Abstract
Nanotoxicological research has shown toxicity of nanomaterials to be inversely related to particle size. However, the contribution of agglomeration to the toxicity of nanomaterials has not been sufficiently studied, although it is known that agglomeration is associated with increased nanomaterial size. In this study, we prepared aerosols of nano-sized carbon black by 2 different ways to verify the effects of agglomeration on the toxicity and deposition of nano-sized carbon black. The 2 methods of preparation included the carbon black dispersion method that facilitated clustering without sonication and the carbon black dispersion method involving sonication to achieve scattering and deagglomeration. Male Sprague-Dawley rats were exposed to carbon black aerosols 6 hr a day for 3 days or for 2 weeks. The median mass aerodynamic diameter of carbon black aerosols averaged 2.08 μm (for aerosol prepared without sonication; group N) and 1.79 μm (for aerosol prepared without sonication; group S). The average concentration of carbon black during the exposure period for group N and group S was 13.08 ± 3.18 mg/m3 and 13.67 ± 3.54 mg/ m3, respectively, in the 3-day experiment. The average concentration during the 2-week experiment was 9.83 ± 3.42 mg/m3 and 9.08 ± 4.49 mg/m3 for group N and group S, respectively. The amount of carbon black deposition in the lungs was significantly higher in group S than in group N in both 3-day and 2-week experiments. The number of total cells, macrophages and polymorphonuclear leukocytes in the bronchoalveolar lavage (BAL) fluid, and the number of total white blood cells and neutrophils in the blood in the 2- week experiment were significantly higher in group S than in normal control. However, differences were not found in the inflammatory cytokine levels (IL-1β, TNF-α, IL-6, etc.) and protein indicators of cell damage (albumin and lactate dehydrogenase) in the BAL fluid of both group N and group S as compared to the normal control. In conclusion, carbon black aerosol generated by sonication possesses smaller nanoparticles that are deposited to a greater extent in the lungs than is aerosol formulated without sonication. Additionally, rats were narrowly more affected when exposed to carbon black aerosol generated by sonication as compared to that produced without sonication.
Keywords: Carbon black, Nano, Deposition, Toxicity
INTRODUCTION
Particles with at least one dimension between 1 and 100 nm are called ultrafine particles or nanomaterials (1). With the rapid development of high-tech industry, many nanomaterials have been introduced to the workplace. Since Ferin et al. and Oberdorster et al. first reported in 1990 that particles smaller than 100 nm in diameter showed greater pulmonary toxicity. The toxicity of nanomaterials has been widely investigated since then (2,3). Reviewing the achievement of 20 years of nanotoxicology studies, Maynard et al. summarized several properties that affect the toxicity of nanomaterials such as particle size, degree of aggregation/agglomeration, internal particle structure, particle shape, and so on (4). In some nanomaterials (especially poorly soluble particles with low cytotoxicity (PSPs) such as titanium dioxide, carbon black, and amorphous silica), it is arguably accepted that the surface area is a major factor in determining the toxicity of nanomaterials (4-6).
Carbon black is an industrial chemical used mainly to reinforce rubber in a tire industry. Carbon black, which is insoluble and tends to lump in an aqueous solution due to rapid aggregation (i.e., particles strongly bonded or fused together) or agglomeration (i.e., collection of weakly bonded particles), makes it complex to explain the toxic effects of carbon black in the workplace: aggregation and/or agglomeration decrease the total surface area whereas they increase the particle size (7,8). In addition, it is not certain whether smaller agglomerates deposit more in the lung than larger agglomerates: until now, it is recognized that respirable particles deposit more in the lung than nanoparticles (9). Therefore, controlling de-agglomeration/agglomeration and dispersion is a prerequisite for studying the toxicity of nano-sized carbon black like other nanomaterials, especially for PSPs (8).
