Abstract
Bronchoalveolar lavage (BAL) is a useful tool in researches and in clinical medicine of lung diseases because the BAL fluid contains biochemical and cytological indicators of the cellular response to infection, drugs, or toxicants. However, the variability among laboratories regarding the technique and the processing of the BAL material limits clinical research. The aim of this study was to determine the suction frequency and lavage fraction number necessary to reduce the variability in lavage using male Sprague-Dawley rats. We compared the total cell number and protein level of each lavage fraction and concluded that more cells and protein can be obtained by repetitive lavage with a suction frequency of 2 or 3 than by lavage with a single suction. On the basis of total cell recovery, approximately 70% of cells were obtained from fractions 1~3. The first lavage fraction should be used for evaluation of protein concentration because fractions 2~5 of lavage fluid were diluted in manifolds. These observations were confirmed in bleomycin-induced inflamed lungs of rats. We further compared the BAL data from the whole lobes with data from the right lobes and concluded that BAL data of the right lobes represented data of the whole lobes. However, this conclusion can only be applied to general lung diseases. At the end, this study provides an insight into the technical or analytical problems of lavage study in vivo.
Keywords: Bronchoalveolar lavage, BAL fluid, Suction frequency number, Lavage fraction number
INTRODUCTION
Bronchoalveolar lavage (BAL) is a useful and commonly implemented tool in research and clinical medicine. BAL was first used to treat a patient with phosgene gas poisoning in 1922 (Gee and Fick, 1980) . This approach has now been extended to investigate the pathogenesis, diagnosis, and therapeutic management of lung diseases in humans (Daniele et al., 1985; Gee and Fick, 1980; Klech and Hutter, 1990) . In laboratory animals, Myrvik obtained alveolar macrophage from rabbit lung lavage to compare physiological and functional ability in 1961 (Gee and Fick, 1980) . Since then, BAL has been commonly analyzed for monitoring the therapeutic efficacy and toxicity in laboratory animals (Tornling et al., 1987; Henderson, 2005) . When the disease or toxicity is limited to the lung, some useful information can be obtained by BAL, whereas blood analysis does not always indicate the state of lung. BAL has been widely used for pre-clinical and clinical research because BAL fluid contains both biochemical and cytological indicators of cellular responses to infection, cancer, or inhaled drugs or toxicants (Hunninghake et al., 1979) .
One of the basic assumptions regarding BAL is that BAL data represent the whole lung status. This concept was supported by reports showing a reasonable correlation between the cellular features of open lung biopsies and the cell population derived from BAL (Haslam et al., 1980; Gee and Fick, 1980) . However, one of the main obstacles for general acceptance of BAL as a clinical or a research tool is the variability regarding the lavage technique and the processing of the BAL material among laboratories (Cordeiro and Cemylyn-Jones, 2008; Crystal et al., 1986; Gee and Fick. 1980; Baughman, 1997; Singletary et al., 2008) . Many investigators, including European Respiratory Society, have tried to establish methods (or guidelines) for the measurement and standardization of BAL in humans (Haslam and Baughman, 1999; Walters and Gardiner, 1991; Crystal et al., 1986) . To reduce variability between each BAL trial, investigators recommend a standard introduction volume of lavage fluid, a standard site, and some general standardized BAL procedures. These trials are necessary for standardization of the BAL method in laboratory animals.
The present study focused on standardizing 3 aspects of the BAL protocol. Firstly, we investigated the optimum number of times lung lavage should be performed. Secondly, we determined which fraction of BAL fluid should be used to measure protein levels. Lastly, we determined whether the BAL fluid of the right or left lobes stood for the whole lungs. Standardization of the BAL protocol will help reduce the variability between laboratories and improve the reproducibility and reliability of the BAL procedure.
MATERIALS AND METHODS
Animals.
Seven-week-old, male, Sprague-Dawley rats were obtained from Orient Bio Inc. (Korea) . Rats were housed in environmentally controlled animal facilities and the animal room was maintained at 22~24℃ with a 12 h light/dark cycle. Rats were provided with rodent chow (PMI Lab Diet, USA) and UV-irradiated tap water. Rats were acclimated for at least 1 week. All experiments were approved by the Institutional Animal Care and Use Committee and conducted in accordance with Association for Assessment and Accreditation of Laboratory Animal Care international guidelines.
Experimental design.
