Abstract
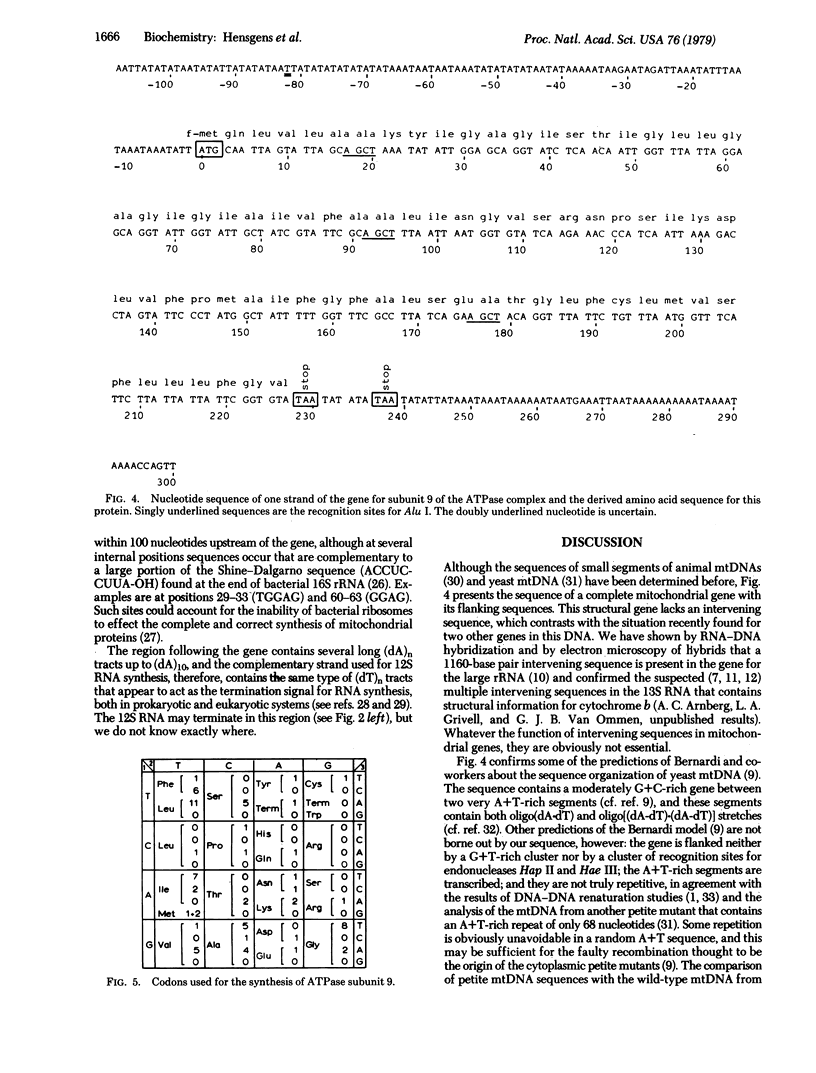
We have determined the nucleotide sequence of a segment of Saccharomyces mtDNA that contains the structural gene for one of the subunits (the dicyclohexylcarbodiimide-binding protein) of the mitochondrial ATPase complex. The sequence fits the known amino acid sequence of this protein with the exception of one amino acid. Codon usage is biased in favor of A + T-rich codons. On both sides of the gene, the nucleotide sequence contains less than 4% (mol/mol) G + C for at least 180 nucleotides; these A + T sequences show no evidence of internal repetition. The gene and all the A + T-rich sequence preceding the gene are present in a 12S RNA that is the major transcript of this segment of mtDNA. The nature of the sequences responsible for binding ribosomes to mitochondrial mRNA and for termination of RNA synthesis is considered.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernardi G., Piperno G., Fonty G. The mitochondrial genome of wild-type yeast cells. I. Preparation and heterogeneity of mitochondrial DNA. J Mol Biol. 1972 Mar 28;65(2):173–189. doi: 10.1016/0022-2836(72)90275-6. [DOI] [PubMed] [Google Scholar]
- Bernardi G., Timasheff S. N. Optical rotatory dispersion and circular dichroism properties of yeast mitochondrial DNA's. J Mol Biol. 1970 Feb 28;48(1):43–52. doi: 10.1016/0022-2836(70)90217-2. [DOI] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. The mitochondrial genome of yeast. Cell. 1978 Nov;15(3):705–723. doi: 10.1016/0092-8674(78)90257-x. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Heyting C., Borst P., Arnberg A. C., Van Bruggen E. F. An insert in the single gene for the large ribosomal RNA in yeast mitochondrial DNA. Nature. 1978 Sep 28;275(5678):336–338. doi: 10.1038/275336a0. [DOI] [PubMed] [Google Scholar]
- Boseley P. G., Tuyns A., Birnstiel M. L. Mapping of the Xenopus laevis 5.8S rDNA by restriction and DNA sequencing. Nucleic Acids Res. 1978 Apr;5(4):1121–1137. doi: 10.1093/nar/5.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen C., Christiansen G., Bak A. L. Heterogeneity of mitochondrial DNA from Saccharomyces carlsbergensis: renaturation and sedimentation studies. J Mol Biol. 1974 Mar 25;84(1):65–82. doi: 10.1016/0022-2836(74)90212-5. [DOI] [PubMed] [Google Scholar]
- DeFilippes F. M. A new method for isolation of a restriction enzyme from Hemophilus parainfluenzae. Biochem Biophys Res Commun. 1974 Jun 4;58(3):586–596. doi: 10.1016/s0006-291x(74)80460-2. [DOI] [PubMed] [Google Scholar]
- Deutsch J., Dujon B., Netter P., Petrochilo E., Slonimski P. P., Bolotin-Fukuhara M., Coen D. Mitochondrial genetics. VI. The petite mutation in Saccharomyces cerevisiae: interrelations between the loss of the p+ factor and the loss of the drug resistance mitochondrial genetic markers. Genetics. 1974 Feb;76(2):195–219. doi: 10.1093/genetics/76.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum A. M., Clayton D. A. Displacement-loop replication initiation sequence in animal mitochondrial DNA exists as a family of discrete lengths. Proc Natl Acad Sci U S A. 1978 Feb;75(2):677–681. doi: 10.1073/pnas.75.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg C. P., Borst P., van Bruggen E. F. Mitochondrial DNA. V. A 25 micron closed circular duplex DNA molecule in wild-type yeast mitochondria. Stucture and genetic complexity. Biochim Biophys Acta. 1970 May 21;209(1):1–15. [PubMed] [Google Scholar]
