Abstract
This study was performed to examine whether elevated activity of cAMP responsive element-binding protein (CREB) attenuates the detrimental effects of acute gamma (γ) -irradiation on hippocampal neurogenesis and related functions. C57BL/6 male mice were treated with rolipram (1.25 mg/kg, i.p., twice a day for 5 consecutive days) to activate the cAMP/CREB pathway against cranial irradiation (2 Gy) , and were euthanized at 24 h post-irradiation. Exposure to γ-rays decreased both CREB phosphorylation and immunohistochemical markers for neurogenesis, including Ki-67 and doublecortin (DCX) , in the hippocampal dentate gyrus (DG) . However, the rolipram treatment protected from γ-irradiation-induced decreases of CREB phosphorylation, and Ki-67 and DCX immunoreactivity in the hippocampal DG. In an object recognition memory test, mice trained 24 h after acute γ-irradiation (2 Gy) showed significant memory impairment, which was attenuated by rolipram treatment. The results suggest that activation of CREB signaling ameliorates the detrimental effects of acute γ-irradiation on hippocampal neurogenesis and related functions in adult mice.
Keywords: CREB, Hippocampus, Irradiation, Neurogenesis, Rolipram
INTRODUCTION
During treatment of brain tumors, some head and neck tumors, and other conditions including arteriovenous malformations, the normal brain is exposed to ionizing radiation (Posner, 1992) . As the number of long-term survivors increases, irradiation-induced side effects, including cognitive impairments, have become a health issue (Stone et al.,2003; Coleman et al., 2004; Meyer and Brown, 2006) . Relatively lower doses of ionizing radiation can be responsible for cognitive impairments, even without any significant morphological changes in the mammalian brain (Crossen et al., 1994) . Although neural precursor cells in the dentate gyrus (DG) of the hippocampus have been suggested to be involved in some animal models, the precise mechanism of the impairment is unclear. (Madsen et al., 2003; Kim et al.,2008) .
The cAMP response element binding protein (CREB) is a member of a transcription factor family belonging to the basic-domain leucine zipper class, which regulates transcription via a specific DNA target, the cAMP-response element (CRE) (Brindle and Montminy, 1992) . Several studies have demonstrated that CREB activation plays an important role in cellular and behavioral models of learning and memory, as well as in neuronal survival and activation (Silva et al., 1992; Finkbeiner, 2000; Nakagawa et al.,2002) . Manipulation of the cAMP/CREB signal pathway can have beneficial effects in the context of age-related memory loss and cognitive impairment in Alzheimer disease (Barad, 2003; Gong et al., 2004) . Rolipram, a specific inhibitor of the phosphodiesterase type 4 (PDE4) isoform, is able to restore the cAMP/CREB pathway activity (Vitolo et al., 2002) . Rolipram has been shown to enhance memory retention in both young and aged rats, and to reverse memory deficits in animal models of cerebral ischemia (Nagakura et al., 2002) , Rubinstein-Taybi syndrome (Bourtchouladze et al., 2003) and Alzheimer disease (Gong et al., 2004) . However, nothing is known about the protective effects of CREB activation against irradiation-induced cognitive impairment and suppression of neurogenesis.
In this study, we evaluated whether gamma (γ) -irradiation could suppress CREB phosphorylation in the adult mouse hippocampus and, if so, whether the suppression may be correlated with irradiation-mediated inhibition of hippocampal neurogenesis. Furthermore, the study examined whether rolipram treatment (CREB activation) exerted beneficial influences on hippocampal neurogenesis and hippocampus-dependent behavior in order to confirm the neuroprotective effects of activated CREB.
MATERIALS AND METHODS
Animals.
Eight-week-old male C57BL/6 mice (n = 60) obtained from the animal center at Oriental Inc. (Korea) were used in this study. The mice were fed a standard animal diet. All animal experiments followed a protocol approved by the Committee for Animal Experimentation at the Chonnam National University.
Irradiation and tissue sampling.
The head of mice were irradiated with 2 Gy using 60Co gamma-rays using a Theratron 780 apparatus (Atomic Energy of Canada Ltd., Ontario, Canada) at a dose-rate of 820 cGy/min. The sham control group were also transported to the irradiation facility but were not irradiated.
To see the effect of irradiation on CREB phosphorylation and neurogenesis in mouse hippocampus, the mice were sacrificed and each brain was dissected from each group at 24 h and 14 days (n = 4 mice per group) after the 2 Gy γ-irradiation.