There are three possible techniques to expose the respiratory track to nanomaterials: inhalation, instillation and aspiration. Of the three, inhalation is the most likely exposure route in a workplace condition and it is the only way that determines NOAEL for the airborne concentration of suspended nanomaterials (10). On the other hand, various agglomeration degrees of nano-sized carbon black aerosols can exist in the workplace. Newly generated aerosols may be small (i.e., low agglomeration degree) and they may become bigger (i.e., high agglomeration degree) in the workplace. Therefore, we prepared aerosols of nano-sized carbon black in two different ways to verify the effects of agglomeration on the toxicity and deposition of nano-sized carbon black: 1) carbon black dispersions to allow clustering without sonication, and 2) carbon black dispersions sonicated to scatter and de-agglomerate. Carbon black aerosols were exposed to SD male rats 6 hrs a day for 3 days or 2 weeks and the extent to which carbon black accumulates in a lung and the consequent toxicity were compared in different groups to explain the health effects of carbon black in the workplace.
MATERIALS AND METHODS
Animals. Five week-old male Specific pathogen-free (SPF) Sprague-Dawley (SD) rats were obtained from Central Lab Animal Inc. (Seoul, Korea) and were acclimatized at least 1 week prior to carbon black exposure. During the acclimation and experimental period, rats were housed at polycarbonate cages in a room with controlled temperature (23 ± 2℃), humidity (55 ± 7%), and a 12-hr light/dark cycle. Rats were fed filtered water and a rodent diet (LabDiet 5053, PMI Nutrition, USA) ad libitum. The study was approved by an animal ethics committee to ensure appropriate animal care for research.
Generation and exposure of carbon black aerosol. Printex-90, nano-sized carbon black, was obtained from Degussa (manufacturer’s specification: primary particle diameter of 14 nm). Carbon black dispersions for both group S (aerosols generated with sonication) and group N (aerosols generated without sonication) were prepared by adding 1.5 g of carbon black in 300 ml of distilled water which then were stirred vigorously for one minute, and additional sonication was applied to dispersion for group S in a probe type ultrasonicator (Vibra cell VC-750, Sonics, USA) at a total power of 1170 J. Carbon black dispersions were then aerosolized through 0.8 mm diameter of an orifice at 8 L of air flow per minute (total flow was adjusted to 20 L per minute) in nose-only inhalation chambers (NITC system, HCT, Korea). Rats were exposed to carbon black aerosols 6 hr/day for 3 days or 6 hr/day, 5 days/week for 2 weeks. Particle size distributions of carbon black aerosols were measured with a small size cascade impactor (Minimoudi M135, USA) and shapes of aerosols were observed with a transmission electron microscopy (TEM). During the exposure period, carbon black aerosols were sampled at Membrane cellulose ester filters and weighted in an electric balance (Kern 770, Germany).
Bronchoalveolar lavage. After rats were anesthetized with isoflurane (Ilsung Pharm, Korea), the trachea was cannulated and the lungs were lavaged five times with 3 ml of calcium- and magnesium-free phosphate buffered saline, pH 7.4. BAL fluids were centrifuged at 1500 rpm for 10 min and the supernatants of the first BAL fluid were stored at −80℃ for albumin, lactate dehydrogenase (LDH) and cytokine assays. The numbers of total cells were counted with a Coulter counter (Hemavet 850, Drew Science, USA). Lavaged cells were centrifuged by Cytospin (Hanil, Korea) and stained with Diff-Quick solutions. Differential counts of macrophages, lymphocytes, and PMNs were determined by counting approximately 300 cells under a microscope at 200x magnification. LDH and albumin were measured with a biochemistry analyzer (TBA 20FR, Toshiba Co. Japan) and cytokines were measured using commercial ELISA kits.
Hematology. Blood samples were collected from the abdominal artery in blood-collecting tubes containing anticoagulant EDTA. Total white blood cells (WBC), Neutrophile (NE), Lymphocyte (LY), Monocyte (MO), Eosinophile (EO), and Basophile (BA) were counted using Veterinary Multi-species Hematology System, Hemavet 850 (Drew Science, USA).
Histology and lung burden of carbon black. Lungs were fixed in a 10% formalin solution containing neutral PBS and embedded in paraffin. After staining with hematoxylin and eosin, histological changes and numbers of carbon black clumps in the lung tissues were examined by light microscopy at 200x magnification. Lung burden of carbon black in the lung tissues was measured according to the method of Lim et al. (11).