- Maat J., Smith A. J. A method for sequencing restriction fragments with dideoxynucleoside triphosphates. Nucleic Acids Res. 1978 Dec;5(12):4537–4545. doi: 10.1093/nar/5.12.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system: partial sequence of a mitochondrial ATPase gene in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Jan;76(1):131–135. doi: 10.1073/pnas.76.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R., Schneller J. M., Stahl A. J., Dirheimer G. Studies of odd bases in yeast mitochondrial tRNA: II. Characterization of rare nucleosides. Biochem Biophys Res Commun. 1976 Jun 7;70(3):997–1002. doi: 10.1016/0006-291x(76)90690-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunell A., Bernardi G. The mitochondrial genome of wild-type yeast cells. VI. Genome organization. J Mol Biol. 1977 Feb 15;110(1):53–74. doi: 10.1016/s0022-2836(77)80098-3. [DOI] [PubMed] [Google Scholar]
- Reijnders L., Kleisen C. M., Grivell L. A., Borst P. Hybridization studies with yeast mitochondrial RNAs. Biochim Biophys Acta. 1972 Jul 20;272(3):396–407. doi: 10.1016/0005-2787(72)90392-9. [DOI] [PubMed] [Google Scholar]
- Sanders J. P., Borst P., Weijers P. J. The organization of genes in yeast mitochondrial DNA II. The physical map of EcoRI and HindII + III fragments. Mol Gen Genet. 1975 Dec 30;143(1):53–64. doi: 10.1007/BF00269420. [DOI] [PubMed] [Google Scholar]
- Sanders J. P., Flavell R. A., Borst P., Mol J. N. Nature of the base sequence conserved in the mitochondrial DNA of a low-density petite. Biochim Biophys Acta. 1973 Jul 13;312(3):441–457. doi: 10.1016/0005-2787(73)90443-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweyen R. J., Weiss-Brummer B., Backhaus B., Kaudewitz F. The genetic map of the mitochondrial genome in yeast: map positions of drug' and mit- markers as revealed from population analyses of rho- clones in Saccharomyces cerevisiae. Mol Gen Genet. 1978 Feb 16;159(2):151–160. doi: 10.1007/BF00270888. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Bell G. I., Venegas A., Sewell E. T., Masiarz F. R., DeGennaro L. J., Weinberg F., Rutter W. J. Ribosomal RNA genes of Saccharomyces cerevisiae. II. Physical map and nucleotide sequence of the 5 S ribosomal RNA gene and adjacent intergenic regions. J Biol Chem. 1977 Nov 25;252(22):8126–8135. [PubMed] [Google Scholar]
- Van Kreijl C. F., Bos J. L. The repeating nucleotide sequence in the repetitive mitochondrial DNA from a "low-density" petite mutant of yeast. Nucleic Acids Res. 1977 Jul;4(7):2369–2388. doi: 10.1093/nar/4.7.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ommen G. J., Groot G. S., Borst P. Fine structure physical mapping of 4S RNA genes on mitochondrial DNA of Saccharomyces cerevisiae. Mol Gen Genet. 1977 Sep 9;154(3):255–262. doi: 10.1007/BF00571280. [DOI] [PubMed] [Google Scholar]




