Abstract
This study was carried out to investigate the short term toxicity of nine phthalate diesters including di-2 (ethylhexyl) phthalate (DEHP) , di (n-butyl) phthalate (DBP) , di-n-octyl phthalate (DnOP) , diethyl phthalate (DEP) , butylbenzyl phthalate (BBP) , dimethyl phthalate (DMP) , di-isodecyl phthalate (DIDP) , diundecyl phthalate (DUP) , and di-isononyl phthalate (DINP) and five phthalate monoesters including mono- (2-ethylhexyl) phthalate (MEHP) , monobutyl phthalate (MBuP) , monobenzyl phthalate (MBeP) , monoethyl phthalate (MEP) , monomethyl phthalate (MMP) and phthalic acid (PA) in Sprague-Dawley male rats. Animals were administered 250 mg/kg/day (monoesters and PA) or 500 mg/kg/day (diesters) of phthalate for two weeks. All animals were examined for body and organ weights, blood hematology, serum biochemistry, and urine analysis. The body weight gain was significantly lower in rats treated with BBP, DBP, DINP, MEHP, MBuP, and PA than that of control. Liver weights were significantly increased in the DEHP,DBP, DnOP, DIDP, and MEHP groups as compared to the control group. Testes weights were significantly decreased only in the DEHP-, DnOP-, and DIDP-treated groups as compared to the control. Significant differences in hematological changes were not observed in any treatment groups. Significant increases in blood glucose levels were observed in the DEHP, MEHP, and MBeP groups. Aspartate aminotransferase (AST) levels were significantly increased in the DBP, DUP, DINP, MBuP, and MBeP groups, whereas alanine aminotransferase (ALT) levels were significantly increased only in the DEHP and MEHP groups. Serum ALP levels were significantly higher in phthalate diester (500 mg/kg/day) -treated rats as compared to control. However, the total cholesterol level was significantly reduced in the DEHP- and DIDP-treated groups, whereas serum triglyceride (TG) levels were higher in the DINP-, MEHP-, and MBuP-treated groups. These results suggest that short term toxicity of phthalate monoesters produces adverse effects as similar to phthalate diesters in Sprague-Dawley rats.
Keywords: Phthalate diesters, Phthalate monoesters, Phthalic acid, Toxicity, Hematological changes
INTRODUCTION
Phthalate esters (PEs) are widely used as plasticizers in commercial products for polyvinyl chloride (PVC) (Autian, 1973). Despite the recent scientific interest on the potential human effects of phthalates, their toxicities have not been clearly characterized. An important concern about phthalate esters relates to their potential to act as reproductive toxicans in rodent species. This compound causes apoptosis and the loss of spermatogenic cells, resulting in testicular atrophy (Li et al., 2000; Ryu et al., 2007; Thomas et al., 1978). For example, the perinatal exposure to di (2-ethylhexyl) phthalate (DEHP) or di (n-butyl) phthalate (DBP) inhibits the production of testicular testosterone in the fetus. These compounds dmasculinizes the males in that they display a reduced anogenital distance (AGD) , retained nipples, a cleft phallus with a hypospadias, undescended testes, a vaginal pouch, epididymal agenesis, and small to absent sex accessory glands as adults (Gray et al., 2000; Kim et al., 2004; Park et al., 2002).
Initial experiments implicated only phthalate diester-mediated toxicity, but the toxicity of phthalate monoester, a primary metabolite, was not assessed. The phthalate monoesters are formed as metabolites from the phthalate diester by various tissue enzymes in mammals (Daniel and Bratt, 1974; Peck et al., 1981; Pollack et al., 1989). DEHP administered to rodents is metabolized to mono (2-ethylhexyl) phthalate (MEHP) and 2-ethylhexanol as the major metabolites (Lhuguenot et al., 1985), and the butylbenzyl phthalate (BBP) administered to rats is metabolized to monobenzyl phthalate (MBeP) and monobutyl phthalate (MBuP) (Eigenberg et al., 1986; Nativelle et al., 1999). MBuP is also formed as a major metabolite in rats given DBP (Albro and Moore, 1974; Tanaka et al., 1978). In addition, dimethyl phthalate (DMP) and diethyl phthalate (DEP) are metabolized to their corresponding monoesters, monomethyl phthalate (MMP) and monoethyl phthalate (MEP) (Okubo et al.,2003).
In order to examine the adverse effects of PEs on human health, the urinary excretion levels of phthalate monoesters were measured (Blount et al., 2000). Although PEs did not show any genotoxicity, there is some evidence suggesting that they can cause systemic toxicity at high concentrations (Fisher, 2004; Gangolli, 1982; Rhee et al., 2002). It has been demonstrated that the reproductive toxicity observed in animals exposed to certain PEs may be due in part to estrogenic or anti-androgenic effects (Gotz et al., 2001; Shono and Suita, 2003; Zacharewski, 1998). However, the short term toxicity of various PEs, including DEHP, DBP, or BBP as well as their monoesters, MEHP, MBuP or MBeP, have yet to be evaluated in their comparative toxicity.
The present study investigated the comparative short term toxicity of nine phthalate diesters in Sprague-Dawley rats, which are commonly present in the environment, five phthalate monoesters and PA, which can be derived from the diesters.
MATERIALS AND METHODS
Chemicals.
Phthalate diesters including DEHP, DBP, di-n-octylphthalate (DnOP) , DEP, BBP, DMP, di-isodecyl phthalate (DIDP) , diundecyl phthalate (DUP) , and diisononyl phthalate (DINP) and phthalate monoesters including MEHP, MBuP, MBeP, MEP, mono-methyl phthalate (MMP) , and PA were purchased from Sigma (St. Louis, MO, USA) , Aldrich (Milwaukee, WI, USA) , Fluka (Steinheim, Germany) , Wako (Osaka, Japan) or Tokyo Kasei Kogyo Co. (TKK, Tokyo, Japan) , respectively.
Experimental animals and treatments.
Sprague-Dawley male rats (at four weeks) were purchased from Charles River Laboratories (Japan) and housed under specific pathogen-free (SPF) conditions. They were housed in clear polycarbonate cages with wood chips for bedding and given a pellet rodent diet and water ad libitum. The animals were maintained under controlled conditions (23 ± 3℃, humidity 55 ± 5%, ventilation frequency ≥ 15 cycles/hr, and under a 12 hr light/dark cycle) , and they were examined for any clinical signs and weighed at predetermined intervals. After one week of acclimatization, animals were randomly allocated to groups based on their body weight. As shown in Fig. 1, the phthalate diesters (500 mg/kg bw/day) , phthalate monoesters and PA (250 mg/kg bw/day) were administered by an oral gavage. The control group was given the vehicle (corn oil) orally. Experimental protocols were approved by the institutional animal ethics committee of Korea Food and Drug Administration.