Statistical analysis. Results are presented as mean ± standard deviation. Data were analyzed by one-way analysis of variance (ANOVA) followed by post hoc analysis based on Duncan’s t-test to determine the differences between the control and two carbon black exposure groups. Statistical analyses were performed using SigmaStat software (Version 3.5, Systat Software, USA). Differences were considered significant when the p-value was < 0.05.
RESULTS
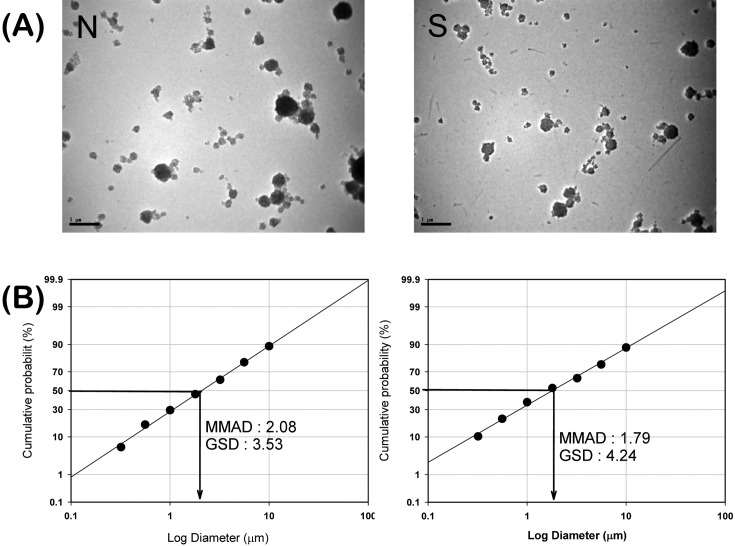
The mass median aerodynamic diameter (MMAD) of carbon black aerosols generated with sonication was not much different from that of aerosols generated without sonication (MMAD for group N and group S were 2.08 μm and 1.79 μm, respectively, Fig. 1B). In contrast, the hydrodynamic diameter of carbon black dispersed in distilled water was distinctly higher in group N than in group S (hydrodynamic diameter for group N and group S were over 5000 nm and approximately 200 nm, respectively, data not shown). Carbon black aerosols generated with and without sonication looked aciniform in TEM, and the agglomerates in the aerosols generated with sonication looked smaller and more uniform than the aerosols without sonication (Fig. 1A). The average concentrations of carbon black during the exposure period for group N and group S were 13.08 ± 3.18 mg/m3 and 13.67 ± 3.54 mg/m3 for a 3 day experiment and 9.83 ± 3.42 mg/m3 and 9.08 ± 4.49 mg/m3 for a 2 week experiment (Table 1).
Fig. 1. Morphologies of transmission electron microscopy (TEM) and particle size distributions of carbon black aerosols in nose-only inhalation chambers. (A) TEM morphologies of carbon black aerosols for group N (without sonication) and group S (with sonication). Images were taken at 10,000x magnification. (B) Particle size distributions of carbon black aerosols in nose-only inhalation chambers.
Table 1.
Concentrations of carbon black in the nose-only inhalation chambers during exposure period Unit : mg/m3
| Experiment period | Group N | Group S |
|---|---|---|
|
| ||
| 3 day | 13.08 ± 3.18 | 13.67 ± 3.54 |
| 2 week | 9.83 ± 3.42 | 9.08 ± 4.49 |
Values presented as mean ± standard deviation. Group N: group of rats exposed to carbon black aerosols without sonication. Group S: group of rats exposed to carbon black aerosols with sonication.