Three individual experiments were performed. Here, we have used the term "suction frequency number" in BAL procedure to mean the administration and aspiration in each fraction with the same lavage fluid. We have used the term "lavage fraction number" to mean the counted fraction number when we use fresh fluid buffer for each separate lavage. The same terms apply correspondingly to the following. First, we compared the cell number and protein concentration of BAL fluid based on suction frequency number (1, 2, or 3 times) with the same lavage fluid and lavage number (up to 5) . Second, 2 groups of rats were exposed to either bleomycin or saline by intratracheal instillation, and then performed BAL at 7 days after intratracheal instillation for cell counting and protein concentration measurement. Last, BAL was performed in the whole lungs or right lung lobes. Five or six rats were used in each group and the experiment was repeated twice.
Bronchoalveolar lavage based on suction times.
Rats were anesthetized with isoflurane. The abdominal cavity was opened, exsanguination was performed via the aorta abdominalis, and the chest cavity was dissected to expose the lung and the trachea. PE-90 polyethylene tubing (BD, Sparks, MD) connected to a 19-gauge needle hub was gently inserted into the trachea and 30 ml/kg of chilled saline was administered and aspirated slowly through the needle hub. To identify the effects of suction frequency on contents of lavage fluid, 3 groups were separated by suction frequency: 1 time, 2 times, or 3 times. Lavage was performed 5 times in all groups and each aspirated BAL fluids was collected in a 15 ml conical tube, respectively. BAL fluids were centrifuged at 300 g for 5 min at 4℃. BAL cells were suspended in 1 ml of saline, stained with Turk's solution, and counted using a hemocytometer (Neubauer, Marien-feld, Germany) . The lavage fluid was collected and stored at-80℃ until the assay.
Intratracheal instillation.
Rats were divided into 2 groups: with or without bleomycin-treatment. Each rat was anesthetized with isoflurane and intratracheal instillation was performed with saline or 2.5 mg/kg bleomycin hydrochloride (Nippon Kayaku, Tokyo, Japan) suspended in saline. After 1 week, we performed BAL with suction 2 times and lavage 5 times as described above.
Determination of total protein and lactate dehydrogenase level from BAL fluid.
Total proteins of BAL fluid were quantified by Bradford reagent (BioRad, Hercules, Ca) as directed by the manufacturer's protocol. Lactate dehydrogenase (LDH) was analyzed using an automated clinical chemistry analyzer, Dri-chem 3500s (Fujifilm, Tokyo, Japan) .
Statistical analysis.
Student's t-test was used to determine whether there was a statistically significant difference among the groups. A p-value ≤ 0.05 was considered statistically significant.
RESULTS
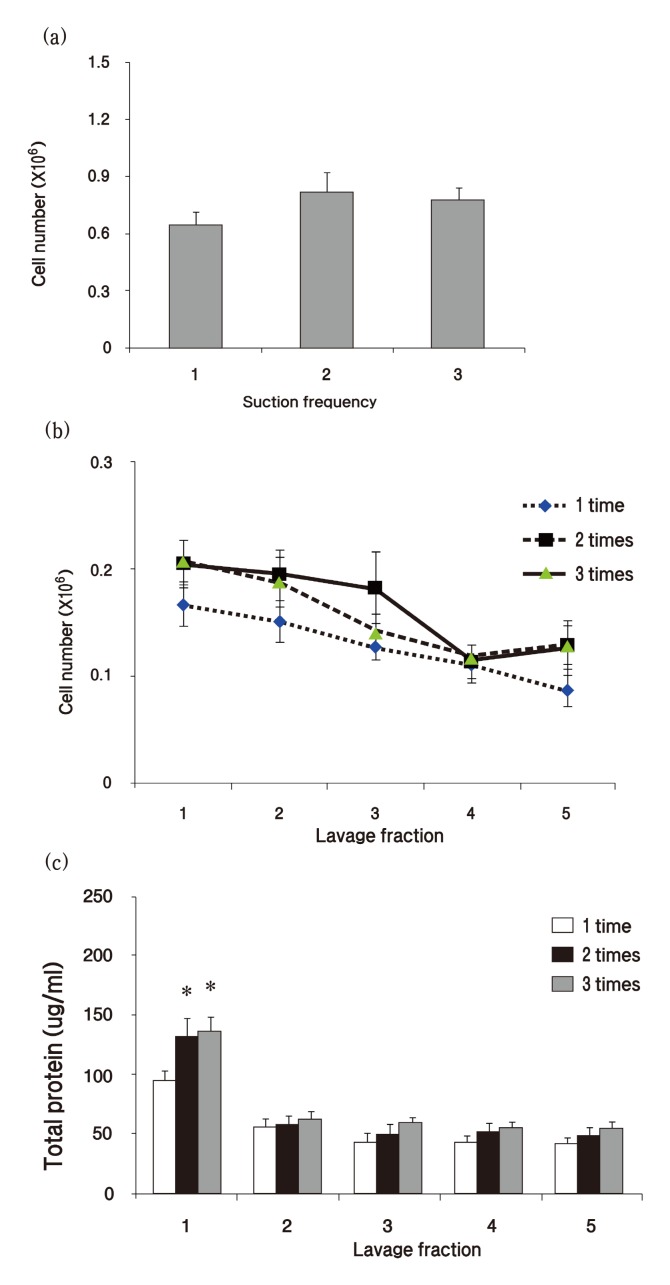
