Abstract
Isaria sinclairii (Cicada Dongchunghacho) was studied as a potential crude natural food in powdered form. The role of tissue fatty acids in relation to the anti-obesity effects of I. sinclairii (IS) was examined by feeding the powder to SD rats ad libitum at 0, 1.25, 2.5, 5 and 10% (calculated about 8 g/kg) of the feed for a period of 3 months and 6 months. The fatty acid composition profile as indicated GC-MS, showed significantly slight dose-dependent increases in the levels of unsaturated fatty acids, particularly, arachidonic acid (C20: 4n6) , oleic acid, linoleic acid, eicosadienoic acid, eicosapentaenoic acid (EPA) (C20: 5) concentration in the the ad libitum IS-fed groups compared to the control group in SD abdominal fat over 6 month period. Over viewing of the SD and Ob mice treated Isaria sinclairii powder; there were increases in the single (mono) unsaturated fatty acids ratio but decreases in polyunsaturated fatty acid. In IS-fed groups in proportion to the treatment period, this Dongchunghacho also induced an increase in the level of same result of unsaturated fatty acid in C57BL/6 obese (ob/ob) mice over a 6-month period treatment compared to those given 10% dry mulberry leaf powder (ML) or silkworm powder mixed with the standard diet.
Keywords: Anti-diabetic effect, Isaria sinclairii, Obese mice, 6 months treatment
INTRODUCTION
Obesity is a co morbid disease that caused circulatory disorders, such as, hypertension, diabetes, arteriosclerosis (Hall et al., 2002) , premature aging (Slawik and Vidal-Puig, 2006) and cancer (Jee et al., 2006) . In very rare situations,human obesity is caused by an absence of the leptin signal (mutations of the leptin gene or leptin receptor gene) , which produces the internal perception of starvation, results in the chronic stimulation of excessive food intake (Jéquier 2002) .Recently, many natural products can help control the bodyweight through specific mechanisms, such as ion channel regulation by AMPK (AMP-activated protein kinase) (Vong et al., 2009) , dyslipidemia action of acetyl-CoA carboxylase (ACC) (Kim and Kim, 2009) and the promotion of glucagons-like peptide-1 secretion (Chen and Reimer, 2009). Among the Dongchunghachoes, Isaria sinclairii (Cicada Dongchunghacho) exhibited possesses selective antihypertensive activity in a spontaneously hypertensive rat (SHR) model (Ahn et al., 2007c) . A previous report suggested that I. sinclairii powder (IS) reduces obesity in Zucker-fa/fa rats by reducing the serum total cholesterol, triglyceride and LDL levels, and by increasing the leptin levels. The body weight was reduced by an I. sinlairrii treatment (Ahn et al., 2007a) . However, the mechanism for the decrease in adipose fat and the change in the I. sinlairii treated tissues is unclear. According a report of United States Department of Agricultural, IS provided new insight into the treatment of lethal horse disease that the agency’s Richard B. Russell Agricultutal Research Center in Athens, reported that I. sinclairii,produces a compound called ISP-I or myriocin that temporarily reduces the sphinganine levels (Riley et al., 1999) . As the known active substances of I. sinclairii, there are FTY720, Myriocin (ISP-1) : potent immunomodulator (Ueda et al., 2005) , N-hydroxyethyl adenosine (Furuya et al., 1983; Ahn et al., 2008) and purine derivatives.
As a sericulture product, mulberry (Morus alba L.) leaves contain many nutritional components. The major components are flavones, steroids, triterpenes, amino acids, vitamins and other trace minerals. Out of the six N-containing sugars isolated from mulberry leaves, 2-O-α-D-galactopyranosyl-Doxynojrimycin (GAL-DNJ) and fagomine have the most potent antihyperglycemic effects (Chen et al., 1995) . They have also been used in traditional Chinese medicine as an antihyperglycemic to treat and prevent diabetes mellitus (Prowlowska et al., 2008) . The administration of DNJ extracted from mulberry suppresses an increase in postprandial blood glucose in humans (Tsuduki et al., 2009) and the lipid-lowering effects of mulberry water extracts (MME) for 12 weeks on hamsters showed hypolipidemic effects of high fat cholesterol diets with 1 and 2% (MME) (Liu et al., 2009) . Conjugated linoleic acid (CLA) provides several health benefits for humans as an anticarcinogenic or antiatherosclerogenic agent (Park et al., 2006) .
In Oriental Asia, silkworm extract has been known for its effectiveness in enhancing of male stamina and improving vitality. Its main ingredients are reported as DNJ (0.22%) ,crude protein (56.76%) , crude fat (9.27%) , crude fiber (6.62%) , amino acid (mg/100 g silkworm: cystine 1.19,methionine 1.39, aspartic acid 3.94, threonine 1.75, serine 2.20, glutamic acid 8.65, glycine 2.34, alanine 2.69, valine 2.35, isoleucine 1.88, leucine 4.50, tyrosine 9.61, phenylalanine 2.32, lysine 2.17, histidine 1.78, arginine 2.71, proline 3.44) and trace element (μg/kg silkworm: Ca 0.44, P 0.86,K 6.38, Na 0.06, Mg 0.38, Fe 139, Mn 35, Zn 61) (Cui et al., 2002) .
Silkworms with conjugated linoleic acid (CLA) incorporated into lipids were produced to enhance the quality of silkworms having a synergistic effect with CLA functions by dietary synthetic CLA (Park et al., 2006).