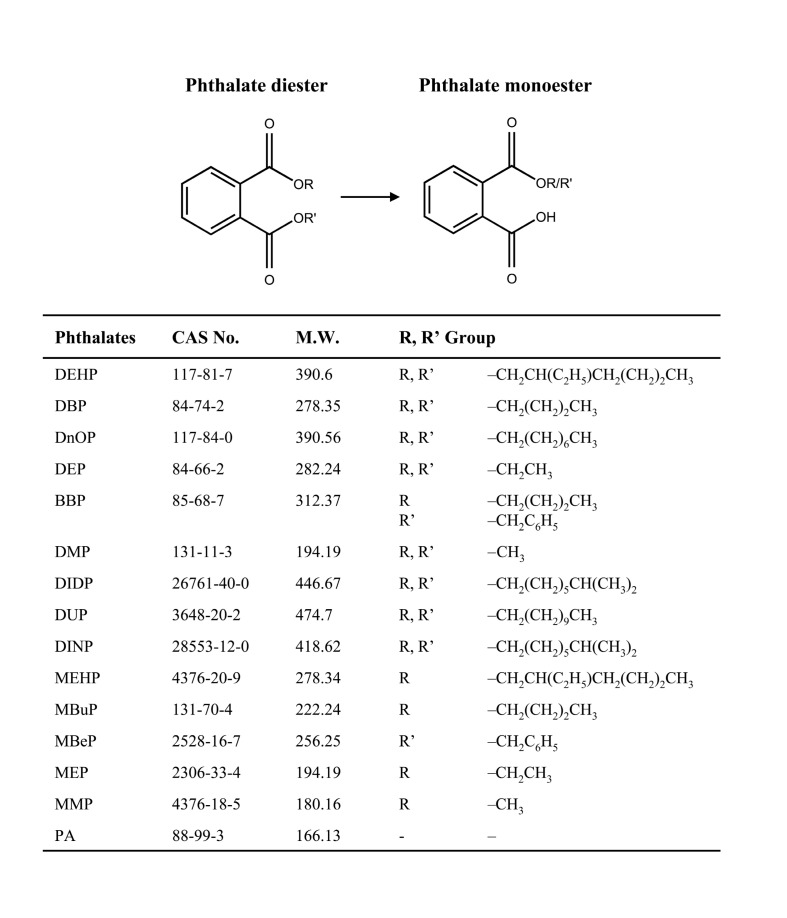
Fig. 1. The used PEs and metabolites used in the present study. The alkyl- or alkylaryl-diesters are hydrolyzed to form monoesters.
Clinical signs, mortality, dietary consumption, and body and organ weights.
The animals were observed thoroughly for the onset of any immediate toxic signs and were examined throughout the observation period to record any delayed acute effects and mortality. The animals were observed once each day after administering the test chemicals for four weeks. All of the rats were weighed on days 0, 3, 6, 9, 12, and 14 (two-week repeated study) . The mean body weight was calculated in the animals that survived up to the end of the period. The dietary consumption in the groups was measured at the beginning of the treatment and twice a week during the two weeks. Under anesthesia, the following vital organs including the heart, lung, liver, kidney, adrenal glands, spleen, thymus, thyroid glands, testes and epididymides were weighed, and the organ-to-body weight ratios were calculated.
Blood and urine analysis.
During the autopsy, the whole blood taken from all of the rats in each group was allowed to clot, the serum was separated and the blood samples for hematological analysis were collected into CBC bottles containing EDTA. The red blood cell (RBC) count, hemoglobin (Hb) concentration, hematocrit (Ht) , mean corpuscular volume (MCV) , mean corpuscular hemoglobin (MCH) , mean corpuscular hemoglobin concentration (MCHC) , platelet (PLT) count and white blood cell (WBC) count were determined using a Coulter counter T-540 (Coulter Counter Electronics, USA) . For serum biochemistry analysis, blood samples were centrifuged at 3000 rpm for 10 min within 1 h after collection. The sera were stored at -80℃ freezer before analysis. The levels of serum biochemistry parameters including calcium, potassium, sodium, albumin, blood urea nitrogen (BUN) , cholesterol, triglyceride (TG) , creatinine, glucose, total cholesterol, total bilirubin, total protein,alkaline phosphatase (ALP) , aspartate aminotransferase (AST) , alanine aminotransferase (ALT) , γ-gluamyl transferase (GGT) and creatine phosphokinase (CPK) were measured using an autoanalyzer, model Hitachi 7170 (Hitachi, Tokyo, Japan) .For urine analysis, six rats from each group were placed in urine-collection cages without food and water, and the 12-h urine outputs were collected. The occult blood, pH, protein, urobilinogen, glucose, nitrite, bilirubin, ketone bodies, leukocytes and urine specific gravity were measured using a Multi-Stick (Miles-Sankyo Co., Tokyo, Japan) and with an Atago Serum Protein Refractometer N (Atago Co., Tokyo, Japan) .
Statistical analysis.
The quantitative differences between the control and treatment groups in terms of the body and organ weights, sperm counts and motility, blood hematology, serum biochemistry and urine parameters were analyzed by a one-way ANOVA. A Dunnett’s test (P < 0.05) was applied for a pair-wise comparison between the control and treatment groups.
RESULTS
Clinical signs, mortality, body weight and food consumption.
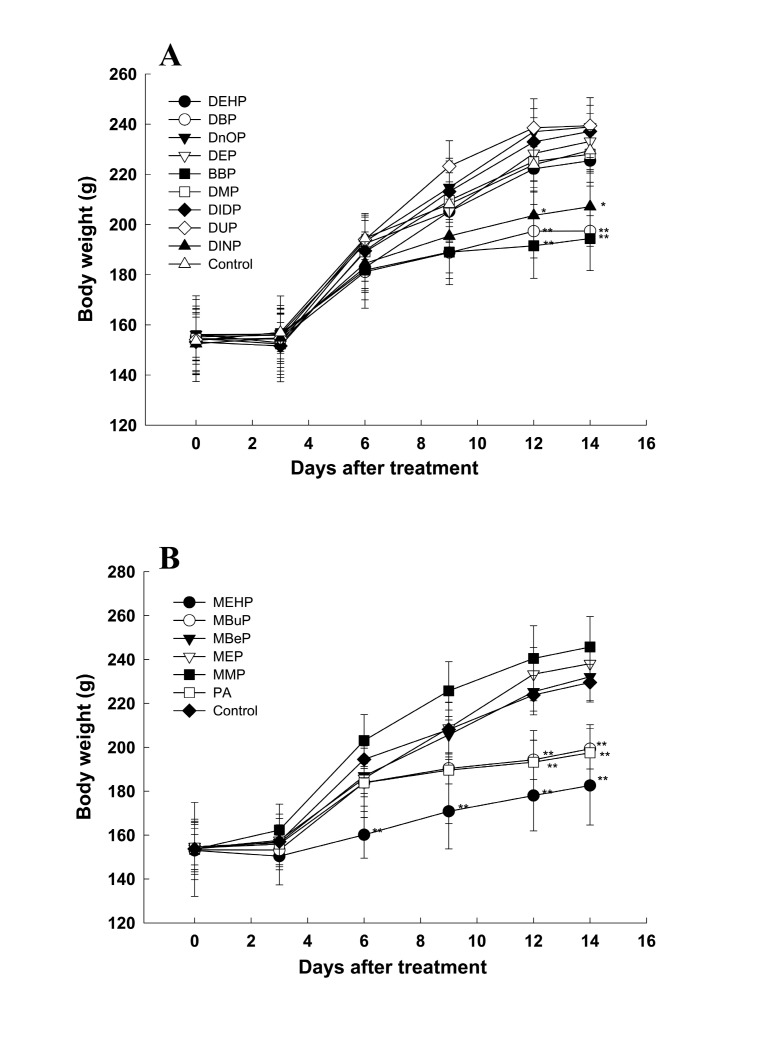
No animals died during the PE administration period until two weeks (data not shown) . Salivation was observed immediately after administering most PEs in all animals (data not shown) . The body weights of animals in the DBP-, DINP-, BBP-, MEHP-, MBuP- and PA-treated groups were significantly decreased after the two-week administration periods (Fig. 2) , whereas there were no significant differences in food consumption between the treatment groups and control throughout the study (data not shown) .
