Abstract
The process of new drug development consists of several stages; after identifying potential candidate compounds, preclinical studies using animal models link the laboratory and human clinical trials. Among many steps in preclinical studies, toxicology and safety assessments contribute to identify potential adverse events and provide rationale for setting the initial doses in clinical trials. Gene modulation is one of the important tools of modern biology, and is commonly employed to examine the function of genes of interest. Advances in new drug development have been achieved by exploding information on target selection and validation using genetically modified animal models as well as those of cells. In this review, a recent trend of genetically modified methods is discussed with reference to safety assessments, and the exemplary applications of gene-modulating tools to the tests in new drug development were summarized.
Keywords: Gene modulation, Toxicological study, Knockout mice, Drug development
IMPORTANCE OF TOXICOLOGY TESTS IN PRECLINICAL STUDIES
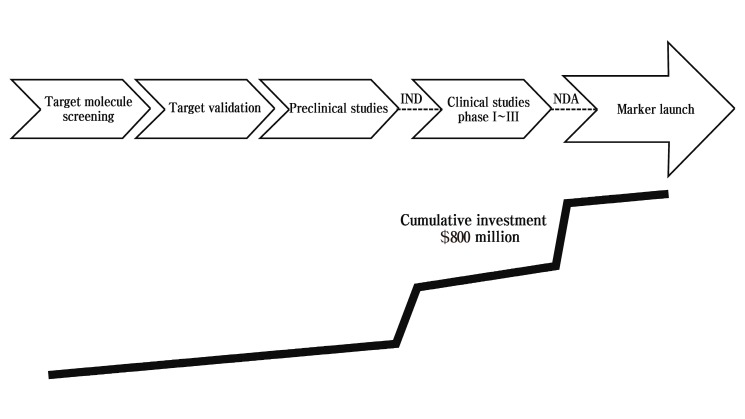
The purpose of pharmaceutical drug development is to discover safer and more effective drug. Preclinical development of a drug is the part of drug development occurring before the drug enters human trials. During this time, studies on the safety, activity, and mechanism of action are necessary. Preclinical toxicology study is a major strategy in the process of drug development for saving costs and is important for the prediction of effectiveness in human. Pharmaceutical companies can decrease the need for expensive and time-consuming clinical studies by doing good preclinical work (Ryan et al., 2008) . In this sense, drug developers continuously face increasing challenges in drug development from shorter product life cycles, higher global competition, and increased consumer demand. Enlarged candidate compound library due to advances in hight- throughput screening system increases the cost and time of pharmaceutical process for new drug development. High attrition rate because of toxicity, lack of efficacy or poor pharmacokinetic profiles makes this situation worse. It usually takes a decade or longer to develop a drug and to reach the market place. Moreover, the cost to identify and develop a therapeutically efficient drug is hundreds of millions of dollars (DiMasi et al., 2003; Fricker, 2008). There are many new drugs investigated in cell or animal model systems every year that are found to be active in various ways. As summarized in Fig. 1, new drug development is an expensive, long-term and arduous task that requires 10 or more years’ endeavor (Woodcock and Woosley, 2008). This begins from basic research including target molecular identification and target validation in laboratories. A couple of hundred target molecules that may be helpful for a specific disease are usually selected from thousands of molecules discovered during basic research. The next step will be preclinical studies to test several kinds of potential compounds on cells and animals and collect data in support of safety as well as efficacy. If the collected data is enough to suggest efficacy and safety on animals, a clinical study is conducted on humans usually for 5~10 years. However, few of these compounds can be translated successfully to human applications, which may be due to unacceptable toxicity, adverse effects, species variation in effect, and cost-benefit ratio in production (Lord et al., 2006). Ultimately, the candidates that show robust efficacy and safety can be brought into the market. Having a standard set of procedures would make the process easier.
Fig. 1. The general steps of new drug development and investment.
Before clinical trials, consistent efforts are made over a long period of time, including preliminary laboratory investigation and animal experiments. The aim of clinical trials is to provide a definitive answer on treatment approach to prevent, detect, or treat diseases, or to improve care for patients (Paul et al., 2005). Clinical trials take place in phases after laboratory studies. A phase I trial is the first step in testing safety and tolerability of a new drug in healthy volunteers (O’Quigley et al., 1990). In this trial, researchers find the best way to give people the new drug (i.e., tablets, capsules or injectables) , and search for the appropriate frequency and dose. In phase I trial, small groups of people with a disease are treated with certain dose of a widely pre-studied new drug at a certain frequency. Among the groups of patients, dosage is gradually increased to find the maximal dose that will not cause harmful side effects. This process identifies safe and appropriate doses for phase II trial. During phase II trials, researchers evaluate whether the investigational drug benefits patients at the dose employed.