There are some reports showing that the CLA content of beef can be increased by vegetable oil (including oil seed) . In addition, dietary CLA-beef may alter the characteristics of adipose tissue by decreasing the number of adipocytes and the size of the tissue (He et al., 2009) . Another report showed that daily oleosyl-estrone without fatty acid, reduced body fat through energy consumption, i.e., the administration of oleoyl-estrone results in a dose-dependent loss of body fat that does not affect in the level of the main plasma energy homeostasis indicators i.e. unaltered glucose, triacylglycerols, free fatty acid and etc. (Grasa et al., 2001).
This study examined the fatty acid composition on treated rat tissue, particularly poly unsaturated fatty acid with conjugated fatty acid versus saturated fatty acid in various murine cases: obese (ob/ob) mice and SD rat treated with I.sinclairii over a 6 month period.
MATERIALS AND METHODS
Materials.
I. sinclairii, endophytically parasitizing on dead or living Cicadae subspecies, was cultivated in the Department of Agricultural Biology, National Academy of Agricultural Science, Suwon, Korea. The same department also supplied mulberry leaves and silkworms.
Preparation of IS, mulberry leaf and silkworm powder.
Each of dried I. sinclairii (IS) , mulberry leaves and silkworms was homogenized in a blender, weighed to 5%IS powder, 10% IS powder, 10% mulberry leaf powder (ML) and 10% silkworm powder (SW) , mixed with normal diet feeder, standard Rat and Mouse 18% 5L79 (PMI Nutrition International, Brentwood, MO, USA) and proper distilled water, made pellets, oven dried at 65oC, and then ad libitum fed at doses of at 0, 1.25, 2.5, 5 and 10% (calculated about 8 g/kg) of the feed over a 13-week or 26-week period.
Animals.
Specific pathogen free SD rats (4 weeks old,weighing 165 ± 5 g, male and female) , were supplied from Samtako Co. Ltd. (Osan, Korea) and fed with standard diet (Samtako Co. Ltd) , and water ad libitum, C57BL/6-ob/ob mice (5 weeks old, female) , being genetically obese due to impaired regulation of leptin expression in the stomach (Pico et al., 2002) , were originally procured from Korea Institute of Toxicology, Daejeon) . The strain has been used extensively in studies of anti-diabetes efficacy in obese mice with obesity and is supplied by Jung Ang Lab animals Co. (Seoul, Korea) . All procedures were in accordance with the NIH Guidelines for Care and Use of Laboratory Animals. The rats were kept for one week at normal physical conditions (23 ± 2℃, 55 ± 10%, humidity and regular day/night cycle) and fed with standard diet (Rat and Mouse 18% 5L79) (PMI Nutrition International) and water ad libitum before obesity testing began. At 7 weeks of age, C57BL/6-ob/ob mice with body weights ranging from 27 to 37 g were randomly divided into one control and four treatment groups (N = 6 for each group) . The mice were distributed into the groups based on similar weights as given below: obese control with normal diet, II: treated with 5% IS powder, III: treated with 10% IS powder, IV: treated with 10% ML and V: treated with 10% SW. Each group was maintained for a 26-week period.
Abdominal fat weights and analysis of fatty acid composition in murine adipose tissue.
The absolute weights of following organs were measured in all rats when they were sacrificed. For epididymidal and abdominal fat analysis,the concentration of free fatty acids and fatty acid composition were analyzed 29 fatty acids in adipose tissues by gas chromatography-mass spectroscopy (GC-MS) . Each cut adipose or epididymidal tissues (0.1 g) were taken and test tissue fatty acid prepared by chloroform: methanol (2 : 1) mixture extraction for overnight and the filtrated solution was removed under nitrogen gas. Then the lipids were saponified by alkaline hydrolysis of phospholipid at 100℃ with 0.5 N methanolic sodium hydroxide and methylated at 100℃ with 14% BF3 for 15 min. Top layer was transferred in petroleum ether and analyzed by GC/MS.
Gas chromatography-mass spectrometry.
GC/MS was performed with Aglient 6890GC coupled to an Aglient 5973N mass selective detector operated in the EI mode, with a HP-5 capillary column (Aglient Technolgies, Palto alto, Ca, USA) . Helium was used as a carrier gas as a constant flow rate of 0.9 ml/min. The inlet temperature was 250℃ and the MS transfer line was kept constant 230℃.The oven temperature was held at 180℃ for 20 min, then programmed at 10℃/min to 230℃ held 10 min. Quantification was achieved using a mixed 37 fatty acid standard: Sigma L9405, 10 rg/mL (Sigma-Aldrich Inc.) and linoleic acid (C18:2n6) as an internal standard.
Statistical analysis.
Mean and standard error values of all the parameters studied were determined for each group. Student’s t-test was conducted to establish significant differences between the final biochemical level in control and treated groups. p < 0.05 was considered statistically significant.
RESULTS
Abdominal fat weights.
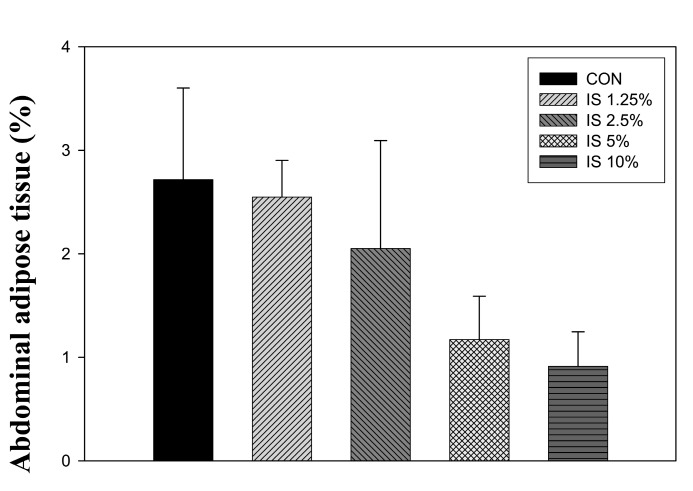
It was reported that the weight of abdominal adipose tissues surrounding epididymides were reduced considerably by Dongchunghacho, in conjunction with a mild increase in body weight gain in a thirteen-week repeated oral toxicity study of I. sinclairii in Sprague-Dawely rats (Ahn et al., 2004) . However, the authors, did not provide, conclusive weight data of the abdominal adipose tissues surrounding epididymides. In the present study, female SD rats and obese ob/ob mice showed a decrease in the weight of abdominal adipose tissues female SD rats over a 3-month (Fig. 1) . There were somewhat differences of abdominal fat weight between normal (SD rat, and obese murine or treatment periods as the cell membrane susceptibility, or the membrane receptor binding ability, is increased in disease- (hypertension or obese) oriented rats by the treatment of I. sinclairii (Ahn et al., 2007d) .