Fig. 2. The body weight changes following the administration of the vehicle control (corn oil) and phthalate esters (diesters, 500mg/kg bw/day; monoesters, 250mg/kg bw/day) to Sprague-Dawley male rats for two weeks (A, phthalate diesters; B, phthalate monoesters) . The data is expressed as the mean ± SD, as calculated for ranges of five to six rats. * Significantly different from the control group at P < 0.05. ** Significantly different from the control group at P < 0.01.
Relative organ weights.
The relative liver weights in the DEHP-, DBP-, DnOP- and DIDP-treated groups were significantly higher than those in the controls. The relative testes weights were significantly decreased in the DEHP-,DnOP-, DIDP- and DUP-treated groups but not in any other PEs. The thymus weights were significantly lower than the controls in the MEHP-treated group only. There were no significant differences in the relative weights of the thyroid, lung, heart, spleen, kidney, adrenal gland and epididymis in any of the PE groups (Table 1) .
Table 1.
Relative organ weights of Sprague-Dawley rats orally treated with phthalate esters for 2 weeks
| Thyroid | Lung | Thymus | Heart | Liver | Spleen | Kidney (paired) | Adrenal (paired) | Testis (paired) | Epididymis (paired) | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| CONa | 0.006 ± 0.001 | 0.58 ± 0.26 | 0.20 ± 0.07 | 0.37 ± 0.01 | 2.84 ± 0.07 | 0.26 ± 0.03 | 0.38 ± 0.02 | 0.010 ± 0.003 | 0.759 ± 0.084 | 0.103 ± 0.012 |
| DEHP | 0.006 ± 0.002 | 0.55 ± 0.05 | 0.25 ± 0.04 | 0.41 ± 0.05 | 4.45 ± 0.39** | 0.29 ± 0.03 | 0.40 ± 0.02 | 0.010 ± 0.003 | 0.676 ± 0.065* | 0.104 ± 0.013 |
| DBP | 0.007 ± 0.001 | 0.49 ± 0.04 | 0.19 ± 0.04 | 0.39 ± 0.04 | 3.19 ± 0.15* | 0.27 ± 0.02 | 0.37 ± 0.01 | 0.013 ± 0.003 | 0.672 ± 0.044 | 0.107 ± 0.009 |
| DnOP | 0.007 ± 0.002 | 0.50 ± 0.03 | 0.25 ± 0.05 | 0.39 ± 0.03 | 3.32 ± 0.32* | 0.26 ± 0.03 | 0.38 ± 0.02 | 0.009 ± 0.003 | 0.627 ± 0.050* | 0.103 ± 0.015 |
| DEP | 0.007 ± 0.002 | 0.52 ± 0.06 | 0.22 ± 0.02 | 0.40 ± 0.06 | 3.13 ± 0.15 | 0.29 ± 0.03 | 0.38 ± 0.03 | 0.010 ± 0.002 | 0.635 ± 0.020 | 0.103 ± 0.007 |
| BBP | 0.006 ± 0.002 | 0.59 ± 0.07 | 0.19 ± 0.08 | 0.40 ± 0.02 | 3.14 ± 0.22 | 0.29 ± 0.03 | 0.40 ± 0.03 | 0.012 ± 0.003 | 0.700 ± 0.021 | 0.112 ± 0.015 |
| DMP | 0.007 ± 0.002 | 0.48 ± 0.06 | 0.21 ± 0.02 | 0.39 ± 0.03 | 2.79 ± 0.11 | 0.25 ± 0.03 | 0.37 ± 0.03 | 0.011 ± 0.003 | 0.739 ± 0.061 | 0.111 ± 0.011 |
| DIDP | 0.008 ± 0.002 | 0.53 ± 0.04 | 0.22 ± 0.02 | 0.43 ± 0.10 | 3.69 ± 0.46** | 0.28 ± 0.02 | 0.39 ± 0.02 | 0.009 ± 0.002 | 0.623 ± 0.074* | 0.101 ± 0.005 |
| DUP | 0.006 ± 0.002 | 0.52 ± 0.06 | 0.24 ± 0.05 | 0.38 ± 0.03 | 3.19 ± 0.17 | 0.26 ± 0.02 | 0.37 ± 0.01 | 0.009 ± 0.001 | 0.630 ± 0.040* | 0.099 ± 0.005 |
| DINP | 0.006 ± 0.001 | 0.50 ± 0.06 | 0.17 ± 0.09 | 0.39 ± 0.04 | 3.32 ± 0.13 | 0.27 ± 0.03 | 0.39 ± 0.02 | 0.011 ± 0.001 | 0.652 ± 0.057 | 0.116 ± 0.008 |
| MEHP | 0.005 ± 0.002 | 0.54 ± 0.10 | 0.12 ± 0.07* | 0.46 ± 0.03 | 3.48 ± 0.43* | 0.24 ± 0.05 | 0.39 ± 0.03 | 0.014 ± 0.004 | 0.678 ± 0.069 | 0.104 ± 0.015 |
| MBuP | 0.006 ± 0.001 | 0.61 ± 0.04 | 0.14± 0.10 | 0.38 ± 0.05 | 3.14 ± 0.27 | 0.25 ± 0.03 | 0.40 ± 0.02 | 0.012 ± 0.002 | 0.672 ± 0.060 | 0.106 ± 0.016 |
| MBeP | 0.007 ± 0.004 | 0.46 ± 0.02 | 0.21 ± 0.03 | 0.38 ± 0.04 | 2.54 ± 1.16 | 0.24 ± 0.02 | 0.40 ± 0.02 | 0.010 ± 0.002 | 0.694 ± 0.061 | 0.111 ± 0.012 |
| MEP | 0.007 ± 0.001 | 0.57 ± 0.16 | 0.19 ± 0.02 | 0.39 ± 0.03 | 2.69 ± 0.23 | 0.27 ± 0.02 | 0.35 ± 0.02 | 0.012 ± 0.002 | 0.745 ± 0.055 | 0.120 ± 0.019 |
| MMP | 0.006 ± 0.000 | 0.47 ± 0.05 | 0.23 ± 0.06 | 0.37 ± 0.03 | 2.86 ± 0.18 | 0.25 ± 0.05 | 0.37 ± 0.03 | 0.010 ± 0.001 | 0.665 ± 0.079 | 0.103 ± 0.014 |
| PA | 0.007 ± 0.000 | 0.55 ± 0.11 | 0.17 ± 0.04 | 0.38 ± 0.03 | 2.90 ± 0.29 | 0.24 ± 0.05 | 0.39 ± 0.02 | 0.012 ± 0.001 | 0.737 ± 0.052 | 0.114 ± 0.010 |
Data are means ± SD (n = 6 animals) .
aTreated with corn oil.