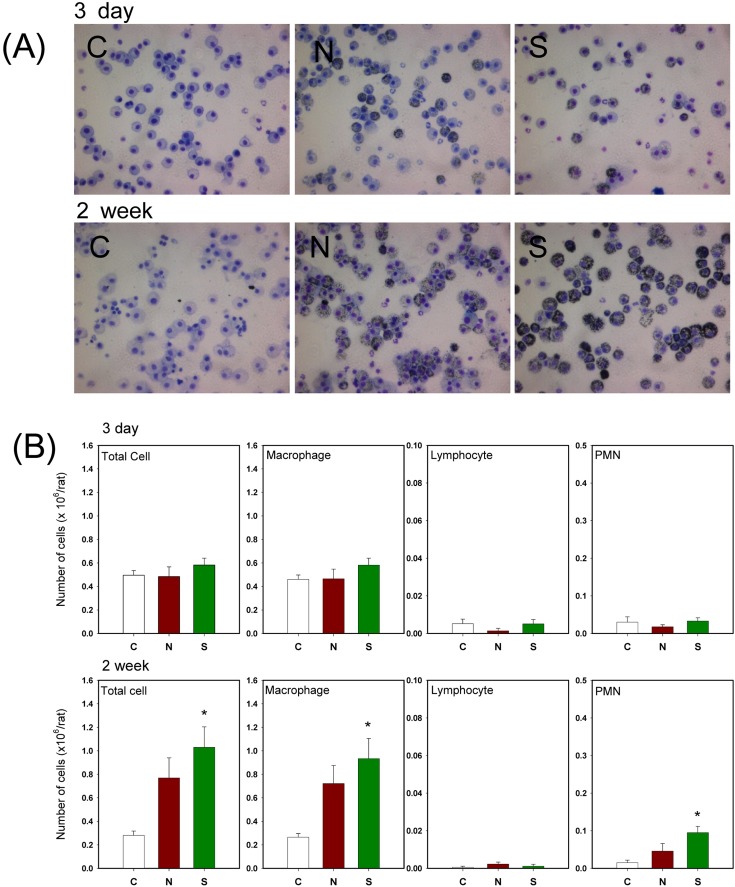
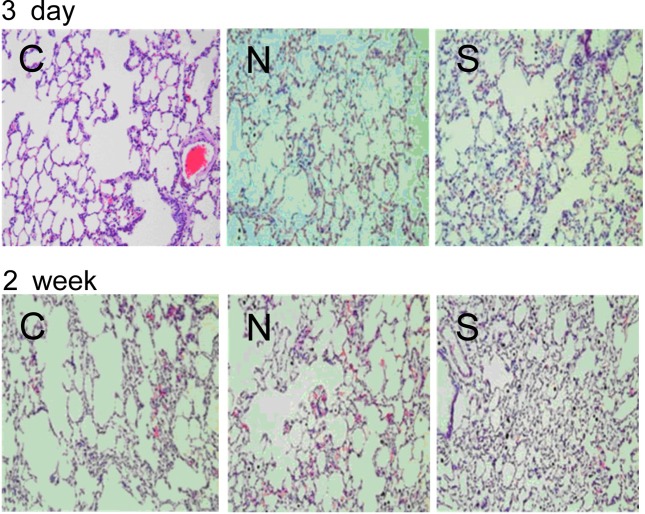
No significant changes in cytokine levels and cell damaging indicator proteins in BAL fluid were found (Table 2) whereas the number of total cells, macrophages, and PMNs exposed to carbon black for 2 weeks was significantly increased (Fig. 2B). The BAL fluid showed more macrophages engulfing carbon black particles in rats exposed to sonicated carbon black (Fig. 2A). White blood cells and neutrophils were also increased in rats exposed to carbon black aerosols generated with sonication for 2 weeks (Table 3). However, no histopathological changes were found in carbon black exposed lungs except for a number of carbon black clumps found in the lung exposed to carbon black (Fig. 3). The number of carbon black clumps in the lungs of group S was higher than in the lungs of group N (Table 4). The concentrations of carbon black in the lungs of group S were also higher than in group N (Table 4): more carbon black was deposited in the lungs of rats exposed to sonicated carbon black. In contrast, the weight changes of lungs due to carbon black exposure were not significant (Table 4).
Table 2.