Fig. 1. Effect of I. sinclairii powder on abdominal adipose tissues of the female SD rats over a 3 month. Each value represents mean ± S.D. for 10 rats. *p< 0.05 vs control (group 1) . Fat percentage means abdominal fat (g) / body weight (g) .
Ageing or abnormal state, super nutrition and a lack of exercise can cause fat accumulation into adipose tissue, particularly abdominal fat. In a previous study, the anti-obesity effects of I. sinclairii (IS) in Zucker-fa/fa rats were examined. The control animals showed higher food intake than the treated animals, and more rapid body weight gain and higher lipid accumulation in the abdominal Zucker rat adipose tissues surrounding the epididymides (Ahn et al.,2007b) . The weight of abdominal adipose tissues surrounding epididymides was reduced significantly by this Dongchunghacho,which was in parallel with a mild increase in body weight gain in the thirteen-week repeated oral toxicity study of I. sinclairii in Sprague-Dawely rats. However, no conclusive weight data on abdominal adipose tissues surrounding the epididymides was provided (Ahn et al.,2004) . Therefore, this report showed the weight change of the abdominal adipose tissues in female SD rats over a 3-month (Fig. 1) . I. sinclairii (IS) contains approximately 7.0% crude fat (crude protein; 65.74; fiber: 6.39; ash: 6.55) (Ji et al., 2001) .
The percentage change in weight of abdominal adipose tissues female SD rats over a 3-months was as follows: control:2.72 abdominal fat%; IS 1.25: 2.55%; IS 2.5: 2.05%;IS 5: 1.17%; IS 10: 0.91% (Fig. 1) . The similar results on the abdominal fat in obese (ob/ob) mice over a 6-months treatment suggested the anti-obesity effect of I. sinclairii; control: 9.20 g; IS 5%: 7.71 g; IS 10%: 6.78 g (Ahn et al., 2010) .
Analysis of fatty acid composition in murine adipose tissue.
The fatty acid profile as indicated GC-MS, showed a slight dose-dependent decrease in the arachidonic acid (C20: 4n6, AFA) concentration in epididymidal and abdominal fat of the male SD rats in the IS-fed groups over a 3-month period compared to the control group (Table 1 and Table 2) . The value of respective fatty acid percentage (%) by wt of total fatty acids was calculated by SIM-GC-MS data area comparison of fatty acid standard curve. The effect of I. sinclairii powder produced an increase in linoleic acid (C18:3n3, LFA) level in the epididymidal fat of male SD rats over a 3 month period (IS 0%, 2.75% LFA;IS 1.25%, 3.20% LFA; IS 2.5%, 3.40% LFA; IS 5%, 3.74 LFA; IS 10%, 4.21% LFA) . On the other hand, there was a slight decrease in arachidonic acid: IS 0%, 0.67%; AFA; IS 10%, 0.50% AFA (Table 1) . Similar results were obtained in the abdominal fat: IS 0% 2.47% LFA; IS 10%, 3.43%LFA, and IS 0% 0.39% AFA; IS 10%, 0.23% AFA (Table 2) . In contrast, the arachidonic acid level was significantly higher in the abdominal fat of SD female rats in the 10% IS group than the control group. The linolenic acid level in the abdominal fat (Table 2) fat was higher in both male and female IS-fed SD rats over a 3-month period (Table 2 and Table 3) . The fatty acid composition of abdominal fat of over a 6 month period in SD male rats given I. sinclairii powder showed the following: arachidonic acid, IS 0, 0.60 μg/mg→IS 10, 1.18 μg/mg; eicosapentaenoicacid (EPA) (C20:5) , IS 0, 0.97 μg/mg→IS 10, 1.54 μg/mg (Table 4) . Arachidonic acid is an unsaturated fatty acid constituent of phospholipids of cell membranes and released from the phospholipid by inflammation, ob mice cell condition was somewhat like inflammation cell state by histopathological consideration. Arachidonic acid diminution could be suggested inflammation modulation in obese mice.
Table 1.