*Significantly different from the control group at P < 0.05.
**Significantly different from the control group at P<0.01.
Hematology.
The WBC levels were significantly lower in the DEHP- and DINP-treated groups, but there were no significant differences, and all of the values were within the normal range (Table 2) .
Table 2.
Hematological values of Sprague-Dawley rats orally treated with phthalate esters for 2 weeks
| WBC (103/mm3) | RBC (106/mm3) | Hb (g/dl) | Ht (%) | MCV (μ3) | MCH (pg) | MCHC (g/dl) | PLT (103/mm3) | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| CONa | 6.45 ± 2.46 | 7.58 ± 0.48 | 15.22 ± 0.66 | 48.73 ± 1.58 | 64.43 ± 2.22 | 20.10 ± 0.43 | 31.22 ± 0.53 | 653.83 ± 82.54 |
| DEHP | 5.40 ± 1.35 | 6.95 ± 0.33 | 15.82 ± 0.76 | 46.28 ± 1.97 | 66.66 ± 1.83 | 19.90 ± 0.71 | 29.84 ± 0.82 | 634.00 ± 82.29 |
| DBP | 6.30 ± 0.89 | 7.67 ± 0.31 | 15.52 ± 0.43 | 49.60 ± 1.33 | 64.72 ± 1.43 | 20.26 ± 0.29 | 31.28 ± 0.43 | 691.00 ± 68.08 |
| DnOP | 6.28 ± 1.45 | 7.29 ± 0.16 | 14.73 ± 0.27 | 48.32 ± 1.15 | 66.28 ± 1.82 | 20.22 ± 0.16 | 30.48 ± 0.65 | 663.17 ± 73.18 |
| DEP | 6.26 ± 0.87 | 6.84 ± 0.18 | 14.12 ± 0.34 | 46.44 ± 0.56 | 67.98 ± 2.59 | 20.68 ± 0.80 | 30.40 ± 0.62 | 672.00 ± 46.51 |
| BBP | 5.96 ± 1.68 | 7.81 ± 0.45 | 15.45 ± 0.63 | 49.70 ± 1.58 | 63.73 ± 2.10 | 19.78 ± 0.63 | 31.08 ± 0.72 | 646.67 ± 53.66 |
| DMP | 7.83 ± 2.75 | 7.87 ± 0.48 | 15.90 ± 0.66 | 50.38 ± 1.76 | 64.17 ± 3.56 | 20.23 ± 0.62 | 31.57 ± 0.99 | 671.17 ± 59.73 |
| DIDP | 6.14 ± 0.32 | 7.18 ± 0.14 | 14.66 ± 0.24 | 48.78 ± 0.34 | 67.96 ± 1.57 | 20.42 ± 0.49 | 30.06 ± 0.57 | 713.80 ± 52.48 |
| DUP | 5.32 ± 0.87 | 7.06 ± 0.30 | 14.68 ± 0.39 | 48.87 ± 1.26 | 66.25 ± 2.80 | 20.82 ± 0.57 | 30.07 ± 0.59 | 678.50 ± 37.63 |
| DINP | 5.16 ± 2.29 | 7.61 ± 0.59 | 15.35 ± 1.04 | 48.75 ± 2.38 | 64.22 ± 2.62 | 20.20 ± 0.65 | 31.48 ± 0.75 | 602.17 ± 52.61 |
| MEHP | 7.22 ± 5.12 | 7.91 ± 0.48 | 15.80 ± 0.94 | 50.10 ± 2.68 | 63.38 ± 0.67 | 19.98 ± 0.17 | 31.55 ± 0.47 | 641.75 ± 66.16 |
| MBuP | 6.76 ± 1.66 | 7.92 ± 0.44 | 16.16 ± 0.94 | 50.50 ± 2.14 | 63.82 ± 1.20 | 20.40 ± 0.35 | 31.98 ± 0.54 | 672.40 ± 55.41 |
| MBeP | 7.66 ± 1.86 | 7.71 ± 0.26 | 15.50 ± 0.50 | 50.37 ± 1.75 | 65.32 ± 0.97 | 20.08 ± 0.19 | 30.77 ± 0.37 | 655.00 ± 81.90 |
| MEP | 7.85 ± 1.91 | 7.94 ± 0.19 | 16.16 ± 0.21 | 50.76 ± 0.44 | 63.98 ± 1.45 | 20.38 ± 0.48 | 31.82 ± 0.48 | 680.05 ± 70.43 |
| MMP | 6.88 ± 0.94 | 7.56 ± 0.37 | 15.55 ± 0.78 | 50.32 ± 2.04 | 66.60 ± 1.38 | 20.57 ± 0.27 | 30.88 ± 0.51 | 719.38 ± 71.16 |
| PA | 6.11 ± 1.28 | 7.94 ± 0.28 | 16.18 ± 0.42 | 50.40 ± 1.90 | 63.50 ± 0.73 | 20.42 ± 0.37 | 32.12 ± 0.53 | 612.03 ± 98.85 |
Data are means ± SD (n = 6 animals) .
aTreated with corn oil.
Serum biochemistry.
The glucose levels in the DEHP,MEHP and MBeP groups and TP in the DUP group were significantly higher compared with the control group. The AST levels in the DBP-, DUP-, DINP-, MBuP- and MBePtreated groups and the ALT levels in the DEHP, MEHP, and MEP groups were significantly higher than those in the control group. The ALP activity in the serum of the DBP-,DnOP-, DMP-, DUP-, DINP-, MEHP-, and MBuP-treated groups was significantly increased as compared to the control group. However, the serum total cholesterol levels were significantly decreased in DEHP- and DIDP-treated rats as compared to the control rats. However, TG levels were significantly increased in the DINP- and MEHP-treated groups, and serum LDH levels in the DEHP and DEP groups were significantly higher than those in the control group (Table 3) .
Table 3.