Effects of carbon black on cytokines and cell damaging indicator proteins in BAL fluid
| Experiment period | Parameters | Control | Group N | Group S |
|---|---|---|---|---|
|
| ||||
| 3 day | IL-1β (pg/ml) | 34.45 ± 0.669 | 34.07 ± 0.614 | 34.03 ± 0.407 |
| TNF-α (pg/ml) | 52.62 ± 29.87 | 38.24 ± 19.16 | 52.83 ± 30.40 | |
| IL-4 (pg/ml) | 23.33 ± 13.70 | 17.66 ± 7.760 | 21.20 ± 15.17 | |
| IL-6 (pg/ml) | 1504 ± 576.1 | 1006 ± 141.0 | 1410 ± 408.2 | |
| INF-γ (pg/ml) | 455.3 ± 316.1 | 247.2 ± 116.7 | 377.0 ± 357.9 | |
| IL-10 (pg/ml) | 225.3 ± 103.4 | 172.2 ± 44.70 | 246.1 ± 122.4 | |
| albumin (mg/dl) | 0.003 ± 0.002 | 0.001 ± 0.001 | 0.002 ± 0.003 | |
| LDH (IU/L) | 37.54 ± 40.69 | 18.28 ± 15.69 | 17.42 ± 7.610 | |
| 2 week | IL-1β (pg/ml) | 33.37 ± 0.288 | 33.32 ± 0.472 | 33.10 ± 0.200 |
| TNF-α (pg/ml) | 96.52 ± 20.81 | 97.33 ± 20.05 | 50.04 ± 9.920 | |
| IL-4 (pg/ml) | 18.15 ± 10.40 | 26.30 ± 8.590 | 10.16 ± 3.560 | |
| IL-6 (pg/ml) | 1351 ± 296.8 | 1599 ± 359.4 | 1066 ± 170.2 | |
| INF-γ (pg/ml) | 250.8 ± 288.8 | 580.1 ± 355.1 | 83.90 ± 60.80 | |
| IL-10 (pg/ml) | 136.3 ± 54.00 | 175.1 ± 34.10 | 102.4 ± 21.70 | |
| albumin (mg/dl) | 0.003 ± 0.001 | 0.001 ± 0.001 | 0.001 ± 0.002 | |
| LDH (IU/L) | 17.43 ± 12.15 | 16.60 ± 12.06 | 31.58 ± 21.25 | |
Values presented as mean ± standard deviation. Group N: group of rats exposed to carbon black aerosols without sonication. Group S: group of rats exposed to carbon black aerosols with sonication.
Fig. 2. Morphologies of leukocytes in BAL fluid and BAL cell differentiation counts. (A) Morphologies of leukocytes in BAL fluid. (B) BAL cell differentiation counts. C: control; N: group N (without sonication); S: group S (with sonication). Error bars indicate the standard error of the mean. * Statistically different from control (p< 0.05).
Table 3.
Effects of carbon black on the blood hematology
| Experiment period | Parameters | Control | Group N | Group S |
|---|---|---|---|---|
|
| ||||
| 3 day | WBC | 5.392 ± 0.712 | 5.132 ± 1.499 | 5.900 ± 2.526 |
| NE | 2.570 ± 0.545 | 2.350 ± 0.762 | 2.804 ± 0.917 | |
| LY | 1.072 ± 0.530 | 0.838 ± 0.428 | 1.412 ± 1.061 | |
| MO | 0.540 ± 0.350 | 0.624 ± 0.231 | 0.398 ± 0.214 | |
| EO | 1.150 ± 0.210 | 1.264 ± 0.427 | 1.204 ± 0.760 | |
| BA | 0.066 ± 0.038 | 0.060 ± 0.012 | 0.082 ± 0.070 | |
| 2 week | WBC | 6.020 ± 0.610 | 6.968 ± 0.408 | 8.388 ± 0.770* |
| NE | 2.858 ± 0.519 | 3.336 ± 0.663 | 4.912 ± 0.922* | |
| LY | 0.892 ± 0.222 | 1.086 ± 0.341 | 0.912 ± 0.126 | |
| MO | 1.072 ± 0.111 | 1.334 ± 0.386 | 1.192 ± 0.262 | |
| EO | 1.140 ± 0.241 | 1.176 ± 0.309 | 1.328 ± 0.270 | |
| BA | 0.058 ± 0.080 | 0.034 ± 0.011 | 0.054 ± 0.027 | |
Values presented as mean ± standard deviation.