Effect of I. sinclairii powder on fatty acid composition ratio of epididymidal fat of SD male rats over a 3-month
| Fatty acid (%) | Group (I. sinclairii dose) | ||||
|---|---|---|---|---|---|
|
| |||||
| Group1 0% | Group 2 1.25% | Group 3 2.5% | Group 4 5% | Group 5 10% | |
|
| |||||
| Myristic acid (C14:0) | 0.81 | 0.76 | 0.72 | 0.85 | 0.79 |
| Palmitic acid (C16:0) | 20.20 | 20.26 | 19.51 | 20.64 | 18.06 |
| Palmitoleic acid (C16:ln7) | 2.22 | 1.34 | 2.07 | 2.65 | 3.38 |
| Stearic acid (C18:0) | 3.02 | 2.75 | 3.40 | 3.25 | 3.14 |
| Oleic acid (C18:ln9) | 25.95 | 24.66 | 26.59 | 26.63 | 27.13 |
| Vaccenic acid (C18:ln7) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Linoleic acid (C18:2n6) | 42.11 | 44.43 | 41.90 | 39.69 | 41.03 |
| γ-Linoleic acid (C18:3n6) | 0.05 | 0.05 | 0.04 | 0.06 | 0.06 |
| Linolenic acid (C18:3n3) | 2.75 | 3.20 | 3.40 | 3.74 | 4.21 |
| Eicosenoic acid (C20:1n9) | 0.28 | 0.23 | 0.29 | 0.28 | 0.26 |
| Eicosadienoic acid (C20:2n6) | 0.31 | 0.25 | 0.31 | 0.27 | 0.23 |
| Eicosatrienoic acid (C20:3n6) | 0.16 | 0.17 | 0.13 | 0.14 | 0.11 |
| Arachidonic acid (C20:4n6) | 0.67 | 0.60 | 0.44 | 0.50 | 0.50 |
| Eicosapentaenoicacid (EPA) (C20:5n3) | 0.13 | 0.18 | 0.12 | 0.15 | 0.14 |
| Docosatetraenoic acid (C22:4n6) | 0.17 | 0.11 | 0.10 | 0.12 | 0.08 |
| Docosapentaenoicacid (C22:5n3) | 0.38 | 0.37 | 0.37 | 0.40 | 0.35 |
| Docosahexaenoicacid (DHA) (C22:6n3) | 0.78 | 0.64 | 0.60 | 0.65 | 0.53 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Saturated fatty acid | 24.03 | 23.77 | 23.63 | 24.74 | 21.99 |
| Unsaturated fatty acid (UFA) | 75.97 | 76.23 | 76.37 | 75.26 | 78.01 |
| Single UFA | 28.45 | 26.22 | 28.96 | 29.56 | 30.77 |
| Poly UFA | 47.52 | 50.00 | 47.42 | 45.71 | 47.24 |
Table 2.
Effect of I. sinclairii powder on fatty acid composition of abdominal fat of the SD male rats over a 3-month
| Fatty acid (%) | Group (I. sinclairii dose) | ||||
|---|---|---|---|---|---|
|
| |||||
| Group 1 0% | Group 2 1.25% | Group 3 2.5% | Group 4 5% | Group 5 10% | |
|
| |||||
| Myristic acid (C14:0) | 0.80 | 0.73 | 0.76 | 0.96 | 0.84 |
| Palmitic acid (C16:0) | 19.26 | 20.25 | 20.43 | 20.26 | 19.26 |
| Palmitoleic acid (C16:ln7) | 3.38 | 1.71 | 1.42 | 3.63 | 4.21 |
| Stearic acid (C18:0) | 3.29 | 3.24 | 3.33 | 3.43 | 3.57 |
| Oleic acid (C18:ln9) | 26.93 | 26.24 | 26.86 | 27.20 | 27.98 |
| Vaccenic acid (C18:ln7) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Linoleic acid (C18:2n6) | 42.00 | 42.89 | 42.57 | 39.43 | 39.54 |
| γ-Linoleic acid (C18:3n6) | 0.06 | 0.06 | 0.04 | 0.04 | 0.05 |
| Linolenic acid (C18:3n3) | 2.47 | 3.09 | 2.92 | 3.34 | 3.43 |
| Eicosenoic acid (C20:1n9) | 0.32 | 0.30 | 0.28 | 0.29 | 0.26 |
| Eicosadienoic acid (C20:2n6) | 0.26 | 0.21 | 0.22 | 0.22 | 0.16 |
| Eicosatrienoic acid (C20:3n6) | 0.11 | 0.12 | 0.10 | 0.09 | 0.05 |
| Arachidonic acid (C20:4n6) | 0.39 | 0.43 | 0.37 | 0.32 | 0.23 |
| Eicosapentaenoicacid (EPA) (C20:5n3) | 0.07 | 0.09 | 0.07 | 0.08 | 0.03 |
| Docosatetraenoic acid (C22:4n6) | 0.13 | 0.10 | 0.13 | 0.11 | 0.06 |
| Docosapentaenoicacid (C22:5n3) | 0.20 | 0.22 | 0.20 | 0.25 | 0.14 |
| Docosahexaenoicacid (DHA) (C22:6n3) | 0.33 | 0.32 | 0.28 | 0.34 | 0.19 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Saturated fatty acid | 23.35 | 24.22 | 24.66 | 24.66 | 23.67 |
| Unsaturated fatty acid (UFA) | 76.65 | 75.78 | 75.34 | 75.34 | 76.33 |
| Single UFA | 30.63 | 28.25 | 31.12 | 31.12 | 32.45 |
| Poly UFA | 46.02 | 47.53 | 44.22 | 44.22 | 43.87 |
Table 3.
