Abstract
The potential role of topical valproate (VPA) in hair regrowth has been recently suggested. However, safety reports of VPA as a topical formulation are lacking. Therefore, in the present study, we investigated whether VPA causes skin irritation in humans. We first performed a cell viability test and showed that VPA did not exhibit toxicity toward HaCaT keratinocytes, fibroblasts, and RBL-3H mast cells. We then performed clinical patch test and skin irritation test through transdermal drug delivery with the help of microneedle rollers. No significant findings were obtained in the clinical patch test. In the skin irritation test, only 1 patient showed erythema at 1 hr, but the irritation reaction faded away within a few hours. Erythema and edema were not observed at 24 hr. We concluded that VPA has minimal potential to elicit skin irritation. Therefore, we consider that VPA can safely be applied to human skin.
Keywords: Valproate, Skin toxicity
INTRODUCTION
Valproic acid (VPA) is a mood stabilizer that has been commonly prescribed for the treatment of epilepsy and bipolar disorder over the last several decades (1). Antiepileptic drugs including VPA frequently cause cutaneous eruptions, especially during initiation of new therapy. In addition to causing common and usually limited morbilliform and urticarial eruptions, which have often mild morbidity, antiepileptic drugs can also induce widespread maculopapular rash, hypersensitivity syndrome, psoriatic dermatitis and occasionally severe reactions such as Steven Johnson’s syndrome, toxic epidermal necrolysis and erythema multiforme (2,3).
In recent studies using animal models, topical application of VPA stimulated hair re-growth (4). In an in vitro study, VPA up-regulated the Wnt/β-catenin pathway and alkaline phosphatase activity in human dermal papilla cells (4). Activation of the Wnt/β-catenin pathway is required for initiation of hair follicle formation (5), and stimulates growth and differentiation of hair by maintaining expression of genes that function at the anagen phase of the hair cycle (6). Consequently, it was suggested that VPA could be developed as a topical drug to stimulate hair re-growth (4), but there are currently no studies on the safety of VPA for skin application. The aim of the present study was to investigate whether VPA exhibits toxicity towards skin. We tested the toxicity of VPA in keratinocytes, fibroblasts and mast cells and performed skin tests in healthy human subjects using a clinical patch test and skin irritation tests through transdermal drug delivery using microneedle rollers.
MATERIALS AND METHODS
Materials. Twenty healthy volunteers aged 21 to 43 years (mean age 30.4) were enrolled for primary skin irritation and toxicity tests. Individuals were excluded if they had current or past history of underlying chronic skin diseases that might interfere with the evaluation of skin reactions. We used sodium valproate (95 mg/ml) and normal saline (as a negative control) for skin tests.
The study was approved by the relevant institutional review boards. All subjects signed informed consent forms, and the study protocol conformed to the guidelines set forth by the Declaration of Helsinki and Korean Good Clinical Practice.
Cell culture. Human HaCaT keratinocytes were obtained from the Korean Cell Line Bank (Seoul). Cells were incubated in DMEM supplemented with 10% FBS, 1 mM sodium pyruvate, 50 μg/ml streptomycin and 50 μg/ml penicillin at 37℃ in 5% CO2.
Determination of cell viability. Human HaCaT keratinocytes were seeded in a 24-well plate at a density of 4 × 105 cells per well and fibroblasts were seeded at 5 × 104 cells per well. After overnight incubation, cells were treated with various concentrations of VPA ranging from 0.1 μM to 100 μM and incubated in a 37℃ CO2 incubator for 24 hr. The percentage of viable human HaCaT keratinocyte cells and fibroblast cells was determined by staining the cells with MTT. The culture medium was removed from each well, and the cells were washed with phosphate-buffered saline (PBS) and treated with the MTT staining solution. After incubation in a 37℃ CO2 incubator for 3 hr, the medium was replaced with 1 ml DMSO and the absorbance was measured at 570 nm. Cytotoxicity towards RBL- 2H3 mast cells was measured using the cell counting kit-8 (CCK-8) assay (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s instructions. RBL-2H3 cells were seeded in a 96-well plate at a density of 2.5 × 105 cells per well. After overnight incubation, cells were treated with various concentrations of VPA ranging from 0.1 μM to 100 μM. Absorbance was measured at 450 nm.
Clinical patch test. A patch test was performed on the skin of all subjects. The test samples (0.2 ml of VPA or normal saline) were placed in a Finn Chamber (Chemotechnique Diagnostics, Sweden) and applied to the ventral side of the upper arm of each subject for 48 hrs in an occlusive condition. Skin reactions were evaluated at 1 hr and 48 hrs after removing the test samples. The reaction was evaluated according to the International Contact Dermatitis Research Group (ISDRG) standard (Table 1) (7).