Biochemical serum values of male Sprague-Dawley rats orally treated with phthalate esters for 2 weeks
| GLU (g/dl) | T.P (g/dl) | ALB (g/dl) | T.BIL (mg/dl) | AST (IU/l) | ALT (IU/l) | ALP (IU/l) | T.CHO (mg/dl) | GGT (IU/l) | TG | CA (mg/dl) | BUN (mg/dl) | CREA (mg/dl) | CPK (IU/l) | LDH | HDL.C | Na (mEq/l) | K (mEq/l) | Cl (mEq/l) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||||
| CONa | 123.01 ± 20.05 | 7.03 ± 0.28 | 2.50 ± 0.09 | 0.11 ± 0.00 | 75.67 ± 7.81 | 41.17 ± 7.03 | 347.04 ± 49.78 | 93.54 ± 11.04 | 0.83 ± 0.02 | 42.17 ± 7.65 | 10.23 ± 0.43 | 17.12 ± 1.41 | 0.58 ± 0.04 | 123.17 ± 24.36 | 83.51 ± 60.40 | 27.33 ± 2.16 | 144.47 ± 1.25 | 4.28 ± 0.32 | 102.23 ± 1.93 |
| DEHP | 174.33 ± 38.60* | 7.05 ± 0.33 | 2.75 ± 0.10 | 0.13 ± 0.00 | 70.57 ± 7.23 | 61.67 ± 10.02* | 532.17 ± 67.67 | 78.37 ± 8.78* | 0.52 ± 0.05 | 56.67 ± 5.54 | 11.00 ± 0.44* | 19.27 ± 2.63 | 0.57 ± 0.08 | 141.02 ± 25.85 | 148.67 ± 23.63* | 33.17 ± 2.79 | 138.67 ± 1.00 | 4.82 ± 0.36 | 97.92 ± 1.10 |
| DBP | 135.22 ± 23.47 | 7.2 ± 0.08 | 2.34 ± 0.28 | 0.12 ± 0.05 | 96.63 ± 6.77* | 54.02 ± 12.11 | 923.21 ± 82.08** | 86.66 ± 7.86 | 1.47 ± 0.04 | 58.28 ± 7.98 | 10.88 ± 0.56 | 15.52 ± 3.35 | 0.56 ± 0.05 | 158.45 ± 48.65 | 135.20 ± 42.71 | 32.22 ± 3.40 | 139.46 ± 1.78 | 4.61 ± 0.82 | 97.72 ± 1.67 |
| DnOP | 144.05 ± 21.13 | 7.06 ± 0.28 | 2.46 ± 0.21 | 0.16 ± 0.05 | 83.65 ± 8.46 | 42.05 ± 8.28 | 500.40 ± 56.78* | 85.22 ± 8.97 | 1.09 ± 0.00 | 46.23 ± 4.32 | 11.18 ± 0.38** | 15.72 ± 2.20 | 0.56 ± 0.09 | 141.23 ± 75.91 | 130.43 ± 37.42 | 26.87 ± 1.92 | 113.52 ± 57.50 | 3.65 ± 1.78 | 80.58 ± 42.26 |
| DEP | 140.26 ± 23.47 | 6.88 ± 0.08 | 2.48 ± 0.28 | 0.14 ± 0.05 | 75.62 ± 6.77 | 49.81 ± 12.11 | 415.65 ± 82.08 | 81.65 ± 7.86 | 0.85 ± 0.04 | 42.21 ± 6.98 | 11.02 ± 0.56* | 17.42 ± 3.35 | 0.54 ± 0.05 | 168.02 ± 48.65 | 153.52 ± 22.71* | 25.75 ± 3.40 | 140.32 ± 1.78 | 5.85 ± 0.82* | 98.72 ± 1.67 |
| BBP | 143.33 ± 17.41 | 6.8 ± 0.28 | 2.57 ± 0.21 | 0.13 ± 0.05 | 79.33 ± 11.20 | 42.53 ± 6.86 | 523.07 ± 110.70 | 80.51 ± 12.01 | 0.67 ± 0.52 | 54.00 ± 13.9 | 10.92 ± 0.23* | 18.53 ± 2.25 | 0.55 ± 0.05 | 175.51 ± 55.63 | 129.67 ± 13.76 | 30.67 ± 5.05 | 141.07 ± 0.74 | 4.36 ± 0.78 | 98.23 ± 0.81 |
| DMP | 141.27 ± 15.47 | 7.4 ± 0.16 | 2.32 ± 0.25 | 0.16 ± 0.13 | 89.40 ± 11.97 | 47.62 ± 14.50 | 764.03 ± 122.48** | 93.03 ± 4.12 | 1.04 ± 0.71 | 50.04 ± 4.00 | 10.68 ± 0.15 | 16.06 ± 3.73 | 0.51 ± 0.00 | 198.42 ± 159.97 | 131.03 ± 15.04 | 31.46 ± 2.51 | 139.88 ± 1.23 | 5.42 ± 0.42 | 100.24 ± 2.06 |
| DIDP | 135.67 ± 19.46 | 6.92 ± 0.16 | 2.47 ± 0.16 | 0.12 ± 0.04 | 77.67 ± 7.93 | 51.17 ± 4.44 | 579.67 ± 57.70* | 80.52 ± 6.06* | 0.67 ± 0.03 | 57.52 ± 12.1 | 10.61 ± 0.44 | 17.23 ± 1.22 | 0.55 ± 0.05 | 116.33 ± 22.50 | 123.62 ± 15.36 | 26.84 ± 2.59 | 140.38 ± 0.99 | 4.76 ± 0.38 | 98.98 ± 1.27 |
| DUP | 134.03 ± 18.11 | 7.67 ± 0.46* | 2.25 ± 0.19 | 0.15 ± 0.05 | 113.83 ± 15.58** | 47.17 ± 4.64 | 626.52 ± 55.83** | 77.67 ± 8.38* | 0.67 ± 0.82 | 41.17 ± 6.49 | 10.33 ± 0.43 | 18.57 ± 2.73 | 0.55 ± 0.08 | 138.83 ± 35.52 | 142.75 ± 12.00 | 27.25 ± 4.65 | 116.93 ± 54.88 | 3.71 ± 1.58 | 83.45 ± 39.86 |
| DINP | 135.25 ± 16.04 | 7.04 ± 0.33 | 2.46 ± 0.46 | 0.12 ± 0.04 | 100.02 ± 8.10* | 54.45 ± 4.61 | 902.47 ± 89.69** | 91.03 ± 7.25 | 0.6 ± 0.75 | 64.22 ± 8.14** | 10.14 ± 0.63 | 20.68 ± 6.37 | 0.54 ± 0.05 | 138.8 ± 27.63 | 159.8 ± 19.10 | 32.8 ± 3.27 | 142.54 ± 1.73 | 4.85 ± 0.34 | 99.94 ± 0.75 |
| MEHP | 167.75 ± 11.98* | 7.35 ± 0.42 | 2.65 ± 0.31 | 0.15 ± 0.00 | 97.05 ± 9.93 | 66.25 ± 6.18* | 979.75 ± 261.81** | 105.75 ± 7.23 | 1.25 ± 0.17 | 77.25 ± 10.02** | 10.73 ± 0.34 | 18.58 ± 1.70 | 0.58 ± 0.05 | 146.0 ± 9.20 | 149.5 ± 22.52 | 30.75 ± 3.10 | 143.87 ± 1.79 | 4.39 ± 0.56 | 101.72 ± 1.26 |
| MBuP | 146.56 ± 47.90 | 6.92 ± 0.49 | 2.17 ± 0.14 | 0.13 ± 0.05 | 111.04 ± 32.53** | 53.17 ± 31.04 | 732.33 ± 107.35** | 83.52 ± 10.09 | 1.67 ± 0.82 | 58.83 ± 2.46* | 10.62 ± 0.41 | 18.86 ± 2.84 | 0.5 ± 0.07 | 153.4 ± 68.29 | 207.2 ± 124.45 | 30.8 ± 4.92 | 141.83 ± 2.70 | 4.63 ± 0.73 | 98.65 ± 1.45 |
| MBeP | 163.67 ± 16.77* | 7.17 ± 0.39 | 2.48 ± 0.12 | 0.13 ± 0.09 | 129.67 ± 17.35** | 55.03 ± 20.88 | 430.59 ± 69.71 | 89.67 ± 11.99 | 1.17 ± 0.75 | 50.05 ± 9.98 | 10.67 ± 0.57 | 16.68 ± 2.37 | 0.58 ± 0.08 | 226.67 ± 171.73 | 140.67 ± 69.92 | 31.17 ± 4.07 | 142.98 ± 2.19 | 4.25 ± 0.23 | 96.82 ± 5.61 |