Fatty acid composition of abdominal fat of the SD female rats treated with I. sinclairii powder over a 3-month
| Fatty acid (%) | Group (I. sinclairii dose) | ||||
|---|---|---|---|---|---|
|
| |||||
| Group 6 0% | Group 7 1.25% | Group 8 2.5% | Group 9 5% | Group 10 10% | |
|
| |||||
| Myristic acid (C14:0) | 0.83 | 0.89 | 0.82 | 0.76 | 1.06 |
| Palmitic acid (C16:0) | 19.59 | 20.20 | 20.56 | 20.25 | 22.46 |
| Palmitoleic acid (C16:ln7) | 4.04 | 2.00 | 1.87 | 1.68 | 4.49 |
| Stearic acid (C18:0) | 3.36 | 3.65 | 3.73 | 3.66 | 3.59 |
| Oleic acid (C18:ln9) | 27.96 | 28.95 | 28.88 | 27.99 | 28.32 |
| Vaccenic acid (C18:ln7) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Linoleic acid (C18:2n6) | 39.68 | 39.32 | 38.98 | 39.53 | 33.48 |
| γ-Linoleic acid (C18:3n6) | 0.09 | 0.11 | 0.12 | 0.13 | 0.16 |
| Linolenic acid (C18:3n3) | 2.19 | 2.45 | 2.73 | 3.19 | 3.55 |
| Eicosenoic acid (C20:1n9) | 0.26 | 0.32 | 0.29 | 0.30 | 0.24 |
| Eicosadienoic acid (C20:2n6) | 0.21 | 0.26 | 0.23 | 0.24 | 0.21 |
| Eicosatrienoic acid (C20:3n6) | 0.19 | 0.20 | 0.18 | 0.22 | 0.20 |
| Arachidonic acid (C20:4n6) | 0.53 | 0.55 | 0.54 | 0.67 | 0.71 |
| Eicosapentaenoicacid (EPA) (C20:5n3) | 0.11 | 0.10 | 0.10 | 0.13 | 0.21 |
| Docosatetraenoic acid (C22:4n6) | 0.17 | 0.19 | 0.16 | 0.20 | 0.15 |
| Docosapentaenoicacid (C22:5n3) | 0.26 | 0.26 | 0.27 | 0.34 | 0.36 |
| Docosahexaenoicacid (DHA) (C22:6n3) | 0.52 | 0.55 | 0.55 | 0.71 | 0.80 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Saturated fatty acid | 23.78 | 24.74 | 25.11 | 24.67 | 27.10 |
| Unsaturated fatty acid (UFA) | 76.22 | 75.26 | 74.89 | 75.33 | 72.90 |
| Single UFA | 32.27 | 31.27 | 31.04 | 29.97 | 33.06 |
| Poly UFA | 43.95 | 43.98 | 43.85 | 45.36 | 39.84 |
Table 4.
Fatty acid composition of abdominal fat of the SD male rats treated with I. sinclairii powder over a 6-month
| Fatty acid (μ/mg) | Group 1 0% | Group 2 1.25% | Group 3 2.5% | Group 4 5% | Group 5 10% |
|---|---|---|---|---|---|
|
| |||||
| Myristic acid (C14:0) | N.D | N.D | 0.25 ± 0.09 | 0.49 ± 0.00 | 0.74 ± 0.16 |
| Palmitic acid (C16:0) | 25.52 ± 4.22 | 24.50 ± 0.38 | 22.75 ± 1.66 | 30.32 ± 0.08 | 28.68 ± 0.21 |
| Palmitoleic acid (C16:l) | 1.79 ± 0.26 | 1.86 ± 0.12 | 1.24 ± 0.09 | 2.53 ± 0.53 | 3.79 ± 0.07* |
| Stearic acid (C18:0) | 5.13 ± 0.77 | 4.89 ± 0.79 | 5,17 ± 0.36 | 5.61 ± 0.08 | 5.00 ± 0.37 |
| Oleic acid (C18:l) | 64.41 ± 10.33 | 66.93 ± 11.19 | 65.74 ± 5.67 | 79.60 ± 0.08 | 75.49 ± 2.92 |
| Linoleic acid (C18:2) | 55.71 ± 8.40 | 62.17 ± 8.47 | 63.36 ± 4.57 | 69.02 ± 1.41 | 58.55 ± 1.10 |
| Linoleic acid (C20:0) | N.D | N.D | 0.22 ± 0 | 0.25 ± 0.11 | 0.18 ± 0.01 |
| Eicosenoic acid (C20:1) | 0.13 ± 0 | 0.16 ± 0.09 | 0.52 ± 0.07* | 0.28 ± 0.05 | 0.11 ± 0.05 |
| Eicosadienoic acid (C20:2) | 0.69 ± 0.06 | 0.79 ± 0.07 | 0.97 ± 0.01* | 1.27 ± 0.05* | 1.04 ± 0.06 |
| Arachidonic acid (C20:4) | 0.60 ± 0.21 | 0.54 ± 0.07 | 1.26 ± 0.12* | 1.58 ± 0.30* | 1.18 ± 0.09 |
| Eicosapentaenoicacid (EPA) (C20:5) | 0.97 ± 0.08 | 1.08 ± 0.00 | 1.59 ± 0.08** | 2.02 ± 0.22* | 1.54 ± 0.14 |
| Docosahexaenoicacid (DHA) (C22:6) | N.D | N.D | 0.44 ± 0.04 | 0.51 ± 0.08 | 0.33 ± 0.02 |
| Total | 152.56 ± 23.96 | 162.52 ± 24.70 | 163.11 ± 12.61 | 192.89 ± 3.85 | 175.91 ± 4.94 |
| FA/UFA | 1.25 | 1.22 | 1.21 | 1.24 | 1.24 |
| Saturated fatty acid | 19.89 | 18.12 | 17.40 | 19.01 | 19.67 |
| Unsaturated fatty acid (UFA) | 80.65 | 82.17 | 82.84 | 81.29 | 80.75 |
| Single UF | 43.04 | 42.43 | 41.38 | 42.72 | 45.13 |
| Poly UFA | 37.62 | 39.74 | 41.45 | 38.57 | 35.62 |
Each value represents mean ± S.D.