Before starting a clinical trial, preclinical toxicology is required to accurately evaluate the efficacy of a new drug and provide critical information for clinical trials (Grieshaber and Marsoni, 1986). Preclinical toxicology analyzes the toxic effect of a substance on cells and animals, and establishes safe starting dose and dose escalation parameters for phase I trial in human volunteers. Thus, preclinical toxicology studies include determination of acute, subacute, and chronic toxicity, carcinogenicity, mutagenicity, teratogenicity, and effects on the reproductive system. In this process, study aims to obtain information on toxic effect as much as possible so that good preparations can be made for further study.
Safety dosage still be tested during phase II trials, and the therapeutic effect on a particular disease come into be evaluated (Simon, 1989). In phase II trials, using the safety dosage found in phase I trials, the new drug candidate is given to groups of patients in question. Only agents that work effectively against the disease and are safe for patients in a phase II trial can enter a phase III trial. In general, people who participate in phase II trials have other treatments, but those treatments have not been effective. Usually, patients who have ever taken a cure before are not allowed to take part in these trials. As a general rule, phase II trials own no more than 100 participants.
Nowadays, pharmacogenomics is a powerful tool for the prediction of pharmacological and toxicological effects based on drug response in different genetic patients, and is increasingly incorporated into phase II trials (Rioux, 2000). Pharmacogenomic technology deals with genetically determined variations in how individuals respond to drugs and designs rational treatment for patients to make sure maximum efficacy with minimal adverse effects. This technology is likely to be among the first clinical applications of the Human Genome Project, and have impacts on the clinical practice of medicine. Progress has been made towards the incorporation of pharmacogenomics information into clinical practice. For example, these include genetic testing for thiopurine methyltransferase variant alleles in patients prior to mercaptopurine administration, and for UGT1A1*28 in patients prior to administration of irinotecan therapy (Marsh, 2007). However, increasingly recognized limitations still exist in this approach. Particularly, low event rates in phase II trial that include less than 100 participants can make inaccurate response-genotype relationship. More importantly, clinical phenotype is usually caused by coordinated expression of thousands of genes, which also limits the application of pharmacogenomics. Thus, there is still a long way to go before pharmacogenomics achieve the goal of individualized selection of drug treatment (Pusztai, 2007).
After phase II treatment shows positive results, phase III can be taken on. Phase III trials compares the newly found treatment to the “standard treatment” (the most classical treatment, based on results of past research) in order to know whether the new treatment surpasses standard treatment. Every volunteer in phase III trials has the same chance of being assigned to one of two or more arms. The participants are randomly assigned to groups, which is referred as randomization (Peto et al., 1976). Many people choose to get their first treatment in a phase III trial. The type of participant varies, depending on the kind of question being asked about a particular disease. In general, phase III trials have hundreds to thousands of participants to determine whether the differences in the effectiveness and safety of the newly found treatments are true. If the drug passes all of these clinical phases, it can be submitted to regulatory authority as a new drug application. The proportion of investigational new drug applications that succeed has been estimated to be less than 50% in phase I and II studies (Caldwell et al., 2001).
Even once clinical trials have begun, further preclinical studies can be performed to answer developing questions. Clinical toxicology, a branch of toxicology, mainly focuses on the diagnosis including toxicological analysis and the treatment of intoxications and poisonings, chemical-induced diseases, environmental and hazardous material exposures, and other toxicological emergencies (Descotes and Testud, 2005). Especially, clinical toxicology is important in promoting drug development, because a concrete plan on counteracting toxicological effects and reducing the body burden of various poisons needs to be established. Individual patients show different tolerabilities to medications, which leads to different responses. So even at standard doses, a drug may have toxic adverse events in some patients, but fail to reach the expected therapeutic effect in others. Genetic differences in drug response may explain inter-individual variations occurring in therapeutic response and toxicity (Weinshilboum, 2003). For example, ~20% of the populations in industrialized countries reveal immunoglobulin E antibodies against physiological components, which may cause allergic reactions (Valenta, 2002). The aim of an improved therapy is the preparation of an individual composition of the components according to the condition of patients. For this reason, the standardization and characterization of the relationship between specific gene mutation and the phenotype may be important.
As reviewed above, developing a new drug is a complex and costly process (DiMasi et al., 1991). If everything goes well according to plan, a new drug will be introduced to market ~10 years after the work first began. Pharmaceutical companies are constantly striving to shorten this time. Up to 10,000 compounds must be screened to get one potential drug. Even after released to market, only about 30% of drugs produce revenues that match average R&D costs. About one in four marketed drugs typically makes sufficient profits to recover the costs of its discovery and development. Thus, the best way to reduce the budgets and time of pharmaceutical discovery and development is reducing drug failures (Stewart et al., 2001). Approximately 10% of the research budget is spent for preclinical studies today. It will be increased to 15% in the next 3~5 years. Target molecule screening, target validation, and preclinical studies are relatively inexpensive compared to the clinical phases, which may be good strategy for cost-effective drug development (Fig. 1) .