To determine the level of BAL, we first compared the total cell number that summated the cell number of 5 lavage fractions based on the suction frequency number. We observed a tendency for the number of lavaged cells to be lower with a single suction frequency than with 2 or 3 times of suction frequencies, but no significant differences were observed in the summation of the cell counts (Fig. 1A) . Next, we evaluated the pattern of counted cells in each fraction of BAL fluid in the normal rats. The decrease in cell count took place over the sequence of lavage, and the first and the second lavage fraction contained similar numbers of cells (Fig. 1B) . Based on the total cell recovery, approximately 70% of cells were obtained from lavage fractions 1~3.
Fig. 1. Measurement of cell number and protein concentration of bronchoalveolar lavage (BAL) fluid based on suction frequency number. (a) Total cells were stained with Turk’s solution and counted by a hemocytometer. (b) Cells were counted in each fraction with different suction frequencies. (c) Total protein concentration was measured with Bradford assay in each BAL fraction based on different suction frequencies. Graph values (mean ± S.E.M.) represent 12 animals in each case (n = 12) . *p ≤ 0.05 vs. 1 time suction frequency group.
To determine which BAL fluid fraction should be used for biochemical assays, we performed lavage 5 times on each suction frequency group and compared the total protein concentration of each lavage fraction. As shown in Fig. 1C, the total protein level of the first fraction of BAL fluid was approximately twice as much as the second lavage fraction. The protein concentration of the first fraction of BAL fluid obtained by a single suction was significantly lower than the protein concentration obtained from 2 or 3 suctions.
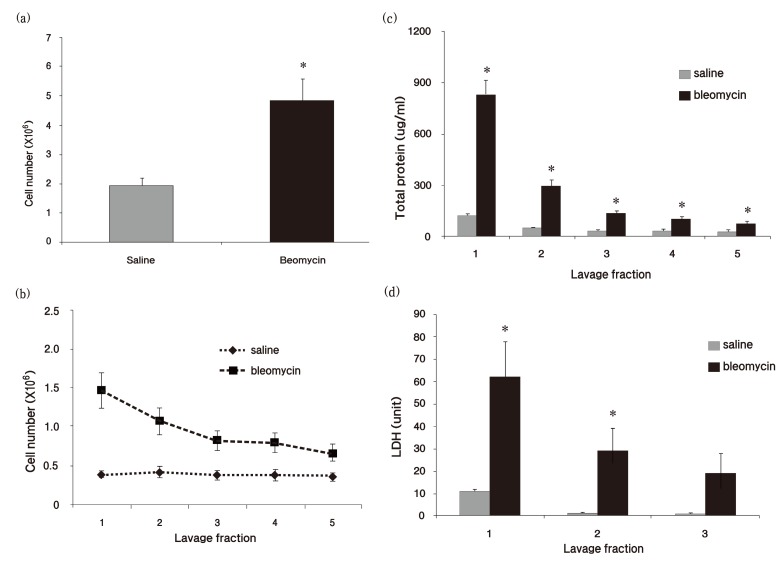
BAL is a time-consuming activity, so we concluded that a suction frequency of 2 times was sufficient for lung lavage based on the above results. To confirm this conclusion, we induced lung inflammation by performing intratracheal instillation of bleomycin hydrochloride (2.5 mg/kg) and evaluated the cell number in BAL fluid 7 days after exposure to bleomycin. A significant increase in cell number was observed in inflamed lungs (Fig. 2A) . The cell number of the first fraction was hugely increased compared to the second fraction in bleomycin-induced inflamed treated lung, whereas no significance was seen between the first and the second fraction in saline-treated lung (Fig. 2B) . Consistent with data from normal rats, approximately 70% of cells were obtained from fractions 1~3 based on the total cell recovery, whereas cell number was decreased depending on the lavage fraction number (Fig. 2B) . Total protein and LDH levels of the first fraction were also elevated 6 times and 5 times, respectively, in bleomycin-treated lung than in saline-treated lung (Fig. 2C and D) . However, decreased total protein and LDH levels were observed over the lavage sequence in the inflamed lungs.