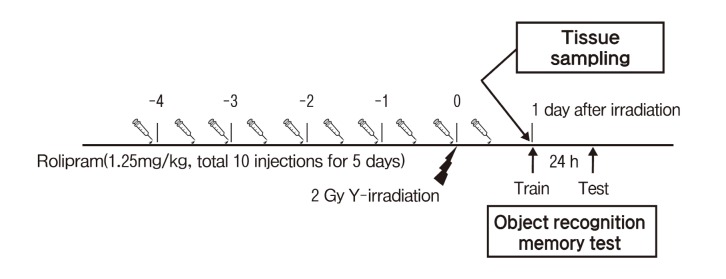
To examine the beneficial effect of rolipram on the CREB activity and neurogenesis in the mouse hippocampus with irradiation, rolipram (1.25 mg/kg, twice a day for 5 consecutive days; Sigma-Aldrich, St. Louis, MO, USA) in saline containing 1% dimethylsulfoxide or the vehicle alone were intraperitoneally administered, as shown in Fig. 1. The mice were sacrificed and the brains were then dissected from each group at 24 h after 2 Gy γ-irradiation (n = 4 mice per group) . Samples of each brain were processed for embedding in paraffin wax after fixation in 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4) .
Fig. 1. Schematic overview of the experimental procedure. Rolipram or vehicle was administered (1.25mg/kg twice a day for 5 consecutive days) . Mice received cranial irradiation using 60CO gamma-rays with 2 Gy. The mice were sacrificed at 24 h after irradiation. Separately mice were trained for object recognition at 24 h after irradiation and tested the recognition memory at 24 h after the training.
Immunohistochemistry.
The fixed brain tissues were processed in paraffin, cut into 4 ㎛-thick coronal sections,and deparaffinized. The sections were incubated in blocking nonspecific binding normal goat serum (Vectastatin Elite ABC kit; Vector Laboratories, Burlingame, CA, USA) for 60 min, and the brain sections were incubated with a 1 : 200 dilution of rabbit monoclonal anti-phosphorylated CREB (pCREB) (87G3; Cell Signaling Technology, Beverly, MA, USA) , a 1 : 400 dilution of rabbit monoclonal anti-Ki-67 (DRM004; Acris Antibodies GmbH, Hiddenhausen, Germany) , or a 1 : 400 dilution of rabbit polyclonal anti-doublecortin (DCX; Cell Signaling Technology) antibodies at 4℃ overnight. The sections then were reacted with biotinylated goat anti-rabbit IgG (Vectastatin Elite ABC kit) . The immunoreactivity was performed using the avidinbiotin peroxidase complex (Vectastatin Elite ABC kit) . The peroxidase reaction was developed using a diaminobenzidine substrate kit (DAB Substrate Kit SK-4100; Vector Laboratories) .As a control, the primary antibodies were omitted for a few test sections in each experiment. The sections were counterstained with hematoxylin before being mounted.
Cell counting.
The number of cells showing specific characteristics of pCREB-positive cells, proliferating cells (positive for Ki-67) and immature progenitor cells (positive for DCX) in the hippocampus was scored by an observer blinded to the identity of the sample using a histomorphometric approach (Kim et al., 2008; Yang et al., 2010) . The brain from each mouse was sampled at approximately 2.12 mm behind the bregma. A standardized counting area containing 5 ㎛-thick coronal sections in a one-in-ten series of sections representing the rostral/mid-hippocampus was used. For each mouse, three non-overlapping sections were analyzed, one from each of the three regions of the hippocampus (approximately 50 ㎛ apart) . All positively-immunolabeled cells within the SGZ of the supra- and infrapyrimidal blades of the DG were quantified. The number of immunopositive cells was determined from the values obtained from each DG in the three brain sections. The mean number of immunopositive cells in the three sections of each mouse was taken as n = 1. The number of immunopositive cells was expressed as the mean ± SEM for each group (n = 4) .
Open-field test.
Open-field analysis was used to measure the activity of the mice in a novel environment. The 8-week-old mice (n = 5 for each group) were placed individually in a brightly-lit arena (26 cm × 26 cm, 250 lux) . Parameters,including the total moving distance (cm) , ambulatory movement time (sec) , and ambulatory movement episodes,were determined over 5 min using the TruScan Photo Beam Activity System (Coulbourn Instruments, Whitehall, PA,USA) .
Object recognition memory test.