* Statistically different from control (p < 0.05). Group N: group of rats exposed to carbon black aerosols without sonication. Group S: group of rats exposed to carbon black aerosols with sonication. WBC: total white blood cell; NE: neutrophile; LY: lymphocyte; MO: monocyte; EO: eosinophile; BA: basophile.
Fig. 3. Histopathology of the lungs exposed to carbon black. C: control; N: group N (without sonication); S: group S (with sonication).
Table 4.
Effects of carbon black on the lung
| Experiment period | Parameters | Control | Group N | Group S |
|---|---|---|---|---|
|
| ||||
| 3 day | Absolute weight (g) | 2.78 ± 0.16 | 2.66 ± 0.20 | 2.70 ± 0.15 |
| Relative weight (g/100 g body weight) | 0.75 ± 0.08 | 0.71 ± 0.06 | 0.72 ± 0.04 | |
| Number of carbon black clumps | ND | 20.20 ± 7.66 | 33.20 ± 9.23 | |
| Concentration of carbon black (mg/g lung) | ND | 1.439 ± 1.571 | 4.551 ± 1.883* | |
| 2 week | Absolute weight (g) | 2.25 ± 1.68 | 2.41 ± 0.20 | 2.38 ± 0.22 |
| Relative weight (g/100g body weight) | 0.74 ± 0.04 | 0.76 ± 0.03 | 0.75 ± 0.08 | |
| Number of carbon black clumps | ND | 22.83 ± 7.65 | 41.83 ± 11.5 | |
| Concentration of carbon black (mg/g lung) | ND | 5.327 ± 2.589 | 13.54 ± 5.666* | |
Values presented as mean ± standard deviation.
* Statistically significant from group N. Group N: group of rats exposed to carbon black aerosols without sonication. Group S: group of rats exposed to carbon black aerosols with sonication. ND, not determined.
DISCUSSION
Carbon black is practically a pure carbon element in the form of colloidal particles generated from incomplete combustion or thermal decomposition of gaseous or liquid hydrocarbons. Its dimension varies from less than 10 nm to more than 300 nm depending on the structural arrangement of carbon elements and it is sometimes regarded as nanomaterials. Nanomaterials are important from toxicity and occupational health perspectives in that they are poorly soluble and relatively low in toxicity, and materials such as carbon black commonly account for severe long-term hazardous effects including tumors and cardiovascular diseases by causing inflammation at high doses (12).
In nanotoxicology, surface area is recognized as one of the major factors in determining the toxicity of nanomaterials (i.e., among shape, size, specific surface properties, and surface area). In this viewpoint, carbon black has been frequently selected as an experimental material to study the role of particle size or surface area due to its various size ranges and rapid aggregation/agglomeration tendency. In this study, Printex 90 was selected as representative nanosized carbon black because the primary particle size (14 nm) is within nano range and the surface area is approximately 300 g/m2.
Much effort has been put to make well-dispersed and less aggregated/agglomerated particles in toxicity studies of PSPs because they practically exist in aggregate/agglomerate form rather than stay in the primary particle size (13-16). Efforts were also made to make well-dispersed and less aggregated/ agglomerated carbon black aerosols to simulate exposure scenarios of nano-sized carbon black in the workplace. Since carbon black particles may exist in small or large agglomerates in the workplace, carbon black aerosols were prepared in two different ways: carbon black dispersions were sonicated or not sonicated before aerosolized. Sonication, which is widely used in nanotoxicology to disrupt inter-particle interactions such as van der Waals force, was used to de-agglomerate carbon black clusters. The hydrodynamic diameter of carbon black in water decreased from size beyond measurable ranges (over 5000 nm) to approximately 200 nm at a total power of 1170 J per 300 ml sonication (i.e., 3.9 J/ml). However, sonication did not make much difference in the mass median aerodynamic diameter (MMAD) of carbon black aerosols (MMAD of carbon black dispersion with sonication was 1.79 μm and 2.08 μm without sonication). The shapes of carbon black agglomerates also looked similar in both aerosols generated with or without sonication in a picture taken by TEM (10,000x magnification): the only difference was that thick and dense lumps of carbon black were found more in aerosols generated without sonication. It is presumed that many large agglomerates in the carbon black dispersion prepared without sonication were eliminated upon passing through the orifice. The size of aerosols generated in this study was similar to that of aerosols in previous studies (Driscoll et al., 0.88 μm (17); Elder et al., 1.2~2.4 μm (18); and Carter et al., 1.2~1.6 μm (19)).
