3.
Cyanogenic compounds in foodstuffs
| Scientific name | Common name | N | Amount of cyanide (ug CN–/g) * | ||
|---|---|---|---|---|---|
|
| |||||
| Range | Mean ± SD † | ||||
|
| |||||
| Hordeum | H. vulgare | Barley | 11 | trace | |
| Malus | M. pumila | Apple, edible parts | 6 | trace | |
| Apple, skin | 6 | trace - 1.27 | 0.42 ± 0.73 | ||
| Apple, seed | 6 | 104.39 - 178.19 | 124.4 ± 31.4 | ||
| Manihot | M. esculenta | Cassava | 1 | 365.44 | |
| Tapioka (starch) | 1 | 0.81 | |||
| Phaseolus | P. angularis | Red bean | 5 | n.d. - trace | trace |
| P. limensis | Lima bean | 1 | trace | ||
| P. radiatus | Mungbean, whole | 2 | trace | ||
| Mungbean, skinned | 4 | trace - 2.23 | 0.56 ± 1.12 | ||
| Mungbean powder | 2 | trace | |||
| P. vulgaris | Kidney bean | 2 | trace | ||
| Prunus | P. armeniaca | Apricot, flesh | 2 | trace - 1.01 | 0.51 |
| Apricot, skin | 2 | trace | trace | ||
| P. aviun | Cherry, flesh | 1 | trace | trace | |
| Cherry, skin | 1 | trace | trace | ||
| Cherry, seed | 1 | 522.78 | |||
| P. communis | Almond | 2 | n.d | ||
| P. domestica | Plum, flesh | 8 | n.d. - trace | trace | |
| Plum, skin | 8 | n.d. - 13.26 | 2.65 ± 5.93 | ||
| Plum, seed | 5 | 505.14 - 1032.96 | 659.1 ± 321.16 | ||
| P. mume | Japanese apricot, flesh | 2 | trace - 0.82 | 0.41 | |
| Japanese apricot, skin | 2 | trace | |||
| Japanese apricot, seed | 2 | 1026.59 - 1155.45 | 1091.02 | ||
| P. persica | Peach, flesh | 11 | n.d. - trace | trace | |
| Peach, skin | 11 | n.d. - trace | trace | ||
| Nectarine, flesh | 4 | n.d. - trace | trace | ||
| Nectarine, skin | 4 | trace | trace | ||
| Sorghum | S. bicolor | Millet | 7 | n.d. - 1.39 | 0.20 ± 0.53 |
| Vitis | V. vinifera | Grape, flesh | 6 | n.d. - trace | trace |
| Grape, skin | 6 | n.d. - trace | trace | ||
| Grape, seed | 5 | n.d. - trace | trace | ||
* N : The number of samples; n.d. : Not Detected. trace : a peak can be seen but its area was less than limit of detection.
† Where there was a single sample, the value represent the mid-point of duplicates for the sample. In such cases C.V. was less than 10%.
As mentioned earlier, some of the medicinal foods are also consumed as food. Purslane, though not cultivated, are gathered and eaten as cooked, and showed average 1150 μg CN−/g. Also flaxseed showed about 300 μg CN−/g. Though cyanogens were detected in other edible plants, the amount was too low to pose health concern.
Acknowledgments
This research was supported by KFDA research grant 06042-036.