Table 1.
Assessment of patch test reactions
| Grading | Description of response |
|---|---|
|
| |
| 0 | No reaction |
| + | Weakly positive reaction (usually characterized by mild erythema or dryness across most of the treatment site) |
| ++ | Moderately positive reaction (usually distinct erythema possibly spreading beyond the treatment site) |
| +++ | Strongly positive reaction (strong, often spreading erythema with edema) |
Skin irritation test. The skin irritation test was performed on three skin test sites of all subjects: frontal scalp, upper arm and upper back. We used the method of transdermal drug delivery by microneedle rollers to perform the skin irritation test. The microneedle rollers used in this study were supplied by DTS Lab Inc. (Disk Microneedles Therapy System Lab, Inc., Seoul, South Korea). The microneedle roller was 0.25 mm long and had 60 circular arrays of nine needles each (a total of 540 needles) in a cylindrical assembly (20-mm diameter, 18.2-mm length). The test samples (2.0 ml of VPA or normal saline) were applied to a 2 cm × 4 cm area of each test site and the microneedle roller was rolled four times on the skin test sites. Skin irritation test sites were examined for the presence of erythema and edema according to the dermal irritation scoring system (Table 2) at 1 hr and 24 hrs (8).
Table 2.
Scoring criteria for dermal reactions
| Evaluation of dermal reactions | |||
|---|---|---|---|
|
| |||
| Erythema and eschar formation | Edema formation | ||
|
| |||
| 0 | No erythema | 0 | No erythema |
| 1 | Very slight erythema (barely perceptible, edges of area not well defined) | 1 | Very slight edema (barely perceptible, edges of area not well defined) |
| 2 | Slight erythema (pale red in color and edges definable) | 2 | Slight edema (edges of area well defined by definite raising) |
| 3 | Moderate to severe erythema (defined in color and area well defined) | 3 | Moderate edema (raised approximately 1 mm) |
| 4 | Severe erythema (beet to crimson red) to slight eschar formation (deep injuries) | 4 | Severe edema (raised more than 1 mm and extending beyond area of exposure) |
RESULTS AND DISCUSSION
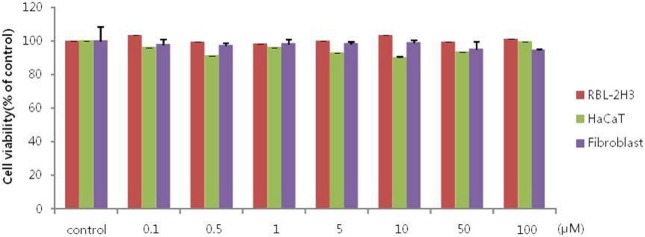
We performed cell viability tests for VPA in HaCaT keratinocytes, fibroblasts, and RBL-3H mast cells. After treatment with various concentrations of VPA, cell toxicity was not observed in RBL-2H3, HaCaT or fibroblast cells (Fig. 1).
Fig. 1. Viability of RBL-2H3 cells, HaCaT keratinocytes and fibroblasts treated with VPA.
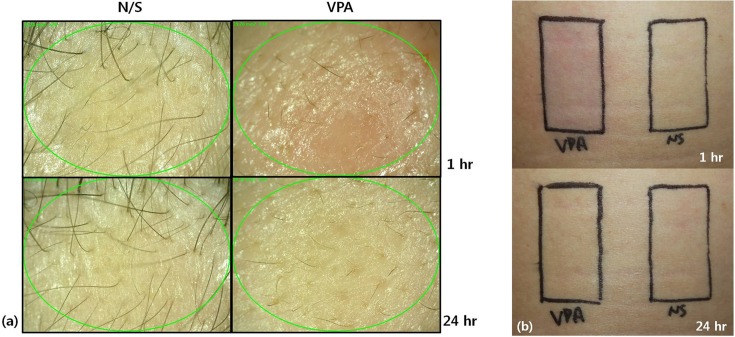
We next performed a clinical patch test on 20 healthy volunteers. No significant clinical findings were observed at 1 hr and 48 hrs after removing the test samples. A skin irritation test was also performed by transdermal drug delivery on 20 healthy volunteers. One patient appeared to have barely perceptible erythema effects on the frontal scalp and upper arm at 1 hr, but there were no erythema or edema effects in any of the patients at 24 hrs (Table 3). The time course of recovery from irritation is depicted in Fig. 2. Erythema faded away quickly and the skin returned to normal within a short period.
Table 3.