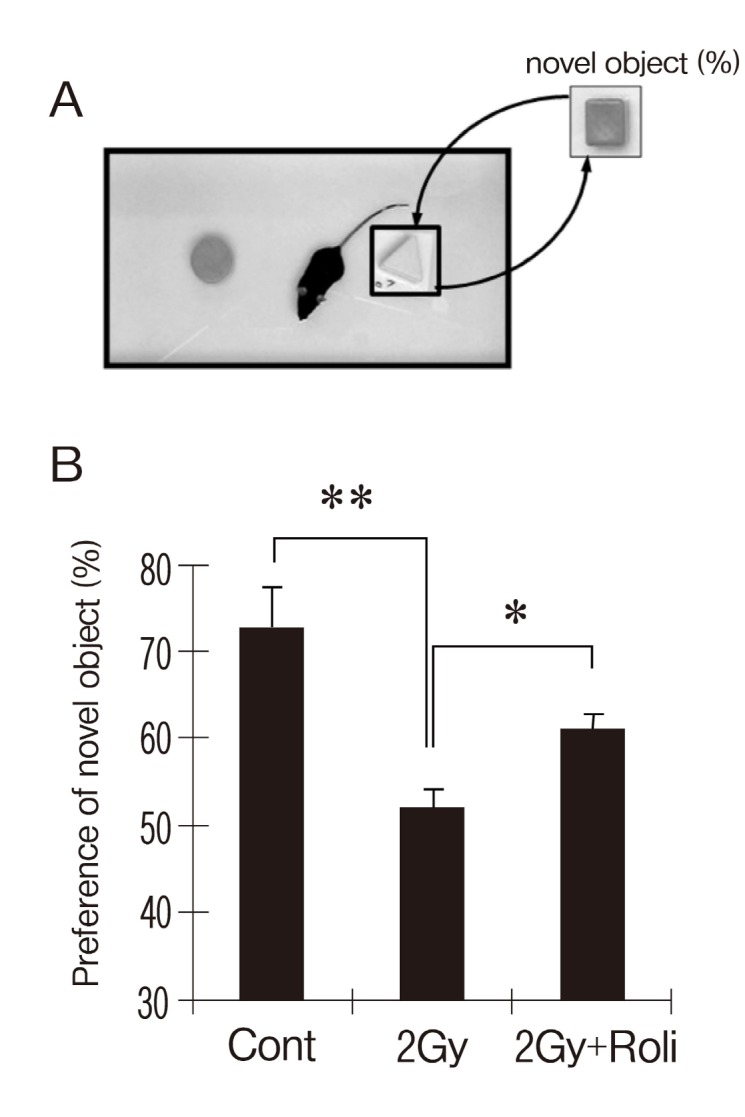
The object recognition memory task (a hippocampus-dependent learning paradigm) has been used for similar purposes (Myhrer, 1989;Kim et al., 2008; Yang et al., 2010) . The mice (n = 7 for each group) were first habituated in the training/testing acryl chamber (42 cm L, 28 cm W, 20 cm H) for 1 week (5 min/day) before training. The 3.5-cm tall plastic objects for discriminative recognition were cubes, pyramids, and cylinders, and they could not be displaced by the mice. The chamber arena and objects were cleaned with 75% ethanol between trials to prevent the build-up of olfactory cues.During training, two differently shaped objects were selected randomly and presented to each mouse for 15 min. At 24 h after training, another set of objects (one old object and one novel object) was presented to the trained mice (Fig. 4A) .The interactions of the mouse with each object, including approaches and sniffing, were scored. If the mouse had memory retention for an old object, it should show preference for the novel object during testing. The percentage of preference was defined as the number of interactions with a specific object divided by the total number of interactions with both objects.
Fig. 4. Rolipram ameliorates γ-irradiation-induced object recognition memory deficit. (A) The cylinder- and pyramid-shaped objects were presented for 15 min during training the cubeshaped object would be used as a novel object during testing (24 h after testing) . The interactions of the mouse with each object including approaches and sniffing were scored. (B) Vehicle-treated irradiated mice (2 Gy) displayed decreased preference to the novel object compared to sham-irradiated controls during testing at 24 h after training. Treatment of rolipram significantly attenuated the reduction in preference to novel object in vehicle-treated irradiation group. Data are shown as mean ± SEM (*p < 0.05 **p < 0.01) .
Statistical analysis.
The data indicate the mean ±SEM. The data were analyzed using one-way analysis of variance (ANOVA) followed by a Student-Newman-Keuls post hoc test for multiple comparisons. In all cases, a p value < 0.05 was considered significant.
RESULTS
Histological change in hippocampal structure using hematoxylin and eosin staining.
No anatomical abnormalities were found at the light microscopic level in 2 Gyirradiated brains (data not shown) . The histological findings of C57BL/6 mice mirrored those of ICR mice in our previous report (Kim et al., 2008) .