Fig. 2. Measurement of cell number and protein concentration in bleomycin-treated rats. Rats were exsanguinated and BAL was performed 7 days after intratracheal instillation of bleomycin. Total cells were stained with Turk’s solution and counted by a hemocytometer. (a) Total cells were counted in the summated fraction from saline- or bleomycin-treated groups. (b) Cell number was assessed in each lavage fraction. (c) Total protein concentration and (d) lactate dehydrogenase (LDH) level were measured in each lavage fraction. LDH level was analyzed by an automated clinical chemistry analyzer Dri-chem 3500s. Graph values (mean ± S.E.M.) represent 12 animals in each case (n = 12) . *p ≤ 0.05 vs. saline-treated group.
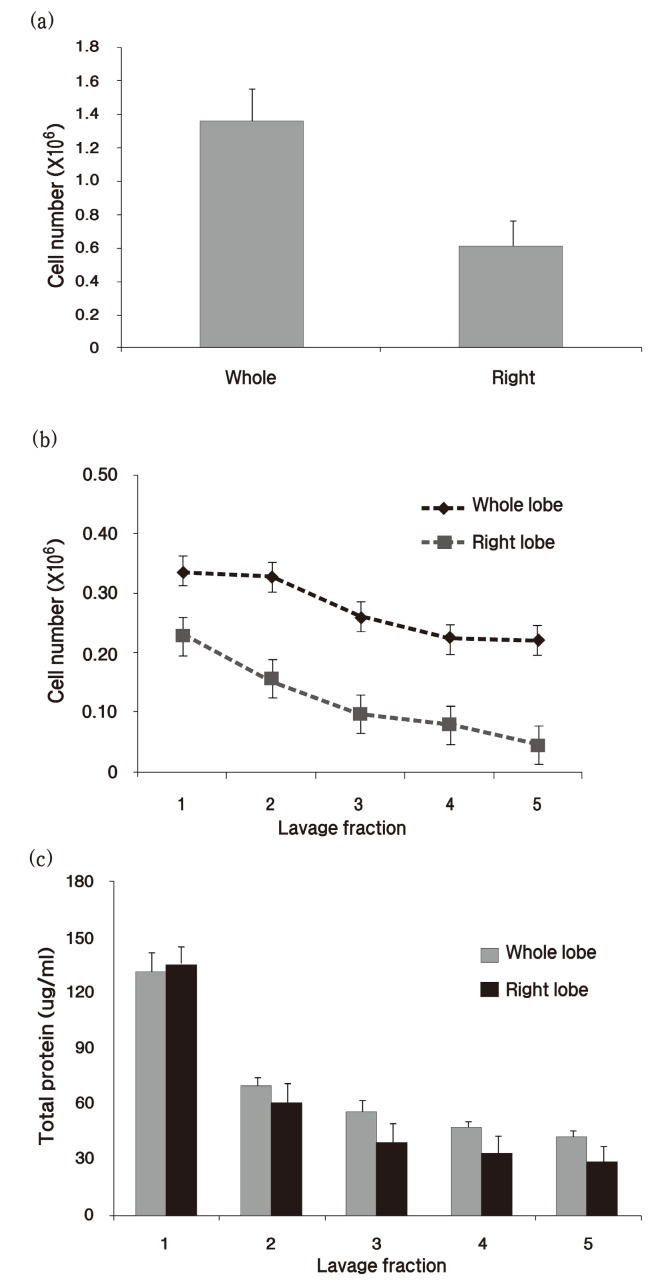
Last, we compared the cell number and protein concentration in the whole lungs and the right lobes of rats. Whole lungs were washed with 30 ml/kg of saline, and right lungs lobes were washed with the half of that volume. Consistent with the total cell number result, the total protein levels in the right lobes and in the whole lobes were the same (Fig. 3) .