Results of skin reactions
| Skin irritation test | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Frontal scalp | Upper arm | Upper back | |||||
|
| |||||||
| Erythema | Edema | Erythema | Edema | Erythema | Edema | ||
|
| |||||||
| Patient | Sex/Age | 1 hr | 24 hr | 1 hr | 24 hr | 1 hr | 24 hr |
|
| |||||||
| 1 | M/41 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | M/42 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | M/43 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | M/35 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | M/36 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | M/39 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | M/37 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | M/32 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | M/42 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | M/21 | 1 | 0 | 0 | 0 | 1 | 0 |
| 11 | M/23 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | M/24 | 0 | 0 | 0 | 0 | 0 | 0 |
| 13 | M/23 | 0 | 0 | 0 | 0 | 0 | 0 |
| 14 | M/23 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15 | M/25 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | M/22 | 0 | 0 | 0 | 0 | 0 | 0 |
| 17 | M/22 | 0 | 0 | 0 | 0 | 0 | 0 |
| 18 | M/31 | 0 | 0 | 0 | 0 | 0 | 0 |
| 19 | M/24 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 | M/23 | 0 | 0 | 0 | 0 | 0 | 0 |
Fig. 2. Clinical signs of skin treated with valproate in patient No. 10. Slight erythema was observed at 1 hr after skin application, but there was no erythema or edema at 24 hrs after application. N/S: normal saline, VPA: valproic acid. (a) Frontal scalp, (b) upper back.
In our study, the skin irritation test was performed not by simple application, but by transdermal drug delivery using microneedle rollers. Therefore, the cause of the erythema that occurred in one patient could be irritation by the microneedles. Microneedle treatment offers a minimally invasive and painless route for transdermal drug application (9) but it causes superficial barrier-related skin damage. However, it has been reported that prompt recovery of barrier function occurs within 72 hrs after microneedle treatment (10).
The potential utility of VPA in the treatment of androgenetic alopecia was recently suggested. Animal studies and in vitro studies revealed that topical VPA stimulated hair regrowth (4). However, the potential of topical VPA for hair re-growth in humans had not been studied in vivo. This is the first safety study of VPA in human skin and we conclude that VPA has minimal potential to elicit an irritation reaction. We therefore believe that VPA can be safely applied to human skin.
References
- 1.Reynolds M.F., Sisk E.C., Rasgon N.L. Valproate and neuroendocrine changes in relation to women treated for epilepsy and bipolar disorder: a review. Curr. Med. Chem. (2007);14:2799–2812. doi: 10.2174/092986707782360088. [DOI] [PubMed] [Google Scholar]
- 2.Tennis P., Stern R.S. Risk of serious cutaneous disorders after initiation of use of phenytoin, carbamazepine, or sodium valproate: a record linkage study. Neurol. . (1997);49:542–546. doi: 10.1212/WNL.49.2.542. [DOI] [PubMed] [Google Scholar]
- 3.Roujeau J.C., Kelly J.P., Naldi L., Rzany B., Stern R.S., Anderson T., Auquier A., Bastuji-Garin S., Correia O., Locati F., et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N. Engl. J. Med. (1995);333:1600–1607. doi: 10.1056/NEJM199512143332404. [DOI] [PubMed] [Google Scholar]
- 4.Lee S.H., Yoon J., Shin S.H., Zahoor M., Kim H.J., Park P.J., Park W.S., Min do S., Kim H.Y., Choi K.Y. Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. PLoS One. (2012);7:e34152. doi: 10.1371/journal.pone.0034152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andl T., Reddy S.T., Gaddapara T., Millar S.E. WNT signals are required for the initiation of hair follicle development. Dev. Cell. (2002);2:643–653. doi: 10.1016/S1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- 6.Kishimoto J., Burgeson R.E., Morgan B.A. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. (2000);14:1181–1185. [PMC free article] [PubMed] [Google Scholar]
- 7.Prausnitz M.R. Microneedles for transdermal drug delivery. Adv. Drug Delivery Rev. (2004);56:581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Draize J.H. The Appraisal of the Safety of Chemicals in Foods, Drugs and Cosmetics, Dermal Toxicity. Association of Food and Drug Officials of the US; Topeka, KA: (1965). pp. 46–59. [Google Scholar]
- 9.Lachapelle J.M. A proposed relevance scoring system for positive allergic patch test reactions: practical implications and limitations. Contact Dermatitis. (1997);36:39–43. doi: 10.1111/j.1600-0536.1997.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 10.Han T.Y., Park K.Y., Ahn J.Y., Kim S.W., Jung H.J., Kim B.J. Facial skin barrier function recovery after microneedle transdermal delivery treatment. Dermatol. Surg. (2012);38:1816–1822. doi: 10.1111/j.1524-4725.2012.02550.x. [DOI] [PubMed] [Google Scholar]