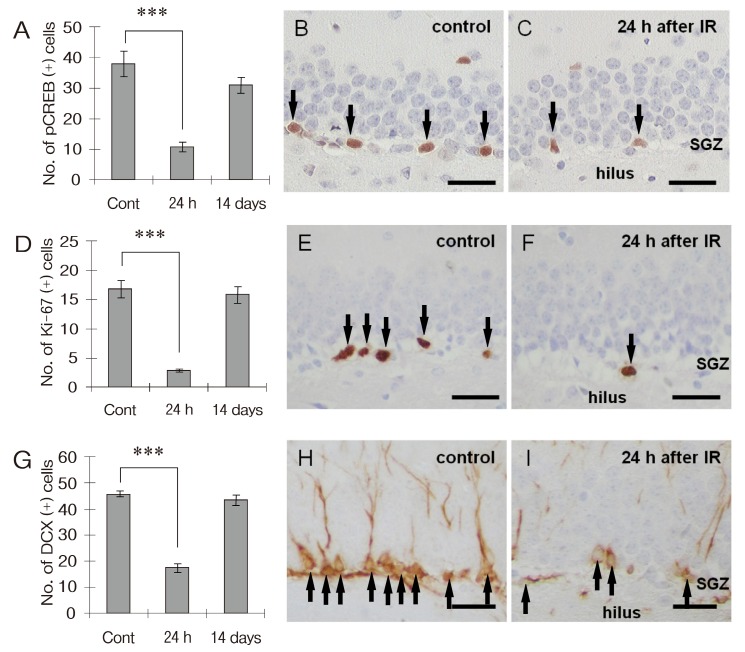
Immunohistochemical changes of pCREB-, Ki-67- and DCX-positive cells in the DG of adult mouse hippocampus following irradiation.
pCREB-positive cells were distributed mainly in the subgranular zone (SGZ) of adult hippocampal DG in sham-irradiated controls (38 ± 4.1 cells/DG, n = 4; Figs. 2A and Figs. 2B) . The number of pCREB-positive cells markedly declined at 24 h post-irradiation (10.75 ±1.38 cells/DG, n = 4; Figs. 2A and Figs. 2C) , and then returned to the control level at 14 days post-irradiation (31 ± 2.55 cells/DG, n = 4; Fig. 2A) .
Fig. 2. Exposure to 2 Gy γ-irradiation significantly decreases pCREB and neurogenesis in hippocampi DG. Bar graphs showing changes of the number of pCREB- (A) , Ki-67- (D) and DCX-positive cells (G) in hippocampal DG after 2Gy irradiation. Representative images showing the pCREB (B and C) , Ki-67 (E and F) and DCX immunoreactivities (H and I) in the hippocampal DG of sham-irradiated controls (B E and H) and γ-irradiated groups (24 h after irradiation; C, F, and I) . The immunoreactivity of pCREB, Ki-67, and DCX markedly declined at 24 h post-irradiation and then returned to the control level at 14 days post-irradiation. Data are shown as mean ± SEM (***p < 0.001) . Counterstained with hematoxylin. Scale bars = 25 ㎛.
The proliferation of hippocampal progenitor cells and the immature progenitor neurons in the DG of the adult mouse hippocampus were assessed by counting the numbers of Ki-67- and DCX-positive cells, respectively. Ki-67- (16.75 ±1.49 cells/DG, n = 4; Figs. 2D and Figs. 2E) and DCX-positive cells (45.75 ± 1.11 cells/DG, n = 4; Figs. 2G and Figs. 2H) were consistently observed in hippocampal DG in sham-irradiated controls. The number of Ki-67-positive cells in the DG markedly declined (2.75 ± 0.25 cells/DG, n = 4; Figs. 2D and Figs. 2F) , but increased to the sham-irradiated control level at 14 days post-irradiation (15.75 ± 1.38 cells/DG, n = 4;Fig. 2D) . The number of DCX-positive cells in the DG significantly declined at 24 h post-irradiation (17.5 ± 1.8 cells/DG, n = 4; Figs. 2G and Figs. 2I) , but increased to the sham-irradiated control level at 14 days post-irradiation (43.5 ± 2.1 cells/DG, n = 4; Fig. 2G) . This suggests that the temporal patterns of Ki-67 and DCX immunoreactivities corresponded to that of pCREB.
CREB activation ameliorates γ-irradiation-induced decrease of hippocampal neurogenesis.