Fig. 3. Total cell count and protein concentration were compared between whole lobes and right lobes. (a) Total cells were counted in the summated fraction of BAL fluid from whole lobes or right lobes. (b) Cell number was assessed in each lavage fraction. (c) Total protein concentration was measured in each lavage fraction. Graph values (mean ± S.E.M.) represent 10 animals in each case (n = 10) .
DISCUSSION
There are many variations in the lavage procedure and a number of factors complicate the quantification of BAL data. The aim of this study was to establish a BAL protocol that correctly reflects the lung status and reduces potential procedural artifacts. BAL can be obtained in many ways, which include massaging the lungs, using gravity, gentle manual aspiration and others (Forget et al., 1983; Kim et al., 2009; Rehn et al., 1992; Walters et al., 2000; Varner et al., 1999) . We adopted the gentle manual aspiration method to reduce individual difference.
In the present study, we investigated the effect of frequency of lung suction during BAL performance. There is no standard recommendation regarding suction frequency, which affects the total cell number and protein level. Our results in this study indicate that a suction frequency of 2 or 3 times results in extraction of lung lining fluid that contain more cells and protein than a single suction frequency. In addition, the protein concentration of the first lavage fraction of BAL fluid obtained by a suction frequency 2 or 3 times was much higher than the protein concentration by 1 time suction, leading us to conclude that a suction frequency of 2 times or 3 times was more suitable than a suction frequency of 1 time. Our laboratory subsequently adopted a suction frequency of 2 times as opposed to 3 times, because BAL fluid is apt to leak if suction is repeatedly performed in a severely injured lung. A further reason to adopt a suction frequency of 2 rather than 3 times is because the extra time and labor required to perform the procedure 3 times did not result in differences in BAL cells and protein levels compared to 2 times in our study.
Lavage fraction number can vary from 2~14 times in different laboratories (Forget et al., 1983; Sung et al., 2004; Majetschak et al., 2009) . To estimate the total cell number in the lung, it is important to recognize which BAL fluid fraction determines the total cell number. The first and the second lavages contained similar number of cells in normal rats, but the first lavage included more cells than the second lavage in bleomycin-treated lungs. Several reports suggested that more cells are present in the second lavage than in the first (Rehn et al., 1992; Kelly et al., 1988) . These studies indicate that a single lavage may not represent the whole lung and may introduce misinterpretation of the results in slightly damaged lungs. Approximately 70% of total lavaged cells were retrieved in fractions 1~3, so we concluded that 3 lavage times are sufficient for collecting cells that reflect the lung status. We confirmed our results in bleomycin-induced inflamed lungs.
A great deal of valuable information can be obtained from the acellular component of BAL fluid including levels of immunoglobulins, enzymes, inflammatory mediators, and surfactant (Henderson, 1984; Eklund et al., 1991; Olsen et al., 1975) . However, variable dilutions of BAL fluid can cause both inaccuracy of quantification and difficulties detecting trace amounts of solute (Walters and Gardiner, 1991) . We measured total protein and LDH levels, which are most commonly measured to detect lung damage (Drent et al., 1996; Henderson, 1984) . Total protein and LDH levels of the first BAL fraction of retrieved BAL components contained 2~3 times more than what was retrieved in the second BAL fraction, indicating an increased dilution effect of BAL components. Based on our results, we recommend that the first fraction of BAL fluid should be used for acellular analysis. However, the small volume of BAL fluid in mice impedes the analysis of acellular components and therefore pooling the BAL fluid of each mouse or combining the first and the second fractions may be a solution for measuring BAL fluid components.
Finally, we compared the cell number and protein concentration in the whole lung and right lobes, because many researchers use one lobe for histopathology and the other for BAL for animal welfare or to simplify the experiments. OECD Guideline No.39 recommends that one half of the lung is used for histopathology, and the other half used for BAL (OECD Guideline No.39, 2009) . In our study, the total cell number of whole lobes was approximately twice the cell number of right lobes but the difference in protein levels between the 2 groups was negligible. We, therefore, concluded that BAL data from right lobes represent the whole lobes. However, this conclusion can be only applied to generalized lung diseases.