| MEP | 141.75 ± 21.01 | 7.04 ± 0.14 | 2.48 ± 0.15 | 0.13 ± 0.05 | 86.54 ± 13.53 | 65.25 ± 29.34 | 368.75 ± 52.96 | 83.25 ± 8.92 | 0.25 ± 0.05 | 51.02 ± 9.13 | 10.43 ± 0.33 | 19.53 ± 2.74 | 0.55 ± 0.06 | 127.75 ± 13.00 | 120.25 ± 36.51 | 30.75 ± 3.30 | 142.95 ± 1.39 | 4.23 ± 0.65 | 100.05 ± 4.68 |
| MMP | 145.33 ± 21.79 | 6.91 ± 0.41 | 2.53 ± 0.27 | 0.12 ± 0.04 | 77.83 ± 10.38 | 38.07 ± 9.38 | 439.67 ± 83.57 | 90.58 ± 9.81 | 0.33 ± 0.02 | 55.07 ± 5.37 | 10.67 ± 0.19 | 16.85 ± 1.11 | 0.5 ± 0.00 | 123.67 ± 22.08 | 124.33 ± 27.20 | 31.0 ± 3.29 | 141.38 ± 1.24 | 5.54 ± 0.34 | 99.64 ± 2.26 |
| PA | 137.07 ± 18.38 | 7.04 ± 0.42 | 2.46 ± 0.28 | 0.21 ± 0.00 | 99.07 ± 16.97 | 61.53 ± 3.54 | 405.56 ± 64.85 | 95.52 ± 2.12 | 1.03 ± 0.01 | 57.03 ± 4.24 | 10.36 ± 0.28 | 20.27 ± 0.01 | 0.52 ± 0.05 | 129.37 ± 1.41 | 129.54 ± 9.19 | 34.01 ± 2.83 | 139.15 ± 1.48 | 5.09 ± 0.15 | 98.72 ± 2.69 |
Data are means ± SD (n = 6 animals) .
aTreated with corn oil.
*Significantly different from the control group at P<0.05.
**Significantly different from the control group at P<0.01.
Urinalysis.
The value of RBC in animals given the DIDP alone was changed, and the urinary protein level in the animals given DINP alone was also changed. Only the leukocytes in three of the phthalates treatment groups (DINP,MBeP, PA) were changed. The specific gravity of the urine was not changed significantly in any of the groups receiving PEs. There were no inter-group differences in the protein, urobilinogen, glucose, bilirubin and ketone levels in the urine (Table 4) .
Table 4.
Urine analysis of Sprague-Dawley rats orally treated with phthalate esters for 2 weeks
| CONa | DEHP | DBP | DnOP | DEP | BBP | DMP | DIDP | DUP | DINP | MEHP | MBuP | MBeP | MEP | MMP | PA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Blood (RBC/μl) | − | − | − | − | − | − | − | 250 | − | − | − | − | − | − | − | − |
| Bilirubin (mg/dl) | − | − | − | 0.5 | − | − | 0.5 | − | 0.5 | − | − | − | − | − | − | − |
| URO | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Ketone (mg/dl) | 10 | 5 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 5 | 5 | − | 5 | 10 | 5 | 10 |
| Protein (mg/dl) | 100 | 30 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 1000 | 100 | 100 | 100 | 100 | 30 | 100 |
| Nitrite | + | − | − | − | + | − | − | − | − | + | − | − | − | − | + | + |
| Glucose (mg/dl) | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| pH | 8 | 5.5 | 5.5 | 5.5 | 6.5 | 5 | 5 | 5 | 5 | 8.5 | 5 | 5 | 5 | 5 | 6.5 | 5.5 |
| Specific gravity (≥) | 1.015 | 1.03 | 1.03 | 1.03 | 1.025 | 1.03 | 1.03 | 1.03 | 1.03 | 1.01 | 1.03 | 1.03 | 1.03 | 1.03 | 1.025 | 1.03 |
| Leukocytes (WBC/μl) | 75 | 25 | 10 | 25 | 75 | 25 | 25 | 25 | 75 | 500 | 25 | 75 | 500 | 75 | 75 | 500 |
Data are means ± SD (n = 6 animals) .
aTreated with corn oil.
DISCUSSION
In the present study, PEs showed moderate toxicity when administered orally to male rats for two weeks. We compared the general toxicities of nine phthalate diesters and five phthalate monoesters in Sprague-Dawley rats exposed to 500 mg/kg/day of DBP, DINP and BBP and 250 mg/kg/day of MEHP, MBuP and PA for two weeks. These results exhibited reduced body weight gain, even though there was no difference in food consumption, which suggests that the reduced body weight gain in those groups may have been compound-related. The major findings in the relative organ toxicity were that short term exposure to DEHP, DBP,DnOP and DIDP increased the relative liver weights in the male rats and that there were decreased relative testes weight in DEHP-, DBP-, and MEHP-treated groups as compared to the control. Increased liver weight could be due to peroxisome proliferator activation leading to increased lysosomal activity, which correlates well with the increased serum enzymes like LDH and ALT. Increased liver enzyme activity could result in the leakage of these enzymes into the serum, resulting in increases in the serum levels. Previous work by others has shown that several phthalate monoesters activate PPARα and PPARγ and that lower concentrations of monoesters are required for the activation of PPARα (Hurst and Waxman, 2003; Maloney and Waxman, 1999).