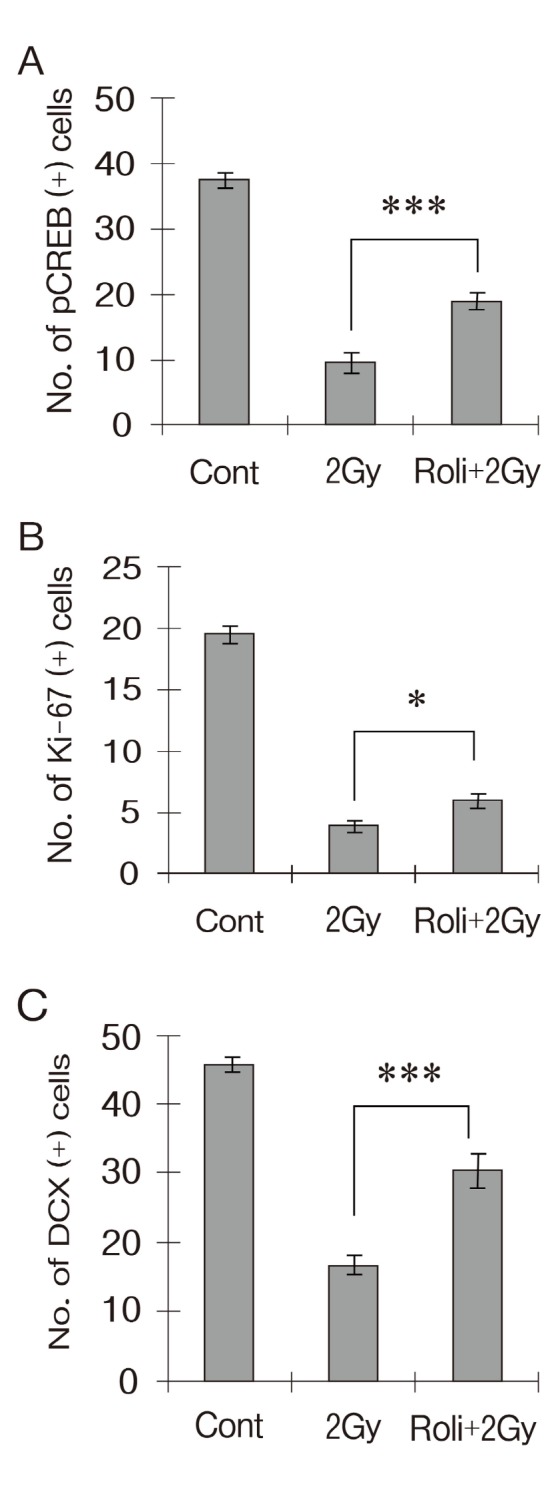
The beneficial effect of rolipram on irradiation-induced reduction of hippocampal neurogenesis in adult mice was investigated. The number of pCREB-positive cells in hippocampal DG was significantly decreased by approximately 75% of the shamirradiated control at 24 h after irradiation (Fig. 3A) . Rolipram treatment produced a significant increase in the number of pCREB-positive cells (18.83 ± 1.17 cells/DG, n = 4) ,compared with the vehicle-treated irradiation group (9.67 ±1.54 cells/DG, n = 4) . The number of Ki-67-positive cells at 24 h after irradiation decreased by approximately 82% that of the sham-irradiated control (Fig. 3B) . Rolipram treatment significantly inhibited the reduction of Ki-67-positive cells caused by irradiation (vehicle-treated irradiation group:3.83 ± 0.48 cells/DG, n = 4; rolipram-treated irradiation group:6 ± 0.63 cells/DG, n = 4) . The number of DCX-positive cells at 24 h after irradiation decreased by approximately 64% that of the sham-irradiated controls (Fig. 3C) . The rolipram treatment significantly increased the number of DCX-positive cells at 24 h after irradiation (30.5 ± 2.46 cells/DG, n = 4) , compared with the vehicle-treated irradiation group (16.5 ± 1.57 cells/DG, n = 4) .
Fig. 3. Rolipram exerts a beneficial influence on γ-irradiation-induced decrease of the number of pCREB- (A) Ki-67- (B) and DCX-positive cells (C) in the hippocampal DG. Rolipram (1.25mg/kg) was administered intraperitoneally twice per day for 5 days. All the tissues were collected at 24 h after 2 Gy γ-irradiation. Rolipram treatment significantly increased the number of pCREB- Ki-67- and DCX-positive cells at 24 h after irradiation compared with the vehicle-treated irradiation group. Data are shown as mean ± SEM (*p < 0.05 ***p < 0.001) .
CREB activation attenuates γ-irradiation-induced recognition memory deficit.
An experiment examined the basal locomotor activity of sham controls and γ-irradiated mice with treatment of either vehicle or rolipram at 24 h after irradiation in a novel environment by open field analysis (n = 5 for each group) , because basal locomotor activity can contribute to potential differences in hippocampusdependent learning and memory behavior tests. The sham controls and γ-irradiated mice with pre-treatment of either vehicle or rolipram showed comparable moving distances,ambulatory movement times and episodes, and resting times (Table 1) .
Table 1.