To verify the toxicity of carbon black, macrophage and PMN infiltration into the alveolar spaces were measured as an indicator of inflammation. There were no significant differences in inflammatory cell infiltration into the alveolar space of rats from both group S and group N in a 3 day exposure experiment. However, BAL fluids from group S in a 2 week exposure experiment showed increased inflammatory cell infiltration (total cells, macrophages and PMNs were significantly increased). The number of white blood cells and neutrophils was also significantly increased only in rats exposed to sonicated carbon black for 2 weeks. In contrast, there were no significant differences in cytokine levels and cell damage indicators in the BAL fluid in both 3 day exposure and 2 week exposure experiment.
The most crucial results were found in the lung burden of carbon black. Deposition of carbon black was significantly higher in the lungs exposed to carbon black aerosols generated with sonication than in the lungs exposed to aerosols generated without sonication. The number of carbon black clumps in the lung was also higher in group S than in group N. In contrast, there were no significant differences in the lung weight or in the lung histology. It is not certain what caused the higher deposition of carbon black in the lungs exposed to carbon black aerosolized with sonication. It might be because large clumps of carbon black that were not eliminated during aerosolization were eliminated in the upper airway of rats. Or, less agglomerated carbon black can be easily engulfed or absorbed by macrophages or epithelial cells in the lung. The results suggest that less agglomerated carbon black is deposited more and has higher toxicity to the carbon black exposed rats.
In summary, less agglomerated nano-sized carbon black shows more lung inflammatory effects than more agglomer-ated carbon black, which was mainly due to more deposition of the lung.
Acknowledgments
The authors thank Dr. Jenny Roberts and Dr. Vincent Castranova at NIOSH in Morgantown, WV, USA for their helpful discussions and for providing carbon black, Printex 90.
References
- 1.NSET. The National nanotechnology Initiative. Research and Development Leading to a Revolution in Technology and Industry Supplement to the Present’s FY 2011 Budget. Subcommittee on nanoscale Science, Engineering and Technology, Committee on Technology, National Science and Technology Council; Washington, DC: (2010). pp. 1–65. [Google Scholar]
- 2.Ferin J., Oberdoster G., Penney D.P., Soderholm S.C., Gelein R., Piper H.C. Increased pulmonary toxicity of ultrafine particles 1. Particle clearance, translocation, morphology. J. Aerosol Sci. (1990);21:381–384. doi: 10.1016/0021-8502(90)90064-5. [DOI] [Google Scholar]
- 3.Oberdorster G., Ferin J., Finkelstein G., Wade P., Corson N. Increased pulmonary toxicity of ultrafine particles. 2. Lung lavage studies. J. Aerosol Sci. (1990);21:384–387. [Google Scholar]
- 4.Maynard A.D., Warheit D.B., Philbert M.A. The new toxicology of sophisticated materials: Nanotoxicology and beyond. Toxicol. Sci. (2011);120:S109–S129. doi: 10.1093/toxsci/kfq372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madl A.K., Pinkerton K.E. Health effects of inhaled engineered and incidental nanoparticles. Crit. Rev. Toxicol. (2009);39:629–658. doi: 10.1080/10408440903133788. [DOI] [PubMed] [Google Scholar]
- 6.Castranova V. Overview of current toxicological knowledge of engineered nanoparticles. J. Occup. Environ. Med. (2011);53:S14–S17. doi: 10.1097/JOM.0b013e31821b1e5a. [DOI] [PubMed] [Google Scholar]