References
- 1.Francisco I.A., Pinotti M.H.P. Cyanogenic glycosides in plants. Braz. Arch. Biol. Technol. (2000);43:487–492. doi: 10.1590/S1516-89132000000500007. [DOI] [Google Scholar]
- 2.Haque M.R., Bradbury J.H. Total cyanide determination of plants and foods using the picrate and acid hydrolysis methods. Food Chem. (2002);77:107–114. doi: 10.1016/S0308-8146(01)00313-2. [DOI] [Google Scholar]
- 3.Vetter J. Plant cyanogenic glycosides. Toxicon. (2000);38:11–36. doi: 10.1016/S0041-0101(99)00128-2. [DOI] [PubMed] [Google Scholar]
- 4.Shibamoto T., Bjeldanes L.F. Introduction to Food Toxicology. Academic Press; San Diego: (1993). pp. 71–74. [Google Scholar]
- 5.Cardoso A.P., Mirione E., Ernesto M., Massaza F., Cliff J., Haque M.R., Bradbury J.H. Processing of cassava roots to remove cyanogens. J. Food Comps. Anal. (2005);18:451–460. doi: 10.1016/j.jfca.2004.04.002. [DOI] [Google Scholar]
- 6.Hong J.H., Lee D.H., Han S.B., Lee D.H., Lee K.B., Park J.S., Chung H.W., Lee S.Y., Park S.G., Park E.R., Hong K.H., Han J.W., Kim M.C., Song I.S. The establishment of analytical method, and monitoring of toxins in food materials; The analysis and decrease of cyanogenic glucoside in apricot Kernel, Peach Kernel, and Flaxseed. Annu.Rep. KFDA. (2004);8:442–452. [Google Scholar]
- 7.Aikman K., Bergman D., Ebinger J., Seigler D. Variation of cyanogenesis in some plants species of the Midwestern united states. Biochem. Syst. Ecol. (1996);24:637–645. [Google Scholar]
- 8.Bradbury J.H, Egan S.V., Lynch M.J. Analysis of cyanide in cassava using acid hydrolysis of cyanogenic glucosides. J. Sci.Food Agric. (1991);55:277–290. doi: 10.1002/jsfa.2740550213. [DOI] [Google Scholar]
- 9.Bradbury J.H., Bradbury M.G., Egan S.V. Comparison of methods of analysis of cyanogens in cassava. Acta Horticul. (1994);37:587–596. [Google Scholar]
- 10.Bradbury M.G, Egan S.V., Bradbury J.H. Picrate paper kits for determination of total cyanogens in cassava roots and all forms of cyanogens in cassava products. J. Sci. Food Agric. (1999);79:593–601. doi: 10.1002/(SICI)1097-0010(19990315)79:4<593::AID-JSFA222>3.0.CO;2-2. [DOI] [Google Scholar]
- 11.Berenguer-Navarro V., Giner-Galván R.M., Arrazola-Teruel G. Chromatographic determination of cyanoglycosides prunasin and amygdalin in plant extracts using a porous graphitic carbon column. J. Agric. Food Chem. (2002);50:6960–6963. doi: 10.1021/jf0256081. [DOI] [PubMed] [Google Scholar]
- 12.Curtis A.J, Grayless C.C., Fall R. Simultaneous determination of cyanide and carbonyls in cyanogenic plants by gas chromatography-electron capture/photoionization detection. Analyst. (2002);127:1446–1449. doi: 10.1039/b205378k. [DOI] [PubMed] [Google Scholar]
- 13.Kobaisy M., Oomah B.D., Mazza G. Determination of cyanogenic glycosides in flaxseed by barbituric acidpyridine, pyridine-pyrazolone, and high-performance liquid chromatography methods. J. Agric. Food Chem. (1996);44:3178–3181. doi: 10.1021/jf950838j. [DOI] [Google Scholar]
- 14.Egan S.V., Yeoh H.H., Bradbury J.H. Simple picrate paper kit for determination of the cyanogenic potential of cassava flour. J. Sci. Food Agric. (1998);76:39–48. doi: 10.1002/(SICI)1097-0010(199801)76:1<39::AID-JSFA947>3.0.CO;2-M. [DOI] [Google Scholar]
- 15.Environmental Protection Agency (EPA). Total and amenable cyanide (Automated colorimetric) EPA; USA: (2004). pp. 1–13. [Google Scholar]
- 16.Oomah B.D., Mazza G., Kenaschuk E.O. Cyanogenic compounds in flaxseed. J. Agric. Food Chem. (1992);40:1346–1348. doi: 10.1021/jf00020a010. [DOI] [Google Scholar]