Open-field analysis of mice placed in a novel environment after exposure of γ-ray
| Distance (cm) | Movement time (s) | Movement episodes | |
|---|---|---|---|
|
| |||
| Control | 553.1 ± 41.8 | 257 ± 6.43 | 30.6 ± 3.08 |
| 2 Gy | 649.6 ± 42.5 | 269.8 ± 1.66 | 24.8 ± 1.28 |
| Roli +2 Gy | 559.6 ± 25.7 | 261.4 ± 1.86 | 28.8 ± 1.74 |
Open-field data for sham-irradiated controls (irradiated with 0 Gy) , 2 Gy-irradiated and rolipram (Roli) -pretreated irradiation groups at 1 day after exposure (n = 5) . No significant differences were found in movement distance, ambulatory movement time, or the number of ambulatory movement episodes between vehicle-treated controls, 2 Gy-irradiated and Roli-pretreated irradiation groups at 1 day after exposure. The data are reported as the mean ± SEM.
Next, sham-irradiated controls and γ-irradiated mice were examined following treatment with either vehicle or rolipram at 24 h after irradiation, using the sensitive hippocampus-dependent paradigm of object recognition memory (n =7 for each group) . Sham-irradiated and γ-irradiated mice treated with either vehicle or rolipram displayed equal preference to the two objects during training (data not shown) . During testing, one conditioned old object was replaced by a novel object. If mice retained memory for the old object, they would show preference to the novel object. When tested 24 h after training, the results indicated that γ-irradiated mice showed impairment of memory, whereas rolipram treatment significantly attenuated the memory deficit in irradiated mice (Fig. 4) . During the test, the preference (mean ± SEM) to the novel object were 72.8 ± 4.7% in sham-irradiated controls, 51.9 ± 2.5% in vehicle-treated irradiation group and 61 ± 1.6% in rolipram-treated irradiation group (Fig. 4) .
There was no significant difference in the total number of interactions during training between the sham-irradiated controls (26.3 ± 3.2) , vehicle-treated irradiation group (27 ± 0.58, p = 0.714 vs. sham-irradiated controls) and rolipramtreated irradiation group (24.8 ± 2.18, p = 0.679 vs. shamirradiated controls, p = 0.9 vs. vehicle-treated irradiation group) . The data was indicative of comparable attention, motivation, and visual perception.
DISCUSSION
The present study has demonstrated that γ-irradiation decreases constitutive CREB activity in the hippocampal DG of adult mice and that the promotion of the CREB activity attenuates γ-irradiation-induced reduction of hippocampal neurogenesis and recognition memory deficit.
It is generally accepted that CREB plays many roles in the development and normal function of the central nervous system. CREB could underlie the action of different neurotransmitters, neurotrophic factors, or conditions known influence neurogenesis (Silva et al., 1992; Barad et al.,1998) . CREB is an important regulator of proliferation, differentiation, and apoptosis, and has been implicated in the regulation of the survival and maturation of newly generated cells in the hippocampus (Bender et al., 2001; Nakagawa et al., 2002) . Down-regulation of CREB activity results in decreased levels of hippocampal neurogenesis (Barad et al., 1998; Nakagawa et al., 2002; Gong et al.,2004; Vitolo et al., 2002) . CREB activity requires phosphorylation at Ser-133 in response to diverse physiological signals (Gonzalez and Montminy, 1989) , and CREB is highly phosphorylated in the granular cell layer of the DG (Bender et al., 2001) . CREB activation is confined to granular cells during the proliferative and differentiation stages (Bender et al., 2001) . In this study, an immunohistochemical analysis revealed that the phosphorylation of CREB was localized mainly in the SGZ of mouse hippocampal DG. This observation is consistent with similar observations in other studies (Bender et al., 2001; Nakagawa et al., 2002) . In aging rats, pCREB levels are very low and limited to cells residing in the SGZ of the DG, where progenitor cells reside, and corresponds to learning and memory impairments during senescence (Bender et al., 2001; Hattiangady et al., 2005) .The disruption of CREB phosphorylation in the hippocampus results in a decreased proliferation rate of progenitor cells (Nakagawa et al., 2002) . The results of this study indicate that γ-irradiation significantly decreases the number of progenitor cells in the SGZ of the DG. Furthermore, the transiently reduced pattern of hippocampal neurogenesis caused by γ-irradiation corresponded to that of pCREB immunoreactivity in adult mouse brains. These results support the suggestion that CREB phosphorylation is associated with neurogenesis in the DG of the mouse hippocampus.