- 7.Zook J.M., MacCuspie R.I., Locascio L.E., Halter M.D., Elliott J.T. Stable nanoparticle aggregates/agglomerates of different sizes and the effect of their size on hemolytic cytotoxicity. Nanotoxicology. (2011);5:517–530. doi: 10.3109/17435390.2010.536615. [DOI] [PubMed] [Google Scholar]
- 8.Stefaniak A.B., Turk G.C., Dickerson R.M., Hoover M.D. Size-selective poorly soluble particulate reference materials for evaluation of quantitative analytical methods. Anal. Bioanal. Chem. (2008);391:2071–2077. doi: 10.1007/s00216-008-1870-x. [DOI] [PubMed] [Google Scholar]
- 9.Kleinstreuer C., Zhang Z., Kim C.S. Combined inertial and gravitational deposition of microparticles in small model airways of a human respiratory system. J. Aerosol Sci. (2007);38:1047–1061. doi: 10.1016/j.jaerosci.2007.08.010. [DOI] [Google Scholar]
- 10.OECD. Guidance on sample preparation and dosimetry for the safety testing of manufactured nanomaterials. OECD Environment, Health and Safety Publications; Paris: (2012). pp. 69–93. [Google Scholar]
- 11.Lim C.H., Kang M., Han J.H., Yang J.S. Effect of agglomeration on the toxicity of nano-sized carbon black in Sprague-Dawley rats. Environ. Health Toxicol. (2012);27:e2012015. doi: 10.5620/eht.2012.27.e2012015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Carbon Black Association. What is Carbon Black. Hebei LongHao Chemical Technology Co., LTD, Shandong; (2012). p. 1. [Google Scholar]
- 13.Porter D., Sriram K., Wolfarth M., Jefferson A., Schwegler-Berry D., Andrew M.E., Castranova V. A biocompatible medium for nanoparticle dispersion. Nanotoxicology. (2008);2:144–154. doi: 10.1080/17435390802318349. [DOI] [Google Scholar]
- 14.Kim S.C., Chen D.R., Qi C., Gelein R.M., Finkelstein J.N., Elder A., Bentley K., Oberdörster G., Pui D.Y. A nanoparticle dispersion method for in vitro and in vivo nanotoxicity study. Nanotoxicology. (2010);4:42–51. doi: 10.3109/17435390903374019. [DOI] [PubMed] [Google Scholar]
- 15.Sager T.M., Porter D.W., Robinson V.A., Lindsley W.G., Schwegler-Berry D.E., Castranova V. Improved method to disperse nanoparticles for in vitro and in vivo investigation of toxicity. Nanotoxicology. (2007);1:118–129. doi: 10.1080/17435390701381596. [DOI] [Google Scholar]
- 16.Bihari P., Vippola M., Schultes S., Praetner M., Khandoga A.G., Reichel C.A., Coester C., Tuomi T., Reberg M., Krombach F. Optimized dispersion of nanoparticles for biological in vitro and in vivo studies. Part. Fibre Toxicol. (2008);5:14. doi: 10.1186/1743-8977-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Driscoll K.E., Carter J.M., Howard B.W., Hassenbein D.G., Pepelko W., Baggs R.B., Oberdörster G. Pulmonary inflammatory, chemokine, and mutagenic responses in rats after subchronic inhalation of carbon black. Toxicol. Appl. Pharmacol. (1996);136:372–380. doi: 10.1006/taap.1996.0045. [DOI] [PubMed] [Google Scholar]
- 18.Elder A., Gelein R., Finkelstein J.N., Driscoll K.E., Harkema J., Oberdorster G. Effects of subchronically inhaled carbon black in three species. 1. Retention kinetics, lung inflammation, and histopathology. Toxicol Sci. (2005);88:614–629. doi: 10.1093/toxsci/kfi327. [DOI] [PubMed] [Google Scholar]
- 19.Carter J.M., Corson N., Driscoll K.E., Elder A., Finkelstein J.N., Harkema J.N., Gelein R., Wade-Mercer P., Nguyen K., Oberdorster G. A comparative doserelated response of several key pro- and antiinflammatory mediators in the lungs of rats, mice, and hamsters after subchronic inhalation of carbon black. J. Occup. Environ. Med. (2006);48:1265–1278. doi: 10.1097/01.jom.0000230489.06025.14. [DOI] [PubMed] [Google Scholar]