*P<0.05, **P<0.01 vs Control (Group 1)
N.D: not detected
In the 6-month IS treated group, the levels of unsaturated fatty acids, particularly, arachidonic acid, EPA and docosahexaenoicacid (DHA) (C22: 6) , increased in a dose dependent manner in case of abdominal fat of the SD male rats over a 6-month (Table 4) . The effects of a long-term treatment of I. sinclairii powder on the fatty acid composition of abdominal fat in the ob/ob female mice over 6 months were as follows: oleic acid (C18:1) , IS 0, 137.50 μg/mg→IS 10,187.08 μg/mg increased level and arachidonic acid, IS 0,0.75 μg/mg→IS 10, 0.38 μg/mg (Table 5) .
Table 5.
Fatty acid composition of abdominal fat of female ob mice treated with I. sinclairii powder over a 6-month
| Fatty acid (μ/fat mg) | Con | IS 5 | IS 10 | ML 10 | SW 10 |
|---|---|---|---|---|---|
|
| |||||
| Myristic acid (C14:0) | 0.77 ± 0.57 | 0.60 ± 0.38 | 1.33 ± 0.70 | 0.58 ± 0.41 | 0.76 ± 0.66 |
| Palmitic acid (C16:0) | 29.44 ± 4.52 | 27.27 ± 6.75 | 43.60 ± 8.53 | 30.04 ± 9.20 | 33.31 ± 5.63 |
| Palmitoleic acid (C16:1) | 12.73 ± 6.60 | 13.57 ± 2.49 | 26.74 ± 5.07* | 13.44 ± 3.39 | 18.39 ± 6.23 |
| Stearic acid (C18:0) | 3.98 ± 1.12 | 2.60 ± 0.52 | 4.06 ± 1.57 | 3.35 ± 0.99 | 3.78 ± 0.37 |
| Oleic acid (C18:1) | 137.50 ± 9.74 | 155.78 ± 43.66 | 193.24 ± 23.61* | 141.24 ± 38.28 | 187.08 ± 22.40* |
| Linoleic acid (C18:2) | 43.43 ± 12.76 | 40.74 ± 10.31 | 63.85 ± 13.40* | 44.48 ± 13.03 | 52.11 ± 6.78 |
| Arachidic acid methyl ester (C20:0) | 0.08 ± 0.03 | 0.04 ± 0.01 | 0.06 ± 0.03 | 0.04 ± 0.00 | 0.03 ± 0.00 |
| Eicosenoic acid (C20:1) | 0.27 ± 0.14 | 0.14 ± 0.08 | 0.09 ± 0.08 | 0.02 ± 0.00 | 0.05 ± 0.03 |
| Eicosadienoic acid (C20:2) | 0.47 ± 0.04 | 0.40 ± 0.13 | 0.42 ± 0.05 | 0.21 ± 0.16 | 0.32 ± 0.12 |
| Eicosatrienoic acid (C20:3) | 0.46 ± 0.23 | 0.19 ± 0.20 | 0.45 ± 0.07* | 0.26 ± 0.20 | 0.41 ± 0.16 |
| Arachidonic acid (C20:4) | 0.75 ± 0.25 | 0.45 ± 0.19 | 0.46 ± 0.09 | 0.35 ± 0.12* | 0.38 ± 0.14 |
| Eicosapentaenoicacid (EPA) (C20:5) | 0.82 ± 0.16 | 0.52 ± 0.08 | 0.61 ± 0.05 | 0.53 ± 0.12 | 0.58 ± 0.10 |
| Docosahexaenoicacid (DHA) (C22:6) | 0.26 ± 0.13 | 0.35 ± 0.12 | 0.36 ± 0.08 | 0.34 ± 0.09 | 0.37 ± 0.14 |
| Total | 230.88 ± 17.51 | 241.82 ± 57.57 | 335.24 ± 51.44* | 234.51 ± 66.15 | 297.46 ± 42.60 |
| FA/UFA | 1.18 ± 0.2 | 1.15 ± 0.01 | 1.17 ± 0.01 | 1.17 ± 0.01 | 1.14 ± 0.01 |
| Saturated fatty acid | 14.85 | 12.62 | 14.63 | 14.50 | 12.73 |
| Unsaturated fatty acid (UFA) | 85.20 | 87.73 | 85.38 | 85.66 | 87.31 |
| Single UFA | 65.18 | 70.09 | 65.65 | 65.97 | 69.09 |
| Poly UFA | 20.01 | 17.64 | 19.73 | 19.68 | 18.21 |
Each value represents mean ± S.D.
*P<0.05 vs Control (Group 1)
Over viewing of the SD and Ob mice treated Isaria sinclairii powder; there were increases in the single (mono) unsaturated fatty acids ratio but decreases in polyunsaturated fatty acid.
DISCUSSION
Recently, it was reported that the primary pharmacological activities of I. sinclairii include selective antihypertensive activity (Ahn et al., 2007c) and anti-obesity activity in rats (Ahn et al., 2007b) . However, I. sinclairii contains polyhydroxylated alkaloids such as 1-deoxynojirimycin (DNJ) that are metabolized from silkworm nutrients (Asano et al., 2001) , and a synthetic long-chain N-alkylated immini sugar, N-buthyl-deoxynojirimycin, was reported, where its membrane disruption and cytotoxicity were dependent on the inhibition of protein and lipid glycosylation, reducing hepatitis virus (Mellor et al., 2003) which might be responsible for the increase in conjugated poly saturated fatty acid level. Recently, sphingosin was reported to be an immunomodulator that inhibits the migration that migrates of lympocytes toward sphingosine 1-phosphate (Chiba, 2009) .
The fatty acid level of the I. sinclairii long-term treated rats or mice showed a dose dependent increase in double bond conjugation (esterfication) . A mechanism for how HDL or reactive oxygen species destroys biologically active lipids in mildly oxidized LDL, and oxidized phospholipids may undergo oxidative fragmentation was reported (Nevab et al., 1996) .