Rolipram, specific PDE4 inhibitor, is known to enhance learning and memory by increasing cAMP levels and stimulating CREB pathway, which is highly conserved across species, regulating hippocampus-dependent memory in mice (Bourtchouladze et al., 2003; Nakagawa et al., 2002) . Previous reports separately demonstrated that rolipram increases hippocampal CREB expression or activity (Nibuya et al.,1996; Nakagawa et al., 2002) , improves hippocampus-dependent memory (Barad et al., 1998; Navakkode et al., 2004) or reverses various types of memory deficits (Bourtchouladze et al., 2003; Vitolo et al., 2002; Barad, 2003; Gong et al., 2004) . It has been suggested that rolipram exerts beneficial effects in various injury models mechanisms that include blocking of the effect of inflammatory cytokines (Beshay et al., 2001; Zhang et al., 2004) and the production of superoxide (Dinter et al., 2000) . Moreover, it is possible that increased phosphorylation of CREB mediated by activation of protein kinase A due to increased cyclic AMP may play a role in the neuroprotective effects (Gong et al., 2004;Monti et al., 2006) . Therefore, rolipram could not only immediately counteract the deficit of pCREB in the DG of adult hippocampus, but also considerably protect from decreased neurogenesis in the DG after irradiation.
Recently, we suggested that a relatively low dose (2 Gy) of irradiation in adult ICR mice is sufficiently detrimental to interrupt the functioning of the hippocampus, including learning and memory, possibly through the inhibition of neurogenesis (Kim et al., 2008) . In the present study, 2 Gyirradiation also inhibited hippocampal neurogenesis and induced a recognition memory deficit in C57BL/6 mice, but rolipram treatment attenuated the inhibition of neurogenesis and the memory deficit. This suggests that the promotion of CREB activity ameliorates neurogenesis-dependent hippocampal functions including learning and memory, possibly via an increased rate of hippocampal neurogenesis by CREB activation. However, it cannot be excluded that other possible mechanisms of the change of CREB activity in other important regions of hippocampus, including CA1 and CA3, influence hippocampal function (Zhang et al., 2004;Hattiangady et al., 2005) . Further, CREB participates in the establishment of plasticity via pre-synaptic mechanisms and dendritic maturation (Davis et al., 1996; Navakkode et al.,2004) , which would be another mechanism of improved learning and memory. Therefore, more studies will be needed to establish precise mechanism of the radioprotective effect by CREB activation in brain.
In this study, we concluded that CREB activity may be attributable to hippocampal neurogenesis, suggesting that the promotion of CREB activity plays a protective role in inhibition of adult hippocampal neurogenesis and related functions caused by γ-irradiation.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) and by the Grant of the Korean Ministry of Education, Science and Technology (The Regional Core Research Program/Biohousing Research Institute) . This work was supported by the Biohousing Research Center.
References
- 1.Barad M. Later developments: molecular keys to age-related memory impairment. Alzheimer Dis. Assoc. Disord. 2003;17:168–176. doi: 10.1097/00002093-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Barad M. Bourtchouladze R. Winder D.G. Golan H. Kandel E. Rolipram a type IV-specific phosphodiesterase inhibitor facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender R.A. Lauterborn J.C. Gall C.M. Cariaga W. Baram T.Z. Enhanced CREB phosphorylation in immature dentate gyrus granule cells precedes neurotrophin expression and indicates a specific role of CREB in granule cell differentiation. Eur. J. Neurosci. 2001;13:679–686. doi: 10.1046/j.1460-9568.2001.01432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beshay E. Croze F. Prud'homme G.J. The phosphodiesterase inhibitors pentoxifylline and rolipram suppress macrophage activation and nitric oxide production in vitro and in vivo. Clin. Immunol. 2001;98:272–279. doi: 10.1006/clim.2000.4964. [DOI] [PubMed] [Google Scholar]
- 5.Brindle P.K. Montminy M.R. The CREB family of transcription activators. Curr. Opin. Genet. Dev. 1992;2:199–204. doi: 10.1016/S0959-437X(05)80274-6. [DOI] [PubMed] [Google Scholar]
- 6.Bourtchouladze R. Lidge R. Catapano R. Stanley J. Gossweiler S. Romashko D. Scott R. Tully T. A mouse model of Rubinstein-Taybi syndrome: defective longterm memory is ameliorated by inhibitors of phosphodiesterase 4. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10518–10522. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourtchuladze R. Frenguelli B. Blendy J. Cioffi D. Schutz G. Silva A.J. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 8.Coleman C.N. Stone H.B. Moulder J.E. Pellmar T.C. Medicine. Modulation of radiation injury. Science. 2004;304:693–694. doi: 10.1126/science.1095956. [DOI] [PubMed] [Google Scholar]