Overall, preclinical studies serve a key role in the drug discovery and development processes. The main goal of preclinical studies is to discover potential drug molecules to provide better candidates to patients faster. Most candidates are failed due to poor pharmacokinetics, lack of efficacy and animal toxicities (Ulrich and Friend, 2002). Pharmacokinetics, bioavailability and efficacy had been the main reasons for the failures. Efficacy problem is still a big issue today. Another major problem that recently came up is the issue of toxicology or adverse effects. These factors can be detected in preclinical phases, implying that well-designed preclinical studies are crucial. These steps take less time and money, but are more important to increase the success rate of drug development. Preclinical data is also useful to choose the right compound, delivery method, and formulation for the overall strategic success.
APPLICATION OF GENETICALLY MODIFIED TOOLS TO PRECLINICAL AND CLINICAL STUDIES
In the toxicological assessments during drug development, a big problem in preclinical studies is the lack of appropriate animal models for disease or toxicology of interest. No single model is likely to be suitable for all studies. Different animal models can help researchers better understand the pathogenesis of various disease and design reasonable therapeutics for patients. Since the first knockout mouse was created in 1989 (Thomas and Capecchi, 1987), transgenic and knockout animal models have become useful tools for the study of human diseases and their treatments.
As an example, Nrf2 is a transcription factor that plays a role in the defense mechanisms against xenobiotic-induced oxidative stress and toxicity. High sensitivity of Nrf2 knockout mice to toxicant-induced liver injury may result from the repression of detoxifying enzymes due to Nrf2 deficiency (Ramos-Gomez et al., 2001; Chan and Kan, 1999; Enomoto et al., 2001) . Moreover, Nrf2-deficient mice exhibited decreased biliary excretion of sulfobromophthalein and decreased bile acid synthesis, which support the concept that the knockout animals may serve as a valuable tool to assess acute toxicity of drug candidate (Table 1) . PPARa is another important transcription factor in the liver which plays a role in fatty acid oxidation and inflammatory processes during hepatic peroxisome proliferation. In PPARα-and Nrf2-null mice, perfluorodecanoic acid-mediated induction of Mrp3 and Mrp4 mRNA was attenuated (Maher et al., 2008), indicating that compensatory hepatoprotective responses might be disrupted. Moreover, PPARα was shown to be associated with down-regulation of liver uptake transporters such as Oatp1a1, 1a4, 1b2, and Ntcp (Cheng and Klaassen, 2008a). Knockouts of other nuclear receptors such as FXR, CAR and PXR may also be useful as toxicological tools as reported in the recent articles of Toxicological Sciences in the years of 2008~2009 (Table 1) .
Table 1.
Frequently applied transgenic and knockout animals used in the field of toxicology
| Knockout genes | Type of animals | Effects on toxicology | References |
|---|---|---|---|
|
| |||
| Nrf2 | C57BL/6 mice | - increased hepatotoxicity to acetaminophen | Reisman et al., 2009a, b; Tanaka et al., 2009; Reisman et al., 2009c; Maher et al., 2008 |
| - decreased biliary excretion of sulfobromophthalein | |||
| - decreased bile acid accumulation | |||
| - decreased expression of detoxification enzymes and transporters | |||
| PPARα | C57BL/6 mice | - decreased expression of multidrug resistance-associated protein transporters | Maher et al., 2008; Cheng and Klaassen, 2008a, b; Faiola et al., 2008 |
| - abrogated downregulation of Oatp1a1, 1a4, 1b2, and Ntcp and induction of Cyp4A14 by perfluorodecanoic acid | |||
| - decreased PPAR-δ agonist-induced skeletal muscle damage and liver injury | |||
| FXR | C57BL/6 mice | - enhanced susceptibility of mice to alpha-naphthyl isothiocyanate-induced liver injury | Cui et al., 2009; Cheng and Klaassen, 2008a, b |
| CAR | C57BL/6 mice | - abrogated induction of Cyp2B10 by perfluorodecanoic acid | Phillips and Goodman, 2009; Cheng and Klaassen, 2008a, b |
| PXR | C57BL/6 mice | - no effect on alpha-naphthyl isothiocyanate- or perfluorocarboxylic acids-induced liver injury | Cui et al., 2009; Cheng and Klaassen, 2008b |
Advances in genomics and proteomics increased possible solutions for improved identification and validation of target molecules (Ohlstein et al., 2000). Gene knockout or silencing, gene expression analysis using microarray technology, and genetics-based approaches of the study population are now commonly used across the industries. These mutants are the tools to directly understand the biological function and utility of the gene and genome. In the past, there were no technologies available to alter the genomic DNA sequences except random mutagenesis. The developed site-directed mutagenesis in the mouse is called “gene targeting” or “knockout mouse” since this technology specifically disrupts the gene of interest. In 1990, many fundamental technologies to elucidate the gene and genome function were set (Collins and McKusick, 2001). The development of site-directed mutagenesis to introduce a single base substitution in the mouse genome has been a dream in the mammalian genetics. The knock-in mouse has now become one of such technologies.