There are many BAL-associated details to be further determined, including the issue of erythrocytes. BAL fluid contains some erythrocytes when lung is not exsanguinated fully and it may contain erythrocytes even when an inflamed lung is exsanguinated fully. Erythrocytes can affect the acellular component of BAL and could lead to faulty cell count results. A possible solution is to separate cells from the fluid as quickly as possible by centrifugation and erythrocytes should be removed completely for cell count.
A vast amount information can be drawn from BAL including levels of important disease markers using analysis methods like ELISA, NMR, or mass spectrometry (Bowler et al., 2006; Hirsch et al., 2004) , and these new analytic techniques will widen and extend the practical usage of BAL in the future. To obtain reliable data from BAL, a validated and standardized lavage process is necessary for future applications
Acknowledgments
This study was supported by the Ministry of Knowledge Economy for General Project Grant of the establishment of the Development of International Inhalation Toxicology Technology in Korea Institute of Toxicology.
References
- 1.Baughman R.P. The uncertainties of bronchoalveolar lavage. Eur. Respir. J. 1997;10:1940–1942. doi: 10.1183/09031936.97.10091940. [DOI] [PubMed] [Google Scholar]
- 2.Bowler R.P. Ellison M.C. Reisdorph N. Proteomics in pulmonary medicine. Chest. 2006;130:567–574. doi: 10.1378/chest.130.2.567. [DOI] [PubMed] [Google Scholar]
- 3.Cordeiro C.R. Cemlyn-Jones J. Bronchoalveolar Lavage - Do We Need It? Eur. Respir. Dis. 2008;4:33–35. [Google Scholar]
- 4.Crystal R.G. Reynolds H.Y. Kalica A.R. Bronchoalveolar lavage. The report of an international conference. Chest. 1986;90:122–131. doi: 10.1378/chest.90.1.122. [DOI] [PubMed] [Google Scholar]
- 5.Daniele R.P. Elias J.A. Epstein P.E. Rossman M.D. Bronchoalveolar lavage: role in the pathogenesis diagnosis and management of interstitial lung disease. Ann. Intern. Med. 1985;102:93–108. doi: 10.7326/0003-4819-102-1-93. [DOI] [PubMed] [Google Scholar]
- 6.Drent M. Cobben N.A. Henderson R.F. Wouters E.F. van Dieijen-Visser M. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur. Respir. J. 1996;9:1736–1742. doi: 10.1183/09031936.96.09081736. [DOI] [PubMed] [Google Scholar]
- 7.Eklund A. Tornling G. Blaschke E. Curstedt T. Extracellular matrix components in bronchoalveolar lavage fluid in quartz exposed rats. Br. J. Ind. Med. 1991;48:776–82. doi: 10.1136/oem.48.11.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forget G. Lacroix M.J. Cadieux A. Calvert R. Grose J.H. Sirois P. An adherent cell perifusion technique to study the overall and sequential response of rat alveolar macrophages to toxic substances. Environ Health Perspect. 1983;51:131–140. doi: 10.2307/3429740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gee J.B. Fick R.B Jr. Bronchoalveolar lavage. Thorax. 1980;35:1–8. doi: 10.1136/thx.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haslam P.L. Turton C.W. Heard B. Lukoszek A. Collins J.V. Salsbury A.J. Turner-Warwick M. Bronchoalveolar lavage in pulmonary fibrosis: comparison of cells obtained with lung biopsy and clinical features. Thorax. 1980;35:9–18. doi: 10.1136/thx.35.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haslam P.L. Baughman R.P. Report of ERS Task Force: guidelines for measurement of acellular components and standardization of BAL. Eur. Respir. J. 1999;14:245–248. doi: 10.1034/j.1399-3003.1999.14b01.x. [DOI] [PubMed] [Google Scholar]