- 9.Crossen J.R. Garwood D. Glatstein E. Neuwelt E.A. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J. Clin. Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 10.Davis G.W. Schuster C.M. Goodman C.S. Genetic dissection of structural and functional components of synaptic plasticity. III. CREB is necessary for presynaptic functional plasticity. Neuron. 1996;17:669–679. doi: 10.1016/S0896-6273(00)80199-3. [DOI] [PubMed] [Google Scholar]
- 11.Dinter H. Tse J. Halks-Miller M. Asarnow D. Onuffer J. Faulds D. Mitrovic B. Kirsch G. Laurent H. Esperling P. Seidelmann D. Ottow E. Schneider H. Tuohy V.K. Wachtel H. Perez H.D. The type IV phosphodiesterase specific inhibitor mesopram inhibits experimental autoimmune encephalomyelitis in rodents. J. Neuroimmunol. 2000;108:136–146. doi: 10.1016/S0165-5728(00)00265-4. [DOI] [PubMed] [Google Scholar]
- 12.Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000;25:11–14. doi: 10.1016/S0896-6273(00)80866-1. [DOI] [PubMed] [Google Scholar]
- 13.Gong B. Vitolo O.V. Trinchese F. Liu S. Shelanski M. Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J. Clin. Invest. 2004;114:1624–1634. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez G.A. Montminy M.R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 15.Hattiangady B. Rao M.S. Shetty G.A. Shetty A.K. Brain-derived neurotrophic factor phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp. Neurol. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Kim J.S. Lee H.J. Kim J.C. Kang S.S. Bae C.S. Shin T. Jin J.K. Kim S.H. Wang H. Moon C. Transient impairment of hippocampus-dependent learning and memory in relatively low-dose of acute radiation syndrome is associated with inhibition of hippocampal neurogenesis. J. Radiat. Res. 2008;49:517–526. doi: 10.1269/jrr.08020. [DOI] [PubMed] [Google Scholar]
- 17.Madsen T.M. Kristjansen P.E. Bolwig T.G. Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/S0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 18.Meyers C.A. Brown P.D. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J. Clin. Oncol. 2006;24:1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 19.Monti B. Berteotti C. Contestabile A. Subchronic rolipram delivery activates hippocampal CREB and arc enhances retention and slows down extinction of conditioned fear. Neuropsychopharmacology. 2006;31:278–286. doi: 10.1038/sj.npp.1300813. [DOI] [PubMed] [Google Scholar]
- 20.Myhrer T. Exploratory behavior, reaction to novelty, and proactive memory in rats with temporo-entorhinal connections disrupted. Physiol. Behav. 1989;45:431–436. doi: 10.1016/0031-9384(89)90151-0. [DOI] [PubMed] [Google Scholar]
- 21.Nagakura A. Niimura M. Takeo S. Effects of a phosphodiesterase IV inhibitor rolipram on microsphere embolism-induced defects in memory function and cerebral cyclic AMP signal transduction system in rats. Br. J. Pharmacol. 2002;135:1783–1793. doi: 10.1038/sj.bjp.0704629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakagawa S. Kim J.E. Lee R. Malberg J.E. Chen J. Steffen C. Zhang Y.J. Nestler E.J. Duman R.S. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J. Neurosci. 2002;22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navakkode S. Sajikumar S. Frey J.U. The type IV-specific phosphodiesterase inhibitor rolipram and its effect on hippocampal long-term potentiation and synaptic tagging. J.Neurosci. 2004;24:7740–7744. doi: 10.1523/JNEUROSCI.1796-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nibuya M. Nestler E.J. Duman R.S. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J. Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Posner J.B. Management of brain metastases. Rev. Neurol. 1992;148:477–487. [PubMed] [Google Scholar]
- 26.Silva A.J. Stevens C.F. Tonegawa S. Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- 27.Stone H.B. Coleman C.N. Anscher M.S. McBride W.H. Effects of radiation on normal tissue: consequences and mechanisms. Lancet. Oncol. 2003;4:529–536. doi: 10.1016/S1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 28.Vitolo O.V. Sant'Angelo A. Costanzo V. Battaglia F. Arancio O. Shelanski M. Amyloid beta-peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang M. Kim J.S. Song M.S. Kim S.H. Kang S.S. Bae C.S. Kim J.C. Wang H. Shin T. Moon C. Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: Possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiol. Learn. Mem. 2010;93:487–494. doi: 10.1016/j.nlm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H.T. Zhao Y. Huang Y. Dorairaj N.R. Chandler L.J. O'Donnell J.M. Inhibition of the phosphodiesterase 4 (PDE4) enzyme reverses memory deficits produced by infusion of the MEK inhibitor U0126 into the CA1 subregion of the rat hippocampus. Neuropsychopharmacol. 2004;29:1432–1439. doi: 10.1038/sj.npp.1300440. [DOI] [PubMed] [Google Scholar]