Human genome is 30 times larger in size and 3 times more in numbers than worm and fly. Since most proteins and their functions are not identified yet, applications of genomics would provide potential drug targets. Currently, only ~800 targets have been identified and more than 7000 molecules are waiting to be explored (Birney et al., 2001). The word ‘leads’ means compounds that have a binding affinity with the micro molar range or less. These are the starting materials for drug development in spite of difficulties presented by targeting protein, cytokine receptors and phosphatases. Thus, ‘drug-like compounds’ means materials that have sufficiently acceptable pharmacokinetic and toxicity properties to survive through the completion of phase I clinical trials. Drug efficacy study is necessary as a proof to ensure the effectiveness with regard to the therapeutic aims. Through drug efficacy study, researchers can evaluate medical effectiveness and narrow the range of “drug-like compounds”. In addition, drug toxicity study is required during drug development to exclude compounds that have unacceptable toxicity or side effects for volunteers in the clinical trials. These compounds have a common feature called ‘the rule of five’; molecular weight less than 500, hydrogen bonds less than 10, hydrogen bond donors less than 5, and calculated logP less than 5 (Lipinski et al., 2001). After approved for marketing for human disease therapy by a regulatory agency (i.e., FDA) , they are called ‘drugs’. Target validation by genomics can save the cost and time for drug development and help avoid unnecessary investments. Moreover, target validation is effective predictors of ‘leads’ generation success.
It is noteworthy that siRNA is a useful tool in drug discovery especially for target identification and validation (Wang et al., 2004). A siRNA-based target identification may involve high throughput transfection of a large siRNA library into cultured cells and observation of the resulting phenotypes, most often by an established assay (Wang et al., 2004). Conditionally replicative adenoviruses also have emerged as a novel and promising approach for a range of advanced neoplasm (Kirn and McCormick, 1996). In this regard, direct translation of this approach from the laboratory to human clinical trials has proceeded at an unprecedented speed.
Previously, drug discovery was usually started from empirical and unselected search for new candidate. These models are not predictable and they often show poor correlation of non-human with human pharmacology. The trend is now changing. Compounds that act through defined molecular mechanisms associated with disease will be developed according to the rationale. Gleevec, a Bcr/Abl kinase inhibitor, was developed using this strategy (Druker and Lydon, 2000). In this case, instead of compound screening, drug development starts with target screening. Target-dependent in vivo model studies using transgenic animals will be followed, and clinical trials include assessment of effect on target. Whether the target of interest is associated with the disease, the frequency of association, mechanism of the link between target and disease, and whether pharmacologic modulation of the target can modify the disease might be important issues. Transgenic mice can help answer these questions on the efficacy and toxicology. For example, PPARα knockout mice revealed the mechanism of hepatotoxic effects of amiodarone (McCarthy et al., 2004). Amiodarone is a widely used antiarrhythmic agent but use of this drug is limited due to hepatotoxicity. Given the similar pathological consequences of PPARα activation and amiodarone-induced hepatotoxicity, PPARα was considered to be relevant to the toxicity associated with amiodarone. As expected, amiodarone did not induce hepatomegaly in PPARα knockout mice, suggesting that the effects of amiodarone were PPARα-dependent. As another example, Pglycoprotein-deficient mice provided valuable information as to the pharmacodynamic consequences of risperidone, a P-glycoprotein substrate (Kirschbaum et al., 2008) . These results are important because applications of these animals provide new mechanistic perspectives. Newly approved drugs and related transgenic knockout animal models are listed in Table 2.
Table 2.