On the other hand, the significantly increased serum triglyceride levels observed in the DINP- and MEHP-treated group indicates an impairment in triglyceride metabolism and enhanced triglyceride storage in the liver, which correlate well with the increased lipid deposits observed in the liver histology in the high-dose DEP-treated groups (Pereira et al., 2006). Cholesterol are synthesized as well as broken down in the liver and the exposure to DEHP at increasing concentration results in higher accumulations of cholesterol in the liver. These results correlate well with an earlier study in which male Sprague-Dawley rats were administered 50 ppm DEP through water; the results showed a significant increase in liver cholesterol levels (Sonde et al., 2000). Impaired cholesterol transport was evident in the higher treated groups due to reduced levels in the serum cholesterol levels. In this study, significant decreases in cholesterol levels were observed in the DEHP- and DIDPtreated groups. Previous studies indicated that steroidogenic acute regulatory protein (StAR) protein plays an important role in the regulation of cholesterol metabolism as well as in steroidogenesis (Nakae et al., 1997; Stocco, 2001). Thus,we suggest that DEHP could affect the StAR protein, hence allowing for the accumulation of excessive cholesterol as observed. Since PPARα is responsible for the regulation of fat metabolism, we suspect that the significantly decreased cholesterol levels in the serum indicate that phthalate-induced toxicity is probably associated with PPARα activation.
PEs have a strong potential for human exposure in the work place and home environment (Tsutsumi et al., 2004). Extensive studies on PE-induced male reproductive toxicity have been reported since Shaffer et al. (1945) first reported the DEHP-induced testicular injury in an animal model. DEHP is a well-known reproductive toxicant, and immature rats are more susceptible to DEHP toxicity than mature rats (Park et al., 2002; Sjoberg et al., 1986; Teirlynck et al., 1988). The major target organ for DBP reproductive toxicity in rodents is the testis in general and the seminiferous tubules in particular (Tsutsumi et al., 2004). In a study, the adverse effects on the development of the reproductive system in male offspring were reported in Wistar rats administered BBP by a gavage at 500 mg/kg on Days 15~17 of pregnancy (Ema et al., 2003). Waterman et al. (2000) reported that DINP exposures are not associated with any detectable effects on reproductive function. However, these studies did not assess the endpoints of reproductive toxicity, which have been shown to be sensitive with other PEs .
There are no reports on the anti-androgenic activity of DINP, DIDP and DnOP. These are general-purpose plasticizers with a broad range of applications in flexible PVC and are used widely in the toys, construction, and general consumer product markets. Because of the physicochemical similarities between these PEs and DEHP, general exposures to PEs are probably quite similar to exposure to DEHP, but there is little monitoring and reproductive toxicity data.
In conclusion, while the specific mechanism of action remains to be identified, DEHP (and MEHP) , DBP (and MBuP) , BBP (and MBuP and MBeP) , DnOP, DINP, DIDP, DUP and MEP treatments exhibited adverse effects in Sprague-Dawley male rats after orally administered at up to 250 or 500 mg/kg/day. These adverse effects included liver enlargement and reduced testes weight and blood parameters. However, a histological finding study is needed to understanding the exact mechanism of phthalate-mediated toxicity.
Acknowledgments
This work was supported by National Toxicology Program grant from National Institute of Food and Drug Safety Evaluation, Korea Food & Drug Administration.
References
- 1.Albro P.W. Moore B. Identification of the metabolites of simple phthalate diesters in rat urine. J. Chlomatgr. 1974;134:209–218. doi: 10.1016/s0021-9673(01)92368-4. [DOI] [PubMed] [Google Scholar]
- 2.Autian J. Toxicity and health threats of phthalate esters: review of the literature. Environ. Health Perspect. 1973;4:3–26. doi: 10.2307/3428178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blount B.C. Silva M.J. Caudill S.P. Needham L.L. Pirkle J.L. Sampson E.J. Lucier G.W. Jackson R.J. Brock J.W. Levels of seven urinary phthalate metabolites in a human reference population. Environ. Health Perspect. 2000;108:979–982. doi: 10.1289/ehp.00108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniel J.W. Bratt H. The absorption metabolism and tissue distribution of di (2-ethylhexyl) phthalate in rats. Toxicology. 1974;2:51–65. doi: 10.1016/0300-483X(74)90042-0. [DOI] [PubMed] [Google Scholar]
- 5.Eigenberg D.A. Bozigian H.P. Carter D.E. Sipes I.G. Distribution excretion and metabolism of butylbenzyl phthalate in the rat. J. Toxicol. Environ. Health. 1986;17:445–456. doi: 10.1080/15287398609530839. [DOI] [PubMed] [Google Scholar]
- 6.Ema M. Miyawaki E. Hirose A. Kamata E. Decreased anogenital distance and increased incidence of undescended testes in fetuses of rats given monobenzyl phthalate a major metabolite of butyl benzyl phthalate. Reprod Toxicol. 2003;17:407–412. doi: 10.1016/S0890-6238(03)00037-6. [DOI] [PubMed] [Google Scholar]
- 7.Fisher J.S. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004;127:305–315. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- 8.Gangolli S.D. Testicular effects of phthalate esters. Environ. Health Perspect. 1982;45:77–84. doi: 10.1289/ehp.824577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotz F. Thieme S. Dorner G. Female infertilityeffect of Perinatal xenoestrogen exposure on reproductive functions in animals and humans. Folia. Histochem. Cytobiol. 2001;39:40–43. [PubMed] [Google Scholar]
- 10.Gray L.E. Ostby J. Furr J. Price M. Veeramachaneni D.N. Parks L. Perinatal exposure to the phthalates DEHP BBP and DINP but not DEP DMP or DOTP alters sexual differentiation of the male rat. Toxicol. Sci. 2000;58:350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- 11.Hurst C.H. Waxman D.J. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol. Sci. 2003;74:297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- 12.Kim H.S. Kim T.S. Shin J.H. Moon H.J. Kang I.H. Kim I.Y. Oh J.Y. Han S.Y. Neonatal exposure to di (nbutyl) phthalate (DBP) alters male reproductive-tract development. J. Toxicol. Environ. Health A. 2004;67:2045–2060. doi: 10.1080/15287390490514859. [DOI] [PubMed] [Google Scholar]