- 12.Henderson R.F. Use of bronchoalveolar lavage to detect lung damage. Environ. Health Perspect. 1984;56:115–129. doi: 10.2307/3429841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson R.F. Use of bronchoalveolar lavage to detect respiratory tract toxicity of inhaled material. Exp. Toxicol. Pathol. 2005;Suppl 1:155–159. doi: 10.1016/j.etp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch J. Hansen K.C. Burlingame A.L. Matthay M.A. Proteomics: current techniques and potential applications to lung disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L1–L23. doi: 10.1152/ajplung.00301.2003. [DOI] [PubMed] [Google Scholar]
- 15.Hunninghake G.W. Gadek J.E. Kawanami O. Ferrans V.J. Crystal R.G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am. J. Pathol. 1979;97:149–206. [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly C.A. Ward C. Stenton S.C. Hendrick D.J. Walters E.H. Assessment of pulmonary macrophage and neutrophil function in sequential bronchoalveolar lavage aspirates in sarcoidosis. Thorax. 1988;43:787–791. doi: 10.1136/thx.43.10.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J.Y. Choeng H.C. Ahn C.M. Cho S.H. Early and late changes of MMP-2 and MMP-9 in bleomycin-induced pulmonary fibrosis. Yonsei Med. J. 2009;50:68–77. doi: 10.3349/ymj.2009.50.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klech H. Hutter C. Clinical guidelines and indications for bronchoalveolar lavage (BAL) : Report of the european society of pneumology task group on BAL. Eur. Respir. J. 1990;3:937–976. [PubMed] [Google Scholar]
- 19.Majetschak M. Sorell L.T. Patricelli T. Seutzm D.H. Knoferl M.W. Detection and possible role of proteasomes in the bronchoalveolar space of the injured lung. Physiol. Res. 2009;58:363–372. doi: 10.33549/physiolres.931526. [DOI] [PubMed] [Google Scholar]
- 20.OECD Environment Health and Safety Publications. Series on Testing and Assessment No. 39 Guidance document on acute inhalation toxicity testing. Environment Directorate OECD; Paris: 2009. 2009. pp. 47–49. [Google Scholar]
- 21.Olsen G.N. Harris J.O. Castle J.R. Waldman R.H. Karmgard H.J. Alpha-1-antitrypsin content in the serum alveolar macrophages and alveolar lavage fluid of smoking and nonsmoking normal subjects. J. Clin. Invest. 1975;55:427–430. doi: 10.1172/JCI107947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rehn B. Bruch J. Zou T. Hobusch G. Recovery of rat alveolar macrophages by broncholaveolar lavage under normal and activated conditions. Environ. Health Perspect. 1992;97:11–16. doi: 10.1289/ehp.929711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singletary M.L. Phillippi-Falkenstein K.M. Scanlon E. Bohm R.P. Jr. Veazey R.S. Gill A.F. Modification of a common BAL technique to enhance sample diagnostic value. J. Am. Assoc. Lab. Anim. Sci. 2008;47:47–51. [PMC free article] [PubMed] [Google Scholar]
- 24.Sung J.H. Choi B.G. Maeng S.H. Kim S.J. Chung Y.H. Han J.H. Song K.S. Lee Y.H. Cho Y.B. Cho M.H. Kim K.J. Hyun J.S. Yu I.J. Recovery from welding-fumeexposure- induced lung fibrosis and pulmonary function changes in sprague dawley rats. Toxicol. Sci. 2004;82:608–613. doi: 10.1093/toxsci/kfh289. [DOI] [PubMed] [Google Scholar]
- 25.Tornling G. Eklund A. Engström-Laurent A. Hällgren R. Unge G. Westman B. Hyaluronic acid in bronchoalveolar lavage in rats exposed to quartz. Br. J. Ind .Med. 1987;44:443–445. doi: 10.1136/oem.44.7.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varner A.E. Sorkness R.L. Kaplan A. Kaplan M.R. Lemanske R.F. Jr. Serial segmental bronchoalveolar lavage in individual rats. J. Appl. Physiol. 1999;87:1230–1233. doi: 10.1152/jappl.1999.87.3.1230. [DOI] [PubMed] [Google Scholar]
- 27.Walters D.M. Wills-Karp. M. Mitzner W. Assessment of cellular profile and lung function with repeated bronchoalveolar lavage in individual mice. Physiol Genomics. 2000;2:29–36. doi: 10.1152/physiolgenomics.2000.2.1.29. [DOI] [PubMed] [Google Scholar]
- 28.Walters E.H. Gardiner P.V. Bronchoalveolar lavage as a research tool. Thorax. 1991;46:613–618. doi: 10.1136/thx.46.9.613. [DOI] [PMC free article] [PubMed] [Google Scholar]