Recently approved new drugs and their targets identified using genetic applications (New Drug Applications Report, FDA, 2008-2009
| Drugs | Target molecule | Active components | Usages | Applications of genetic modulation |
|---|---|---|---|---|
|
| ||||
| EXTAVIA | Unknown | Interferon β-IB | multiple sclerosis | IFN-α/β receptor knockout mice (Müller et al., 1994) |
| MULTAQ | Unknown | Dronedarone HCl | antiarrhythmic | PPARα knockout mice (McCarthy et al., 2004) |
| EFFIENT | P2Y12 | Prasugrel HCl | thrombotic cardiovascular | P2Y12 knockout mice (Andre et al., 2003) |
| INVEGA SUSTENNA | Unknown | Paliperidone palmitate | schizophrenia | P-glycoprotein (mdr1a/1b) knockout mice (Kirschbaum et al., 2008) |
| ONGLYZA | DPP-4 | Saxagliptin HCl | glycemic control in adults with type 2 diabetes mellitus | DPP-4 knockout mice and DPP-4 deficient Fischer rats (Fuchs et al., 2009) |
| ILARIS | IL-1 | Canakinumab | cryopyrin-associated periodic syndromes | IL-1 receptor knockout mice (Pineau et al., 2009) |
| SAMSCA | vasopressin V2 receptor | Tolvaptan | hypervolemic and euvolemic hyponatremia | Only vasopressin V1 receptor (Avpr1a, b) knockout mice available |
| COARTEM | endoperoxide moiety, inhibits the formation of β-hematin | Artemether; Lumefantrine | acute, uncomplicated malaria infections | Increased quinine uptake in mdr1a knockout mice (Pussard et al., 2007) |
| AFINITOR | mTOR | Everolimus | advanced renal cell carcinoma | Pten knockout mice (Hernando et al., 2007) |
| ULORIC | xanthine oxidase | Febuxostat | hyperuricemia in patients with gout | No transgenic animal model available |
| LUSEDRA | prodrug of propofol | Fospropofol disodium | monitored anesthesia care sedation | Glutamate decarboxylase 65 knockout mice (Kubo et al., 2009) |
| FIRMAGON | GnRH receptor | Degarelix acetate | advanced prostate cancer | Only G protein-coupled receptor 54 knockout mice available |
| BANZEL | sodium channel | Rufinamide | seizures associated with Lennox-Gastaut syndrome | No transgenic animal model available |
| PROMACTA | TPO receptor | Eltrombopag olamine | thrombocytopenia | Mpl (thrombopoietin receptor) knockout mice (Jin et al., 2006) |
| TOVIAZ | M receptor | Fesoterodine fumarate | overactive bladder | Muscarinic receptor subtype knockout mice (Ito et al., 2009) |
Nowadays, compounds entering development are increasing every year (Feher and Schmidt, 2003). Along with it, identified innovative targets also increase, which carry increased risk of failure due to problems associated with toxicity and adverse effects (Nebeker et al., 2004) . Limited range of efficient decision making tools to pursue late phase drug development might be another barrier. Increasing pressure to shorten development cycle times and decreasing R&D budgets are the reasons for the necessity of efficient research on target validation and evaluation of preclinical efficacy and toxicity. More efficient decision making tools, higher rates of early attrition and lower rates of late phase attrition in drug development, and shorter early development cycle times would be needed. To discard those compounds which will most likely fail as early as possible and concentrate on the resources which will most likely succeed, introduction of well-designed models becomes more important. It will speed up drugs to the market and produce additional income to companies.
Nonetheless, well-designed animal studies still have some problems because of the differences in etiology, natural history, and/or molecular pathogenetic events between animal model and human disease. Moreover, animals and humans have different pharmacokinetics/pharmacodynamics in the way drugs interact with each other (Graham and Lake, 2008). These limits make it difficult to predict outcomes in human. To overcome these problems, transgenic mice engineered to express human homologs called ‘humanized’ mice had been developed. These transgenic mice can be used in drug development for assessment of efficacy and toxicity (Yoshizato and Tateno, 2009). Knockout mice of CYP450 genes or phase II enzymes might be useful to study pharmacokinetics and pharmacodynamics properties (McKinnon and Nebert, 1998). Moreover, using the Cre recombinase, conditional gene deletion would also be possible. Gene knockout modulation is now increasingly used; the usage of knockout technique has been increased ~4-fold in the past 10 years. Certainly, it will grow faster in the next decade. Nowadays, siRNA technologies are also used in preclinical study to evaluate the mechanism of actions and specificity of drug candidates, and to identify better candidates earlier in the drug development process. The number of articles describing the applications of genetic modulations including siRNA technique to new drug development is depicted in Fig. 2.
Fig. 2. A trend of applications of genetic modulations to toxicological studies published in Toxicological Sciences. 1998; 45 (2) -1999; 51 (1) , 2008; 105 (2) -2009; 111 (1) .
CONCLUSIONS AND FUTURE DIRECTIONS
Pharmaceutical industries are facing productivity crisis over the last decade, despite advances in technologies for developing new candidate molecules and steady increase in R&D investment. The principal problem is the difficulty of developing efficient and safe drug with no adverse events; adverse event is the main reason for drug withdrawal and high rate of drop-out in clinical trials. Simplified and operationally efficient preclinical testing processes can dramatically reduce the high attrition rate and cost in new drug development steps. Today, multi-disciplinary approaches involving novel technologies are available; these technologies may improve in designing a pre-clinical study, and supply an effective framework to test alternative assumptions and facilitate the collection and interpretation of relevant information. Moreover, as all genes are potentially drug targets, strategies must be developed to gain insight that allows rate-limited R&D resources to be focused on genes with the greatest therapeutic potential.
Gene modulation is one of the most important tools of modern biology, and is commonly employed to examine the function of genes of interest (Debouck and Metcalf, 2000). Advances in new drug development have been achieved by exploding information on target selection and validation using transgenic knockout mice. These days, information obtained from gene knockout is accepted as a viable cost-effective alternative for mutagenicity and carcinogenicity testing. In addition, gene knockout can be used to define the biological mode of action by helping to discriminate between the closely related members of a gene family. The examples shown in this article illustrate the potential power of transgenic knockout animal model as a source of functional information and tools that can be used in studies at various other stages of the drug discovery process. New models by which we can evaluate in vivo toxicity will allow us to extrapolate therapeutic efficacy and toxicity to translational purposes that are used as therapeutic indexes for human trials.