There is a related hypothesis that PPARS (peroxisome proliferator activated receptors) stimulates fatty acid catabolism at several levels: fatty acids and cholesterol transport, lipoprotein lipase (hydrolysis of triglycerides) , fatty acid uptake and etc. (Neve et al., 2000)
A diet enriched in the n-3 family poly unsaturated fatty acid (PUFA) decreases the adipose tissue mass and suppresses that development of obesity in rodents (Madsen et al., 2005) , In addition, this study, other components in I.sinclairii, including approximately 7% fat may reduce the level of fat and enriched the levels of PUFA in rodent adipose tissues.
References
- 1.Ahn M.Y. Han J.W. Jee S.D. Hwang J.S. Hwang S.J. Hong Y.N. Kim S.N. A Thirteen-week oral dose sub-chronic toxicity study of Isaria sinclairii in rats. J. Toxicol. Pub. Health. 2007a;23:363–371. [Google Scholar]
- 2.Ahn M.Y. Heo J.E. Ryu J.H. Jeong H.K. Antioxidant activity of N-hydroxyethyl adenosine from Isaria sinclairii. Int. J. Indusr. Entomol. 2008;17:197–200. [Google Scholar]
- 3.Ahn M.Y. Jee S.D. Kim J.Y. Han J.W. Lee Y.K. Lee Y.W. Ryu K.S. Lee B.M. Jung N.J. Kim S.N. Thirteen week repeated oral toxicity study of Paecilomyces sinclairii in Sprague-Dawely rats. J. Toxico. Pub. Health. 2004;20:339–348. [Google Scholar]
- 4.Ahn M.Y. Jee S.D. Lee B.M. Antiobesity effects of an Isaria sinclairii by repeated oral treatment in obese Zucker rats over a 4-month period. J. Toxicol. Environ. Health Part A. 2007b;70:1395–1401. doi: 10.1080/15287390701428556. [DOI] [PubMed] [Google Scholar]
- 5.Ahn M.Y. Jee S.D. Lee B.M. Yeon J.H. Park K.K. Hwang J.S. Yu E.Y. Anti-diabetic effect and gene expression profiling in obese mice treated with Isaria sinclairii over a 6-month period. J. Toxicol. Environ. Health Part A. 2010;73:1511–1520. doi: 10.1080/15287394.2010.511575. [DOI] [PubMed] [Google Scholar]
- 6.Ahn M.Y. Jung Y.S. Jee S.D. Kim C.S. Lee S.H. Moon C.H. Cho S.I. Lee B.M. Ryu K.S. Antihypertensive effect of the Dongchunhacho Isaria sinclairii in the spontaneously hypertensive rats. Arch. Pharm. Res. 2007c;30:493–501. doi: 10.1007/BF02980225. [DOI] [PubMed] [Google Scholar]
- 7.Ahn M.Y. Kim J.Y. Han J.W. Jee S.D. Hwang J.S. Cho S.I. Yun E.Y. Isaria sinclairii extract reduces bodyweight and ameliorates metabolic abnormalities. Int. J. Indust.Entomol. 2007d;14:121–126. [Google Scholar]
- 8.Asano N. Yamashita T. Yasuda K. Ikeda K. Kizu H. Kameda Y. Kato A. Nash R.J. Lee H.S. Ryu K.S. Polyhydroxylated alkaloids isolated from mulberry trees (Morus alba L.) and silk worms (Bombyx mori L.) . J.Agric. Food Chem. 2001;49:4208–4213. doi: 10.1021/jf010567e. [DOI] [PubMed] [Google Scholar]
- 9.Chen F.J. Nakashima N. Kimura M. Hypoglycemic activity and mechanisms of extracts from mulberry leaves (Folium Mori) and Cortex Mori Radicis in streptozotocininduced diabetic mice. Yakugaku Zasshi. 1995;115:476–482. doi: 10.1248/yakushi1947.115.6_476. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q. Reimer R.A. Dairy protein and leucine alter GLP-1 release and mRNA of genes involved in intestinal lipid metabolism in vitro. Nutrition. 2009;25:340–349. doi: 10.1016/j.nut.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui Z. Ahn M.Y. Lee Y.B. Ryu K.S. Bombycidae in Materia Medica from insects. Shinilbooks; Seoul: 2002. pp. 137–151. [Google Scholar]
- 12.Chiba K.A. New therapeutic approach for autoimmune diseases by the spingosine 1-phosphate receptor modulator fingolimod (FTY720) . Jpn. J. Clin. Immunol. 2009;32:92–101. doi: 10.2177/jsci.32.92. [DOI] [PubMed] [Google Scholar]
- 13.Furuya T. Hirotani M. Matsuzuwa M. N6- (2-hydroxyethyl) adenosine a biologically active compound from cultured mycelia of cordyceps and Isaria species. Pytochemistry. 1983;22:2509–2512. doi: 10.1016/0031-9422(83)80150-2. [DOI] [Google Scholar]
- 14.Grasa M.M. Cabot C. Esteve M. Yubero P. Masanes R.M. Blay M.T. Vila R. López-Marti J. Fernández-López J.A. Remesar X. Alemany M. Daily oral oleoyl-estrone gavage induced a dose-dependent loss of fat in Wistar rats. Obes. Res. 2001;9:202–209. doi: 10.1038/oby.2001.22. [DOI] [PubMed] [Google Scholar]