- 13.Lhuguenot J.C. Mitchell A.M. Milner G. Lock E.A. Elcombe C.R. The metabolism of di (2-ethylhexyl) phthalate (DEHP) and mono (2-ethylhexyl) phthalate (MEHP) in rats: in vivo and in vitro dose and time dependency of metabolism. Toxicol. Appl. Pharmacol. 1985;80:11–22. doi: 10.1016/0041-008X(85)90096-1. [DOI] [PubMed] [Google Scholar]
- 14.Li L.H. Jester W.F. Laslett A.L. Orth J.M. A single dose of di- (2-ethylhexyl) phthalate in neonatal rats alters gonocytes reduces sertoli cell proliferation and decreases cyclin D2 expression. Toxicol. Appl. Pharmacol. 2000;166:222–229. doi: 10.1006/taap.2000.8972. [DOI] [PubMed] [Google Scholar]
- 15.Maloney E.K. Waxman D.J. Trans-activation of PPARalpha and PPARgamma by structurally diverse environmental chemicals. Toxicol. Appl. Pharmacol. 1999;161:209–218. doi: 10.1006/taap.1999.8809. [DOI] [PubMed] [Google Scholar]
- 16.Nakae J. Tajima T. Sugawara T. Arakane F. Hanaki K. Hotsubo T. Igarashi N. Igarashi Y. Ishii T. Koda N. Kondo T. Kohno H. Nakagawa Y. Tachibana K. Takeshima Y. Tsubouchi K. Strauss J.F.3rd. Fujieda K. Analysis of the steroidogenic acute regulatory protein (StAR) gene in Japanese patients with congenital lipoid adrenal hyperplasia. Human Mol. Genetics. 1997;6:571–576. doi: 10.1093/hmg/6.4.571. [DOI] [PubMed] [Google Scholar]
- 17.Nativelle C. Picard K. Valentin I. Lhuguenot J.C. Chagnon M.C. Metabolism of n-butyl benzyl phthalate in the female wistar rat. Identification of new metabolites. Food Chem. Toxicol. 1999;37:905–917. doi: 10.1016/S0278-6915(99)00071-X. [DOI] [PubMed] [Google Scholar]
- 18.Okubo T. Suzuki T. Yokoyama Y. Kano K. Kano I. Estimation of estrogenic and anti-estrogenic activities of some phthalate diesters and monoesters by MCF-7 cell proliferation assay in vitro. Biol. Pharm. Bull. 2003;26:1219–1224. doi: 10.1248/bpb.26.1219. [DOI] [PubMed] [Google Scholar]
- 19.Park J.D. Habeebu S.S. Klaassen C.D. Testicular toxicity of di- (2-ethylhexyl) phthalate in young Sprague-Dawley rats. Toxicology. 2002;171:105–115. doi: 10.1016/S0300-483X(01)00567-4. [DOI] [PubMed] [Google Scholar]
- 20.Peck C.C. Odom D.G. Albro P.W. Jess D.A. Barrett B.B. Effect of heat on the conversion of di-2-ethylhexyl phthalate to mono-2-ethylhexyl phthalate in human plasma. Transfusion. 1981;21:163–166. doi: 10.1046/j.1537-2995.1981.21281178151.x. [DOI] [PubMed] [Google Scholar]
- 21.Pereira C. Mapuskar K. Rao C.V. Chronic toxicity of diethyl phthalate in male Wistar rats--a dose-response study. Regul. Toxicol. Pharmacol. 2006;45:169–177. doi: 10.1016/j.yrtph.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Pollack G.M. Shen D.D. Dorr M.B. Contribution of metabolites to the route- and time-dependent hepatic effects of di- (2-ethylhexyl) phthalate in the rat. J. Pharmacol. Exp. Ther. 1989;248:176–181. [PubMed] [Google Scholar]
- 23.Rhee G.S. Kim S.H. Kim S.S. Sohn K.H. Kwack S.J. Kim B.H. Park K.L. Comparison of embryotoxicity of ESBO and phthalates esters using an in vitro battery system. Toxicol. In Vitro. 2002;16:443–448. doi: 10.1016/S0887-2333(02)00026-7. [DOI] [PubMed] [Google Scholar]
- 24.Ryu J.Y. Whang J. Park H. Im J.Y. Kim J. Ahn M.Y. Lee J. Kim S.H. Lee B.M. Yoo S.D. Kwack S.J. Oh J.H. Park K.L. Han S.Y. Kim H.S. Di (2-ethylhexyl) phthalate induces apoptosis through peroxisome proliferatorsactivated receptor-gamma and ERK 1/2 activation in testis of Sprague-Dawley rats. J. Toxicol. Environ. Health A. 2007;70:1296–1303. doi: 10.1080/15287390701432160. [DOI] [PubMed] [Google Scholar]
- 25.Shaffer C.B. Carpenter C.P. Smyth H.F. Acute and subacute toxicity of DEHP with note upon its metabolism. J. Indust. Hyg. Toxicol. 1945;27:130–135. [Google Scholar]
- 26.Shono T. Suita S. Dose-dependent effect of phthalate ester on testicular descent in pre- and post natal rats. Urol. Res. 2003;31:293–296. doi: 10.1007/s00240-003-0330-5. [DOI] [PubMed] [Google Scholar]
- 27.Sjoberg P. Lindqvist N.G. Ploen L. Age-dependent response of the rat testes to di (2-ethylhexyl) phthalate. Environ. Health Perspect. 1986;C.V.:237–242. doi: 10.1289/ehp.65-1474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonde V. D’Souza A. Tarapore R. Pereira L. Khare M.P. Sinkar P. Krishnan S. Rao C.V. Simultaneous administration of diethylphthalate and ethyl alcohol and its toxicity in male Sprague Dawley rats. Toxicology. 2000;147:23–31. doi: 10.1016/S0300-483X(00)00164-5. [DOI] [PubMed] [Google Scholar]
- 29.Stocco D.M. Tracking the role of a StAR in the sky of the new millennium. Mol. Endocrinol. 2001;15:1245–1254. doi: 10.1210/me.15.8.1245. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka 85. Matsumoto A. Yamaha T. Biochemical studies on phthalic esters. III. Metabolism of dibutyl phthalate (DBP) in animals. Toxicology. 1978;9:109–123. doi: 10.1016/0300-483X(78)90036-7. [DOI] [PubMed] [Google Scholar]
- 31.Teirlynck O. Kaufman J.M. Bogaert M.G. Roels H. Testicular toxicity induced by single dosing of di- and mono- (2- ethylhexyl) phthalate in the rat. Toxicol. Lett. 1988;40:85–91. doi: 10.1016/0378-4274(88)90186-5. [DOI] [PubMed] [Google Scholar]
- 32.Thomas J.A. Darby T.D. Wallin R.F. Garvin P.J. Martis L. A review of the biological effects of di- (2-ethylhexyl) phthalate. Toxicol. Appl. Pharmacol. 1978;45:1–27. doi: 10.1016/0041-008X(78)90024-8. [DOI] [PubMed] [Google Scholar]
- 33.Tsutsumi T. Ichihara T. Kawabe M. Yoshino H. Asamoto M. Suzuki S. Shirai T. Renal toxicity induced by folic acid is associated with the enhancement of male reproductive toxicity of di (n-butyl) phthalate in rats. Reprod. Toxicol. 2004;18:35–42. doi: 10.1016/j.reprotox.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Waterman S.J. Keller L.H. Trimmer G.W. Freeman J.J. Nikiforov A.I. Harris S.B. Nicolich M.J. McKee R.H. Two-generation reproduction study in rats given diisononyl phthalate in the diet. Reprod. Toxicol. 2000;14:21–36. doi: 10.1016/S0890-6238(99)00067-2. [DOI] [PubMed] [Google Scholar]
- 35.Zacharewski T. Identification and assessment of endocrine disruptors: limitations of in vivo and in vitro assays. Environ. Health Perspect. 1998;106:577–582. doi: 10.2307/3433808. [DOI] [PMC free article] [PubMed] [Google Scholar]