Acknowledgments
This research was supported by a grant (10182KFDA992) from Korea Food & Drug Administration in 2010.
References
- 1.Andre P. Delaney S.M. LaRocca T. Vincent D. DeGuzman F. Jurek M. Koller B. Phillips D.R. Conley P.B. P2Y12 regulates platelet adhesion/activation thrombus growth and thrombus stability in injured arteries. J. Clin. Invest. 2003;112:398–406. doi: 10.1172/JCI17864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birney E. Bateman A. Clamp M.E. Hubbard T.J. Mining the draft human genome. Nature. 2001;409:827–828. doi: 10.1038/35057004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caldwell G.W. Ritchie D.M. Masucci J.A. Hageman W. Yan Z. The new pre-preclinical paradigm: compound optimization in early and late phase drug discovery. Curr. Top.Med. Chem. 2001;1:353–366. doi: 10.2174/1568026013394949. [DOI] [PubMed] [Google Scholar]
- 4.Chan K. Kan Y.W. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc. Natl. Acad. Sci.U.S.A. 1999;96:12731–12736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng X. Klaassen C.D. Critical role of PPARalpha in perfluorooctanoic acid- and perfluorodecanoic acidinduced downregulation of Oatp uptake transporters in mouse livers. Toxicol. Sci. 2008a;106:37–45. doi: 10.1093/toxsci/kfn161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng X. Klaassen C.D. Perfluorocarboxylic acids induce cytochrome P450 enzymes in mouse liver through activation of PPAR-alpha and CAR transcription factors. Toxicol. Sci. 2008b;106:29–36. doi: 10.1093/toxsci/kfn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins F.S. McKusick V.A. Implications of the Human Genome Project for medical science. JAMA. 2001;285:540–544. doi: 10.1001/jama.285.5.540. [DOI] [PubMed] [Google Scholar]
- 8.Cui Y.J. Aleksunes L.M. Tanaka Y. Goedken M.J. Klaassen C.D. Compensatory induction of liver efflux transporters in response to ANIT-induced liver injury is impaired in FXR-null mice. Toxicol. Sci. 2009;110:47–60. doi: 10.1093/toxsci/kfp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debouck C. Metcalf B. The impact of genomics on drug discovery. Annu. Rev. Pharmacol. Toxicol. 2000;40:193–208. doi: 10.1146/annurev.pharmtox.40.1.193. [DOI] [PubMed] [Google Scholar]
- 10.Descotes J. Testud F. Toxicovigilance: a new approach for the hazard identification and risk assessment of toxicants in human beings. Toxicol. Appl. Pharmacol. 2005;207:599–603. doi: 10.1016/j.taap.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 11.DiMasi J.A. Hansen R.W. Grabowski H.G. Lasagna L. Cost of innovation in the pharmaceutical industry. J.Health Econ. 1991;10:107–142. doi: 10.1016/0167-6296(91)90001-4. [DOI] [PubMed] [Google Scholar]
- 12.DiMasi J.A. Hansen R.W. Grabowski H.G. The price of innovation: new estimates of drug development costs. J. Health Econ. 2003;22:151–158. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 13.Druker B.J. Lydon N.B. Lessons learned from the development of an abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J. Clin. Invest. 2000;105:3–7. doi: 10.1172/JCI9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enomoto A. Itoh K. Nagayoshi E. Haruta J. Kimura T. O’Connor T. Harada T. Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- 15.Faiola B. Falls J.G. Peterson R.A. Bordelon N.R. Brodie T.A. Cummings C.A. Romach E.H. Miller R.T. PPAR alpha more than PPAR delta mediates the hepatic and skeletal muscle alterations induced by the PPAR agonist GW0742. Toxicol. Sci. 2008;105:389–394. doi: 10.1093/toxsci/kfn130. [DOI] [PubMed] [Google Scholar]
- 16.Feher M. Schmidt J.M. Property distributions: differences between drugs natural products andmolecules from combinatorial chemistry. J. Chem. Inf. Comput. Sci. 2003;43:218–227. doi: 10.1021/ci0200467. [DOI] [PubMed] [Google Scholar]
- 17.Fricker J. Time for reform in the drug-development process. Lancet. Oncol. 2008;9:1125–1126. doi: 10.1016/S1470-2045(08)70297-3. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs H. Tillement J.P. Urien S. Greischel A. Roth W. Concentration-dependent plasma protein binding of the novel dipeptidyl peptidase 4 inhibitor BI 1356 due to saturable binding to its target in plasma of mice rats and humans. J.Pharm. Pharmacol. 2009;61:55–62. doi: 10.1211/jpp/61.01.0008. [DOI] [PubMed] [Google Scholar]