- 15.Hall J.E. Crook E.D. Jones D.W. Wofford M.R. Dubbert P.M. Mechanisms of obesity-associated cardiovascular and renal disease. Am. J. Med. Sci. 2002;324:127–137. doi: 10.1097/00000441-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 16.He M.L. Mir P.S. Okine E.K. Napadajlo H. Effect of conjugated linoleic acids from beef or industrial hydrogenation on growth and adipose tissue characteristics of rats. Nutr. Metab. 2009;6:19. doi: 10.1186/1743-7075-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jee S.H. Sull J.W. Park J. Lee S.Y. Ohrr H. Gualler E. Samet J.M. Body-mass index and mortality in Korean men and women. New Engl. J. Med. 2006;355:779–787. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 18.Jéquier E. Leptin signaling adiposity and energy balance. Ann. N.Y. Acad. Sci. 2002;967:379–388. doi: 10.1111/j.1749-6632.2002.tb04293.x. [DOI] [PubMed] [Google Scholar]
- 19.Ji S.D. Nam S.H. Jung I.Y. Cho S.Y. Song B.K. Lee H.S. Choi Y.S. The characteristics and nutrient content of entomopathogenic fungus Isaria sinclairii. in Sericulture and Entomology Research. National Institute of Agricultural Science & Entomolgy RDA; Suwon: 2001. pp. 177–183. [Google Scholar]
- 20.Kim M.J. Kim H.K. Perilla leaf extract ameliorates obesity and dyslipidemia induced by high-fat diet. Phytother. Res. 2009;23:1685–1690. doi: 10.1002/ptr.2811. [DOI] [PubMed] [Google Scholar]
- 21.Liu L.K. Chou F.P. Chen Y.C. Chayau C.C. Ho H.H. Wang C.J. Effects of mulberry (Morus alba L.) extracts on lipid homeostasis in vitro and in vivo. J. Agri. Food Chem. 2009;57:7605–7611. doi: 10.1021/jf9014697. [DOI] [PubMed] [Google Scholar]
- 22.Madsen L. Petersen R.K. Kristiansen K. Regulation of adipocyte differentiation and function by polyunsaturated fatty acids. Biochim. Biophys. Acta. 2005;1740:266–286. doi: 10.1016/j.bbadis.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Mellor H.R. Platt F.M. Dwek R.A. Butters T.D. Membrane disruption and cytotoxicity of hydrophobic N-alkylated iminosugars is independent of the inhibition of protein and lipid glycosylation. Biochem. J. 2003;374:307–314. doi: 10.1042/BJ20030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nevab M. Berliner J.A. Watson A.D. Hama S.Y. Terito M.C. Lusis A.J. Shih D.M. Van Lenten B.J. Frank J.S. Demer L.L. Edwards P.A. Fogelman A.M. The Yin and Yang oxidation in the development of the fatty streak. Arteriosclerosis Thromb. Vas. Biol. 1996;16:831–842. doi: 10.1161/01.atv.16.7.831. [DOI] [PubMed] [Google Scholar]
- 25.Neve B.P. Fruchart J.C. Staels B. Role of the peroxisome proliferator-activated receptors (PPAR) in atherosclerosis. Biochem. Pharmacol. 2000;60:1245–1250. doi: 10.1016/S0006-2952(00)00430-5. [DOI] [PubMed] [Google Scholar]
- 26.Park C.G. Park G.B. Kim Y.S. Kim S.J. Min D.B. Ha Y.L. Production of silkworm with conjuagated linoleic acid (CLA) incopotared into their lipids by dietary CLA. J.Agri. Food Chem. 2006;54:6572–6577. doi: 10.1021/jf052579w. [DOI] [PubMed] [Google Scholar]
- 27.Pico C. Sanchez J. Oliver P. Palou A. Leptin production by the stomach is up-regulation in obese (fa/fa) Zûcker rats. Obes. Res. 2002;10:932–938. doi: 10.1038/oby.2002.127. [DOI] [PubMed] [Google Scholar]
- 28.Prowlowska A.M. Oleszek W. Braca A. Qualiquantitative analyses of flavonoids of Morus nigra L. and Morus alba L. (Moraceae) Fruits. J. Agric. Food Chem. 2008;56:3377–3380. doi: 10.1021/jf703709r. [DOI] [PubMed] [Google Scholar]
- 29.Riley R.T. Voss K.A. Norred W.P. Bacon C.W. Meredith F.I. Sharma R.P. Serine palmitoyltransferase inhibition reverses anti-proliferative effects of ceramide synthase inhibiton in cultured renal cells and suppresses free sphingoid base accumulation in kidney of BALBc mice. Environ. Toxicol.Pharmacol. 1999;7:109–118. doi: 10.1016/s1382-6689(98)00047-7. [DOI] [PubMed] [Google Scholar]
- 30.Slawik M. Vidal-Puig A.J. Lipotoxicity overnutrition and energy metabolism in aging. Ageing Res. Rev. 2006;5:144–164. doi: 10.1016/j.arr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Tsuduki T. Nakamura Y. Honma T. Nakagawa K. Kimura T. Ikeda I. Miyazawa T. Intake of 1-deoxynojirimycin suppresses lipid accumulation through activation of the β-oxidation system in rat liver. J. Agri. Food. Chem. 2009;57:11024–11029. doi: 10.1021/jf903132r. [DOI] [PubMed] [Google Scholar]
- 32.Ueda H. Takahara S. Itoh S. Nomi H. Shibahara N. Katsuoka Y. Preoperative administration of FTY720 prolonged renal allograft survival. Transpl. Immunol. 2005;14:1–8. doi: 10.1016/j.trim.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Vong T. Benhaddou-Andaloussi A. Brault A. Harbilas D. Martineau L.C. Vallerand D. Ramassamy C. Matar C. Haddad P.S. Antiobesity and antidiabetic effects of biotransformed blueberry juice in KKA mice. Int. J. Obesity. 2009;33:1166–1173. doi: 10.1038/ijo.2009.149. [DOI] [PubMed] [Google Scholar]