- 19.Graham M.J. Lake B.G. Induction of drug metabolism: species differences and toxicological relevance. Toxicology. 2008;254:184–191. doi: 10.1016/j.tox.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Grieshaber C.K. Marsoni S. Relation of preclinical toxicology to findings in early clinical trials. Cancer Treat.Rep. 1986;70:65–72. [PubMed] [Google Scholar]
- 21.Hernando E. Charytonowicz E. Dudas M.E. Menendez S. Matushansky I. Mills J. Socci N.D. Behrendt N. Ma L. Maki R.G. Pandolfi P.P. Cordon-Cardo C. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat. Med. 2007;13:748–753. doi: 10.1038/nm1560. [DOI] [PubMed] [Google Scholar]
- 22.Ito Y. Oyunzul L. Yoshida A. Fujino T. Noguchi Y. Yuyama H. Ohtake A. Suzuki M. Sasamata M. Matsui M. Yamada S. Comparison of muscarinic receptor selectivity of solifenacin and oxybutynin in the bladder and submandibular gland of muscarinic receptor knockout mice. Eur. J. Pharmacol. 2009;615:201–206. doi: 10.1016/j.ejphar.2009.04.068. [DOI] [PubMed] [Google Scholar]
- 23.Jin D.K. Shido K. Kopp H.G. Petit I. Shmelkov S.V. Young L.M. Hooper A.T. Amano H. Avecilla S.T. Heissig B. Hattori K. Zhang F. Hicklin D.J. Wu Y. Zhu Z. Dunn A. Salari H. Werb Z. Hackett N.R. Crystal R.G. Lyden D. Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat. Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirn D.H. McCormick F. Replicating viruses as selective cancer therapeutics. Mol. Med. Today. 1996;2:519–527. doi: 10.1016/S1357-4310(97)81456-6. [DOI] [PubMed] [Google Scholar]
- 25.Kirschbaum K.M. Henken S. Hiemke C. Schmitt U. Pharmacodynamic consequences of P-glycoproteindependent pharmacokinetics of risperidone and haloperidol in mice. Behav. Brain Res. 2008;188:298–303. doi: 10.1016/j.bbr.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Kubo K. Nishikawa K. Hardy-Yamada M. Ishizeki J. Yanagawa Y. Saito S. Altered responses to propofol but not ketamine in mice deficient in the 65-kilodalton isoform of glutamate decarboxylase. J. Pharmacol. Exp. Ther. 2009;329:592–599. doi: 10.1124/jpet.109.151456. [DOI] [PubMed] [Google Scholar]
- 27.Lipinski C.A. Lombardo F. Dominy B.W. Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001;46:3–26. doi: 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 28.Lord P.G. Nie A. McMillian M. The evolution of gene expression studies in drug safety assessment. Toxicol.Mech. Methods. 2006;16:51–58. doi: 10.1080/15376520600558200. [DOI] [PubMed] [Google Scholar]
- 29.Maher J.M. Aleksunes L.M. Dieter M.Z. Tanaka Y. Peters J.M. Manautou J.E. Klaassen C.D. Nrf2- and PPAR alpha-mediated regulation of hepatic Mrp transporters after exposure to perfluorooctanoic acid and perfluorodecanoic acid. Toxicol. Sci. 2008;106:319–328. doi: 10.1093/toxsci/kfn177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marsh S. Impact of pharmacogenomics on clinical practice in oncology. Mol. Diagn. Ther. 2007;11:79–82. doi: 10.1007/BF03256226. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy T.C. Pollak P.T. Hanniman E.A. Sinal C.J. Disruption of hepatic lipid homeostasis in mice after amiodarone treatment is associated with peroxisome proliferator-activated receptor-alpha target gene activation. J. Pharmacol.Exp. Ther. 2004;311:864–873. doi: 10.1124/jpet.104.072785. [DOI] [PubMed] [Google Scholar]
- 32.McKinnon R.A. Nebert D.W. Cytochrome P450 knockout mice: new toxicological models. Clin. Exp. Pharmacol. Physiol. 1998;25:783–787. doi: 10.1111/j.1440-1681.1998.tb02153.x. [DOI] [PubMed] [Google Scholar]
- 33.Müller U. Steinhoff U. Reis L.F. Hemmi S. Pavlovic J. Zinkernagel R.M. Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 34.Nebeker J.R. Barach P. Samore M.H. Clarifying adverse drug events: a clinician’s guide to terminology documentation and reporting. Ann. Intern. Med. 2004;140:795–801. doi: 10.7326/0003-4819-140-10-200405180-00009. [DOI] [PubMed] [Google Scholar]
- 35.Ohlstein E.H. Ruffolo R.R. Jr. Elliott J.D. Drug discovery in the next millennium. Annu. Rev. Pharmacol. Toxicol. 2000;40:177–191. doi: 10.1146/annurev.pharmtox.40.1.177. [DOI] [PubMed] [Google Scholar]
- 36.O’Quigley J. Pepe M. Fisher L. Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics. 1990;46:33–48. doi: 10.2307/2531628. [DOI] [PubMed] [Google Scholar]
- 37.Paul J. Seib R. Prescott T. The Internet and clinical trials: background online resources examples and issues. J. Med. Internet. Res. 2005;7:e5. doi: 10.2196/jmir.7.1.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peto R. Pike M.C. Armitage P. Breslow N.E. Cox D.R. Howard S.V. Mantel N. McPherson K. Peto J. Smith P.G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br. J. Cancer. 1976;34:585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips J.M. Goodman J.I. Multiple genes exhibit phenobarbital-induced constitutive active/androstane receptormediated DNA methylation changes during liver tumorigenesis and in liver tumors. Toxicol. Sci. 2009;108:273–289. doi: 10.1093/toxsci/kfp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pineau I. Sun L. Bastien D. Lacroix S. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL-1 receptor/MyD88-dependent fashion. Brain. Behav.Immun. 2009 doi: 10.1016/j.bbi.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Pussard E. Merzouk M. Barennes H. Increased uptake of quinine into the brain by inhibition of P-glycoprotein. Eur. J. Pharm. Sci. 2007;32:123–127. doi: 10.1016/j.ejps.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Pusztai L. Limitations of pharmacogenomic predictor discovery in Phase II clinical trials. Pharmacogenomics. 2007;8:1443–1448. doi: 10.2217/14622416.8.10.1443. [DOI] [PubMed] [Google Scholar]
- 43.Ramos-Gomez M. Kwak M.K. Dolan P.M. Itoh K. Yamamoto M. Talalay P. Kensler T.W. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficientmice. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reisman S.A. Csanaky I.L. Aleksunes L.M. Klaassen C.D. Altered disposition of acetaminophen in Nrf2-null and Keap1-knockdown mice. Toxicol. Sci. 2009a;109:31–40. doi: 10.1093/toxsci/kfp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reisman S.A. Csanaky I.L. Yeager R.L. Klaassen C.D. Nrf2 activation enhances biliary excretion of sulfobromophthalein by inducing glutathione-S-transferase activity. Toxicol. Sci. 2009b;109:24–30. doi: 10.1093/toxsci/kfp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reisman S.A. Yeager R.L. Yamamoto M. Klaassen C.D. Increased Nrf2 activation in livers from Keap1-knockdown mice increases expression of cytoprotective genes that detoxify electrophiles more than those that detoxify reactive oxygen species. Toxicol. Sci. 2009c;108:35–47. doi: 10.1093/toxsci/kfn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rioux P.P. Clinical trials in pharmacogenetics and pharmacogenomics: methods and applications. Am. J. Health Syst. Pharm. 2000;57:887–898. doi: 10.1093/ajhp/57.9.887. [DOI] [PubMed] [Google Scholar]
- 48.Ryan T.P. Stevens J.L. Thomas C.E. Strategic applications of toxicogenomics in early drug discovery. Curr. Opin.Pharmacol. 2008;8:654–660. doi: 10.1016/j.coph.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 49.Simon R. Optimal two-stage designs for phase II clinical trials. Control. Clin. Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 50.Stewart J.J. Allison P.N. Johnson R.S. Putting a price on biotechnology. Nat. Biotechnol. 2001;19:813–817. doi: 10.1038/nbt0901-813. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka Y. Aleksunes L.M. Cui Y.J. Klaassen C.D. ANIT-induced intrahepatic cholestasis alters hepatobiliary transporter expression via Nrf2-dependent and independent signaling. Toxicol. Sci. 2009;108:247–257. doi: 10.1093/toxsci/kfp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas K.R. Capecchi M.R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- 53.Ulrich R. Friend S.H. Toxicogenomics and drug discovery: will new technologies help us produce better drugs? Nat. Rev. Drug Discov. 2002;1:84–88. doi: 10.1038/nrd710. [DOI] [PubMed] [Google Scholar]
- 54.Valenta R. The future of antigen-specific immunotherapy of allergy. Nat. Rev. Immunol. 2002;2:446–453. doi: 10.1038/nri824. [DOI] [PubMed] [Google Scholar]
- 55.Wang S. Sim T.B. Kim Y.S. Chang Y.T. Tools for target identification and validation. Curr. Opin. Chem. Biol. 2004;8:371–377. doi: 10.1016/j.cbpa.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 56.Weinshilboum R. Inheritance and drug response. N. Engl. J. Med. 2003;248:529–537. doi: 10.1056/NEJMra020021. [DOI] [PubMed] [Google Scholar]
- 57.Woodcock J. Woosley R. The FDA critical path initiative and its influence on new drug development. Annu. Rev.Med. 2008;59:1–12. doi: 10.1146/annurev.med.59.090506.155819. [DOI] [PubMed] [Google Scholar]
- 58.Yoshizato K. Tateno C. A human hepatocyte-bearing mouse: an animal model to predict drug metabolism and effectiveness in humans. PPAR Res. 2009;2009:476217. doi: 10.1155/2009/476217. [DOI] [PMC free article] [PubMed] [Google Scholar]